Investigation of Different Methods of Intraoperative Graft Perfusion Assessment during Kidney Transplantation for the Prediction of Delayed Graft Function: A Prospective Pilot Trial
Abstract
1. Introduction
2. Materials and Methods
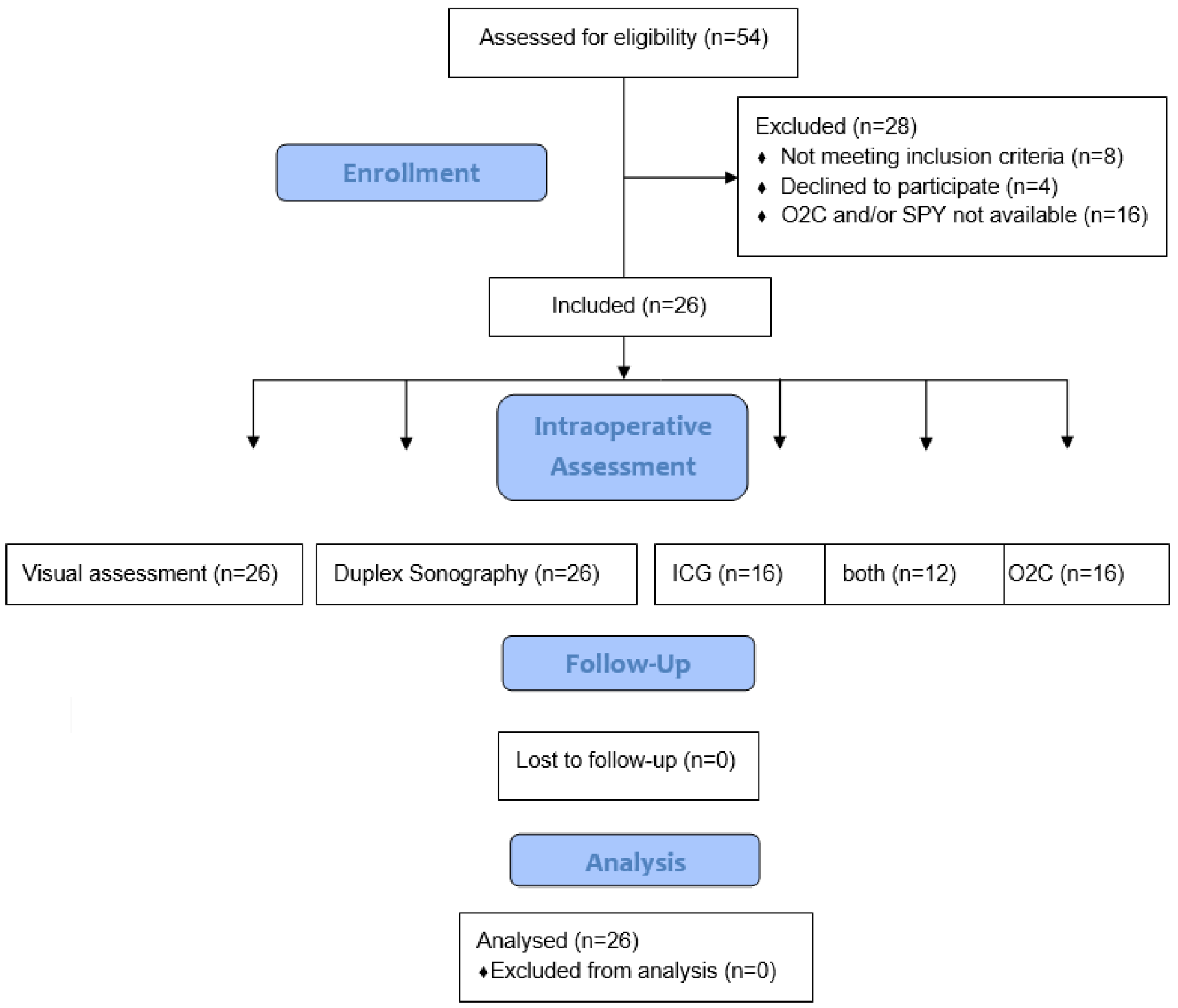
2.1. Inclusion Criteria and Patient Selection
2.2. Study Design and Procedure
2.3. Visual and Formal Perfusion Assessment
2.4. Macroperfusion Assessment by Ultrasound
2.5. Intraoperative Spectrometry of Allograft Microperfusion with Oxygen to See (O2C)
2.6. Intraoperative Fluorescence Angiography with ICG
2.7. Clinical Parameters of Graft Function
2.8. Statistical Analysis
3. Results
3.1. Patients and Procedure Characteristics
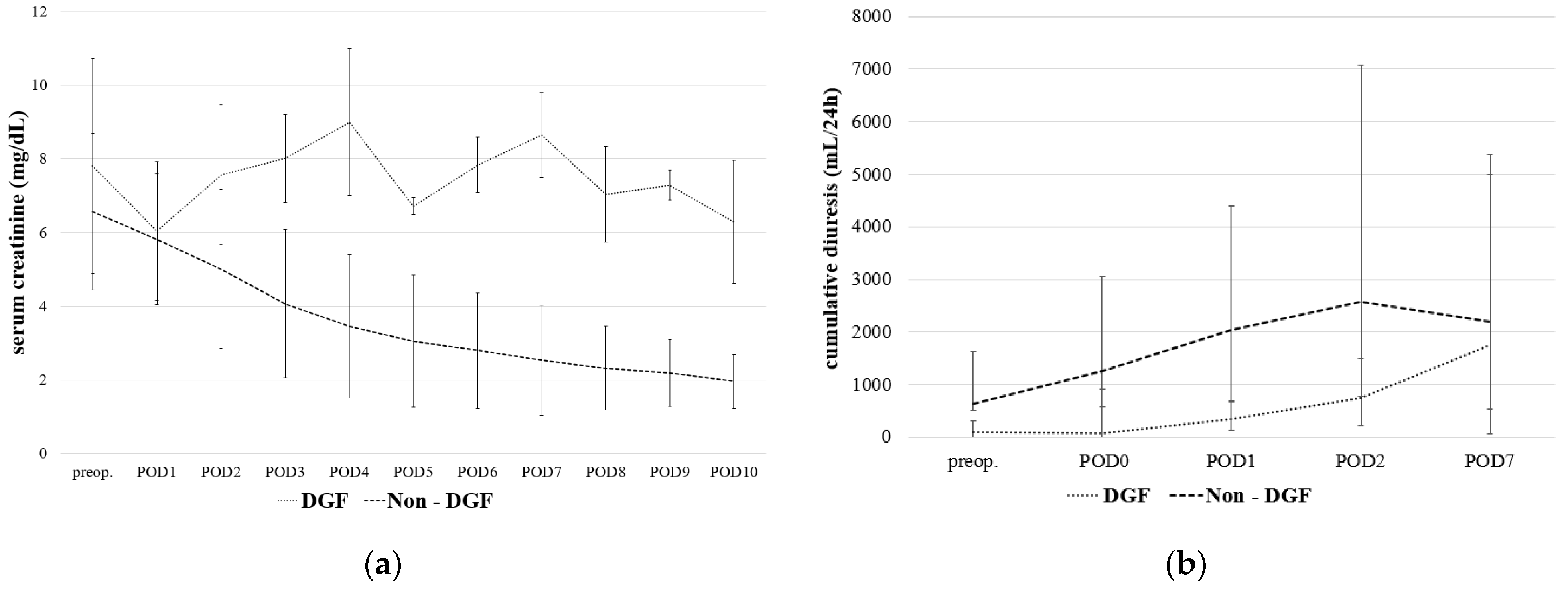
3.2. Postoperative Results and Delayed Graft Function
3.3. Formal and Visual Perfusion Assessment
3.4. Perfusion Assessment by Ultrasound
3.5. Association between Intraoperative Perfusion Analysis with ICG and Delayed Graft Function
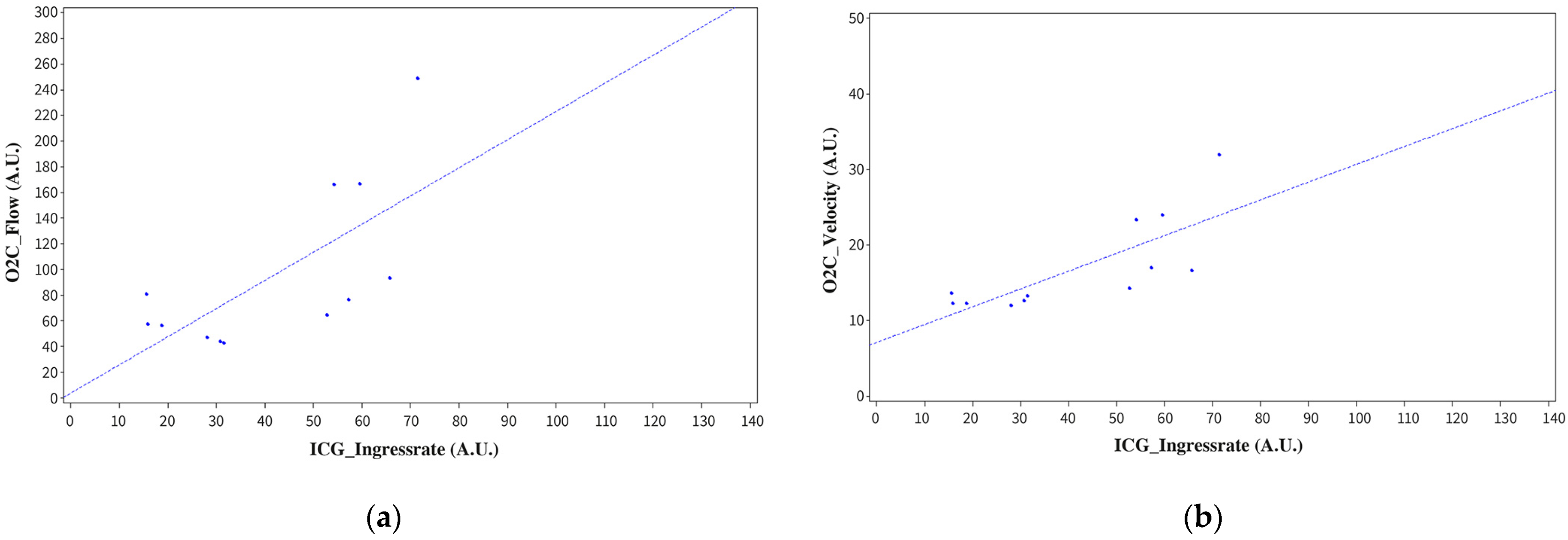
3.6. Correlation of Intraoperative Perfusion Assessment with O2C and ICG
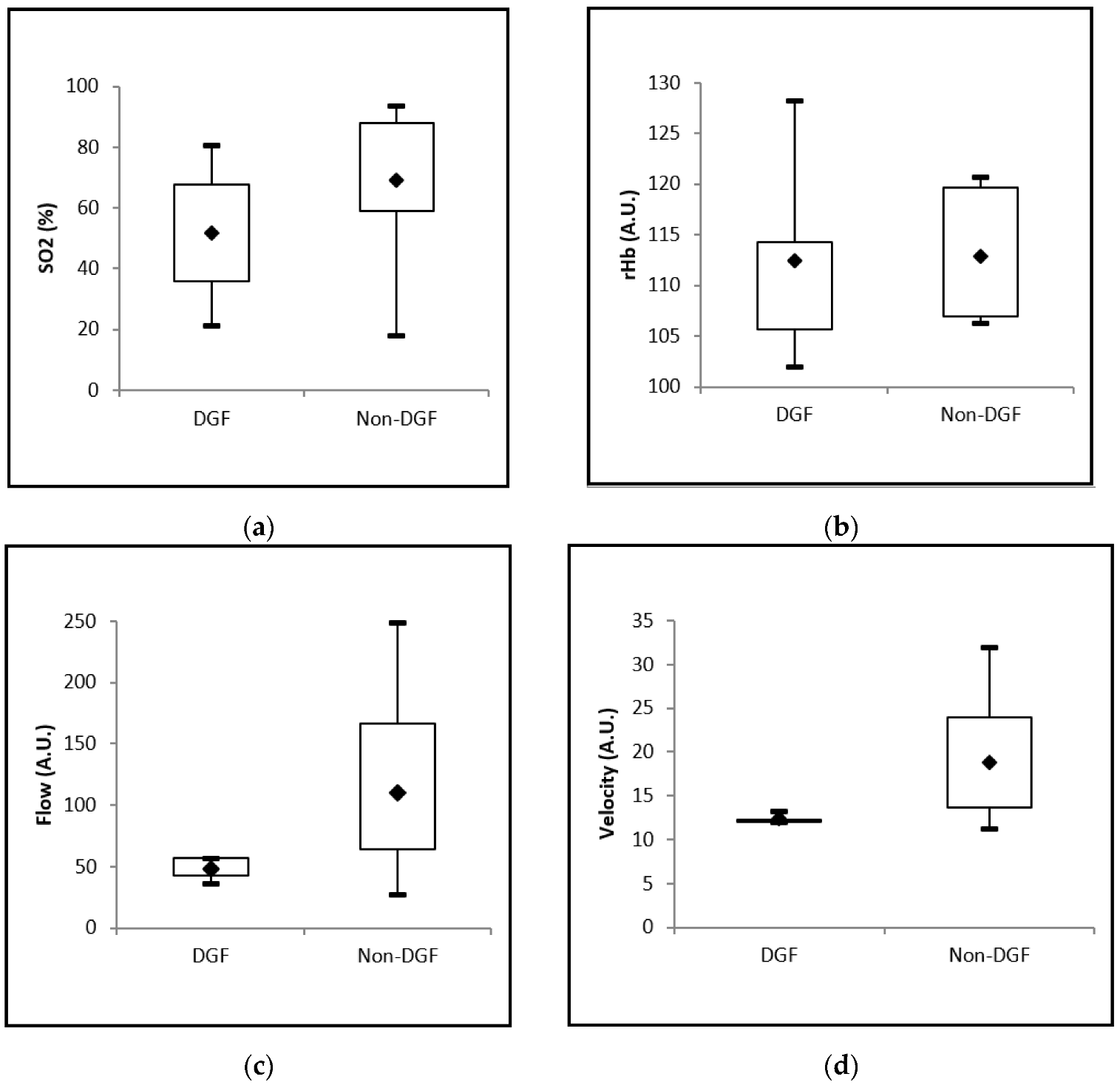
3.7. Association between Intraoperative Perfusion Analysis with O2C and Delayed Graft Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siedlecki, A.; Irish, W.; Brennan, D.C. Delayed graft function in the kidney transplant. Am. J. Transplant. 2011, 11, 2279–2296. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.O.; Wolfe, R.A.; Held, P.J.; Port, F.K.; Schmouder, R.L. Delayed graft function: Risk factors and implications for renal allograft survival. Transplantation 1997, 63, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Koning, O.H.; Ploeg, R.J.; van Bockel, J.H.; Groenewegen, M.; van der Woude, F.J.; Persijn, G.G.; Hermans, J. Risk factors for delayed graft function in cadaveric kidney transplantation: A prospective study of renal function and graft survival after preservation with University of Wisconsin solution in multi-organ donors. European Multicenter Study Group. Transplantation 1997, 63, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Perico, N.; Cattaneo, D.; Sayegh, M.H.; Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 2004, 364, 1814–1827. [Google Scholar] [CrossRef]
- Doshi, M.D.; Garg, N.; Reese, P.P.; Parikh, C.R. Recipient risk factors associated with delayed graft function: A paired kidney analysis. Transplantation 2011, 91, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Dahmane, D.; Audard, V.; Hiesse, C.; Pessione, F.; Bentaarit, B.; Barrou, B.; Rondeau, E.; Cohen, S.; Lang, P.; Grimbert, P. Retrospective follow-up of transplantation of kidneys from ‘marginal’ donors. Kidney Int. 2006, 69, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Lin, M.Z.; Zhou, H.L.; Li, H.; Sun, Q.P.; Huang, Z.Y.; Hong, L.Q.; Wang, G.; Cai, R.M.; Sun, Q.Q. Delayed graft function is correlated with graft loss in recipients of expanded-criteria rather than standard-criteria donor kidneys: A retrospective, multicenter, observation cohort study. Chin. Med. J. 2020, 133, 561–570. [Google Scholar] [CrossRef]
- Metzger, R.A.; Delmonico, F.L.; Feng, S.; Port, F.K.; Wynn, J.J.; Merion, R.M. Expanded criteria donors for kidney transplantation. Am. J. Transplant. 2003, 3 (Suppl. 4), 114–125. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Zamora, J.; Pirsch, J.D. A systematic review of kidney transplantation from expanded criteria donors. Am. J. Kidney Dis. 2008, 52, 553–586. [Google Scholar] [CrossRef] [PubMed]
- Querard, A.H.; Foucher, Y.; Combescure, C.; Dantan, E.; Larmet, D.; Lorent, M.; Pouteau, L.M.; Giral, M.; Gillaizeau, F. Comparison of survival outcomes between Expanded Criteria Donor and Standard Criteria Donor kidney transplant recipients: A systematic review and meta-analysis. Transpl. Int. 2016, 29, 403–415. [Google Scholar] [CrossRef]
- Yarlagadda, S.G.; Coca, S.G.; Formica, R.N., Jr.; Poggio, E.D.; Parikh, C.R. Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2009, 24, 1039–1047. [Google Scholar] [CrossRef]
- Fonseca, I.; Teixeira, L.; Malheiro, J.; Martins, L.S.; Dias, L.; Castro Henriques, A.; Mendonca, D. The effect of delayed graft function on graft and patient survival in kidney transplantation: An approach using competing events analysis. Transpl. Int. 2015, 28, 738–750. [Google Scholar] [CrossRef]
- Redfield, R.R.; Scalea, J.R.; Zens, T.J.; Muth, B.; Kaufman, D.B.; Djamali, A.; Astor, B.C.; Mohamed, M. Predictors and outcomes of delayed graft function after living-donor kidney transplantation. Transpl. Int. 2016, 29, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.A.; Brabrand, K.; Egge, T.S.; Hartmann, A. Doppler examination of the allografted kidney. Acta Radiol. 2003, 44, 3–12. [Google Scholar] [CrossRef] [PubMed]
- van Weel, V.; van Bockel, J.H.; van Wissen, R.; van Baalen, J.M. Intraoperative renal duplex sonography: A valuable method for evaluating renal artery reconstructions. Eur. J. Vasc. Endovasc. Surg. 2000, 20, 268–272. [Google Scholar] [CrossRef][Green Version]
- Mocny, G.; Bachul, P.; Chang, E.S.; Kulig, P. The value of Doppler ultrasound in predicting delayed graft function occurrence after kidney transplantation. Folia Med. Cracov. 2016, 56, 51–62. [Google Scholar] [PubMed]
- Rother, U.; Gerken, A.L.H.; Karampinis, I.; Klumpp, M.; Regus, S.; Meyer, A.; Apel, H.; Kramer, B.K.; Hilgers, K.; Lang, W.; et al. Dosing of indocyanine green for intraoperative laser fluorescence angiography in kidney transplantation. Microcirculation 2017, 24, e12392. [Google Scholar] [CrossRef]
- Rother, U.; Amann, K.; Adler, W.; Nawroth, N.; Karampinis, I.; Keese, M.; Manap, S.; Regus, S.; Meyer, A.; Porubsky, S.; et al. Quantitative assessment of microperfusion by indocyanine green angiography in kidney transplantation resembles chronic morphological changes in kidney specimens. Microcirculation 2019, 26, e12529. [Google Scholar] [CrossRef]
- Gerken, A.L.H.; Nowak, K.; Meyer, A.; Weiss, C.; Kruger, B.; Nawroth, N.; Karampinis, I.; Heller, K.; Apel, H.; Reissfelder, C.; et al. Quantitative Assessment of Intraoperative Laser Fluorescence Angiography with Indocyanine Green Predicts Early Graft Function after Kidney Transplantation. Ann. Surg. 2020, 276, 391–397. [Google Scholar] [CrossRef]
- Rother, U.; Krenz, K.; Lang, W.; Horch, R.E.; Schmid, A.; Heinz, M.; Meyer, A.; Regus, S. Immediate changes of angiosome perfusion during tibial angioplasty. J. Vasc. Surg. 2017, 65, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Rother, U.; Grussler, A.; Griesbach, C.; Almasi-Sperling, V.; Lang, W.; Meyer, A. Safety of medical compression stockings in patients with diabetes mellitus or peripheral arterial disease. BMJ Open Diabetes Res. Care 2020, 8, e001316. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, L.P.; Schroeder, T.V. Micro-lightguide spectrophotometry for tissue perfusion in ischemic limbs. J. Vasc. Surg. 2012, 56, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Forst, T.; Hohberg, C.; Tarakci, E.; Forst, S.; Kann, P.; Pfutzner, A. Reliability of lightguide spectrophotometry (O2C) for the investigation of skin tissue microvascular blood flow and tissue oxygen supply in diabetic and nondiabetic subjects. J. Diabetes Sci. Technol. 2008, 2, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Beckert, S.; Witte, M.B.; Konigsrainer, A.; Coerper, S. The impact of the Micro-Lightguide O2C for the quantification of tissue ischemia in diabetic foot ulcers. Diabetes Care 2004, 27, 2863–2867. [Google Scholar] [CrossRef]
- Harrison, D.K.; McCollum, P.T.; Newton, D.J.; Hickman, P.; Jain, A.S. Amputation level assessment using lightguide spectrophotometry. Prosthet. Orthot. Int. 1995, 19, 139–147. [Google Scholar] [CrossRef][Green Version]
- Bierbach, B.; Scheewe, J.; Derfuss, T.; Krug, A.; Schramm, R.; Dahm, M.; Kuroczynski, W.; Kempski, O.; Horstick, G. Continuous regional myocardial blood flow measurement: Validation of a near-infrared laser Doppler device in a porcine model. Microcirculation 2012, 19, 485–493. [Google Scholar] [CrossRef]
- Ladurner, R.; Feilitzsch, M.; Steurer, W.; Coerper, S.; Konigsrainer, A.; Beckert, S. The impact of a micro-lightguide spectrophotometer on the intraoperative assessment of hepatic microcirculation: A pilot study. Microvasc. Res. 2009, 77, 387–388. [Google Scholar] [CrossRef]
- Martini, M.; Rohrig, A.; Wenghoefer, M.; Schindler, E.; Messing-Junger, A.M. Cerebral oxygenation and hemodynamic measurements during craniosynostosis surgery with near-infrared spectroscopy. Childs Nerv. Syst. 2014, 30, 1367–1374. [Google Scholar] [CrossRef]
- Fechner, G.; von Pezold, J.; Luzar, O.; Hauser, S.; Tolba, R.H.; Muller, S.C. Modified spectrometry (O2C device) of intraoperative microperfusion predicts organ function after kidney transplantation: A pilot study. Transplant. Proc. 2009, 41, 3575–3579. [Google Scholar] [CrossRef]
- Scheeren, T.W.; Martin, K.; Maruschke, M.; Hakenberg, O.W. Prognostic value of intraoperative renal tissue oxygenation measurement on early renal transplant function. Transpl. Int. 2011, 24, 687–696. [Google Scholar] [CrossRef]
- Schwarzbach, M.; Bonninghoff, R.; Harrer, K.; Weiss, J.; Denz, C.; Schnulle, P.; Birck, R.; Post, S.; Ronellenfitsch, U. Effects of a clinical pathway on quality of care in kidney transplantation: A non-randomized clinical trial. Langenbeck’s Arch. Surg. 2010, 395, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Schnuelle, P.; Schmitt, W.H.; Weiss, C.; Habicht, A.; Renders, L.; Zeier, M.; Druschler, F.; Heller, K.; Pisarski, P.; Banas, B.; et al. Effects of Dopamine Donor Pretreatment on Graft Survival after Kidney Transplantation: A Randomized Trial. Clin. J. Am. Soc. Nephrol. CJASN 2017, 12, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Karliczek, A.; Harlaar, N.J.; Zeebregts, C.J.; Wiggers, T.; Baas, P.C.; van Dam, G.M. Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int. J. Colorectal. Dis. 2009, 24, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.J.; Chu, S.H.; Chuang, C.K.; Chen, H.W.; Chou, C.C.; Chen, Y.; Wu, C.T. Resistive index cannot predict transplant kidney function. Transplant. Proc. 2003, 35, 94–95. [Google Scholar] [CrossRef]
- Chudek, J.; Kolonko, A.; Krol, R.; Ziaja, J.; Cierpka, L.; Wiecek, A. The intrarenal vascular resistance parameters measured by duplex Doppler ultrasound shortly after kidney transplantation in patients with immediate, slow, and delayed graft function. Transplant. Proc. 2006, 38, 42–45. [Google Scholar] [CrossRef]
- Gomez, V.; Orosa, A.; Rivera, M.; Diez-Nicolas, V.; Hevia, V.; Alvarez, S.; Carracedo, D.; Ramos, E.; Burgos, F.J. Resistance index determination in the pre and post kidney transplantation time points in graft dysfunction diagnosis. Transplant. Proc. 2015, 47, 34–37. [Google Scholar] [CrossRef]
- Krol, R.; Chudek, J.; Kolonko, A.; Ziaja, J.; Pawlicki, J.; Wiecek, A.; Cierpka, L. Intraoperative resistance index measured with transsonic flowmeter on kidney graft artery can predict early and long-term graft function. Transplant. Proc. 2011, 43, 2926–2929. [Google Scholar] [CrossRef]
- Angelescu, M.; Kraus, T.; Wiesel, M.; Hergesell, O.; Haberkorn, U.; Klar, E. Assessment of renal graft function by perioperative monitoring of cortical microcirculation in kidney transplantation. Transplantation 2003, 75, 1190–1196. [Google Scholar] [CrossRef]
- Lisik, W.; Gontarczyk, G.; Kosieradzki, M.; Lagiewska, B.; Pacholczyk, M.; Adadynski, L.; Kobryn, A.; Kwiatkowski, A.; Chmura, A.; Kahan, B.; et al. Intraoperative blood flow measurements in organ allografts can predict postoperative function. Transplant. Proc. 2007, 39, 371–372. [Google Scholar] [CrossRef]
- Schmitz, V.; Schaser, K.D.; Olschewski, P.; Neuhaus, P.; Puhl, G. In vivo visualization of early microcirculatory changes following ischemia/reperfusion injury in human kidney transplantation. Eur. Surg. Res. 2008, 40, 19–25. [Google Scholar] [CrossRef]
- Goncalves, L.N.; van den Hoven, P.; van Schaik, J.; Leeuwenburgh, L.; Hendricks, C.H.F.; Verduijn, P.S.; van der Bogt, K.E.A.; van Rijswijk, C.S.P.; Schepers, A.; Vahrmeijer, A.L.; et al. Perfusion Parameters in Near-Infrared Fluorescence Imaging with Indocyanine Green: A Systematic Review of the Literature. Life 2021, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Karampinis, I.; Gerken, A.L.H. Application of Fluorescent Dyes in Visceral Surgery: State of the Art and Future Perspectives. Visc. Med. 2020, 36, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Azuma, H.; Nadeau, K.; Takada, M.; Mackenzie, H.S.; Tilney, N.L. Cellular and molecular predictors of chronic renal dysfunction after initial ischemia/reperfusion injury of a single kidney. Transplantation 1997, 64, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef]
| Recipient Characteristics | |
|---|---|
| Age (years) | 56 (±15) |
| Gender (♀;♂) | 6 (23); 20 (77) |
| Body mass index (kg/m2) | 25 (±4) |
| Preoperative eGFR (mL/min/1.73 m2) | 8.5 (5–26) |
| Preoperative hemoglobin | 12 (±1.8) |
| Time on dialysis (months) | 47 (0–158) |
| Smoker | 4 (15) |
| Chronic kidney disease stage 4 | 3 (12) |
| Chronic kidney disease stage 5 | 23 (88) |
| Renal anemia | 18 (69) |
| Diabetes mellitus | 7 (27) |
| Dyslipidemia | 12 (48) |
| Hypertension | 23 (88) |
| Peripheral arterial occlusive disease | 1 (4) |
| Chronic heart failure | 3 (12) |
| Periprocedural characteristics | |
| Living-donor transplantations | 4 (15) |
| Deceased-donor transplantations (all DBD) | 22 (85) |
| 1 artery | 20 (77) |
| 2 arteries | 6 (23) |
| Separate pole artery | 3 (12) |
| 1 vein | 26 (100) |
| Operating time (minutes) | 129 (±47) |
| Cold ischemia time (minutes) | 602 (±308) |
| Warm ischemia time (minutes) | 26 (±8) |
| Postoperative characteristics | |
| Diuresis POD 1 (mL/24 h) | 1880 (30–9000) |
| Diuresis POD 2 (mL/24 h) | 2240 (100–12,300) |
| Diuresis POD 7 (mL/24 h) | 1770 (1050–5500) |
| Recipient Characteristics | DGF | Non-DGF | p-Value |
|---|---|---|---|
| Age (years) | 61 (±9) | 55 (±16) | 0.5803 |
| Gender (♀;♂) | 0 (0); 5 (100) | 6 (29); 15 (71) | 0.2981 |
| Body mass index (kg/m2) | 27 (±2) | 25 (±5) | 0.3657 |
| Smoker | 0 | 4 (19) | 0.5552 |
| Preoperative eGFR (mL/min/1.73 m2) | 6 (5–24) | 9 (5–26) | 0.5984 |
| Comorbidities | |||
| Renal anemia | 4 (80) | 14 (67) | 1.0000 |
| Diabetes | 2 (40) | 5 (24) | 0.5875 |
| Dyslipidemia | 1 (20) | 11 (55) | 0.3217 |
| Hypertension | 5 (100) | 18 (86) | 1.0000 |
| Peripheral arterial occlusive disease | 0 | 1 (5) | 1.0000 |
| Chronic Heart Failure | 0 | 3 (14) | 1.0000 |
| Donor characteristics | |||
| Age (years) | 70 (±11) | 67 (±14) | 0.7641 |
| Gender (♀;♂) | 3 (19); 2 (20) | 13 (81); 8 (80) | 1.0000 |
| First donor creatinine (mg/dL) | 1.0 (0.3–1.7) | 0.9 (0.4–2.6) | 0.5156 |
| Last donor creatinine (mg/dL) | 1.0 (0.3–5.2) | 0.8 (0.4–3.4) | 0.3652 |
| Smoker | 1 (20) | 3 (30) | 1.0000 |
| Cause of death (cerebral hypoxia) | 3 (60) | 2 (12) | 0.0549 |
| Procurement and periprocedural characteristics | |||
| Donation (living; postmortem) | 0 (0); 5 (100) | 4 (19); 17 (81) | 0.5552 |
| Arterial supply 1/2 arteries | 4 (80)/1 (20) | 16 (77)/5 (24) | 1.0000 |
| Operating time (minutes) | 130 (95–183) | 110 (64–239) | 0.3313 |
| Cold ischemia time (minutes) | 554 (430–1431) | 611 (96–1129) | 0.3162 |
| Warm ischemia time (minutes) | 21 (16–28) | 26 (15–40) | 0.5801 |
| Intraoperative urine production (none, moderate or good) | 2 (50); 2 (50) | 3 (19); 13 (82) | 0.2487 |
| Perfusion Parameter | DGF | Non-DGF | p-Value |
|---|---|---|---|
| Ingress (A.U.) | 172 (100–233) | 147.5 (77–252) | 0.7500 |
| IngressRate (A.U.) | 23.5 (15.9–31.5) | 41.8 (8.4–71.5) | 0.5819 |
| Egress (A.U.) | 54.5 (48–80) | 83 (32–196) | 0.3963 |
| EgressRate (A.U.) | 3.9 (1.3–7.8) | 4.7 (2.5–31) | 0.2161 |
| SO2 (%) | 52.5 (21.3–80.7) | 70.3 (18.0–93.7) | 0.1700 |
| rHb (A.U.) | 111.7 (102.0–128.3) | 113.0 (106.3–120.7) | 0.5240 |
| Flow (A.U.) | 47.0 (36.7–57.3) | 81.0 (27.3–249.0) | 0.0275 |
| Velocity (A.U.) | 12.3 (12.0–13.3) | 17.0 (11.3–32.0) | 0.0119 |
| Visual * (homogeneous/marbled) | 4 (80); 1 (20) | 20 (95); 1 (5) | 0.3538 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerken, A.L.H.; Keese, M.; Weiss, C.; Krücken, H.-S.; Pecher, K.A.P.; Ministro, A.; Rahbari, N.N.; Reissfelder, C.; Rother, U.; Yazdani, B.; et al. Investigation of Different Methods of Intraoperative Graft Perfusion Assessment during Kidney Transplantation for the Prediction of Delayed Graft Function: A Prospective Pilot Trial. J. Pers. Med. 2022, 12, 1749. https://doi.org/10.3390/jpm12101749
Gerken ALH, Keese M, Weiss C, Krücken H-S, Pecher KAP, Ministro A, Rahbari NN, Reissfelder C, Rother U, Yazdani B, et al. Investigation of Different Methods of Intraoperative Graft Perfusion Assessment during Kidney Transplantation for the Prediction of Delayed Graft Function: A Prospective Pilot Trial. Journal of Personalized Medicine. 2022; 12(10):1749. https://doi.org/10.3390/jpm12101749
Chicago/Turabian StyleGerken, Andreas L. H., Michael Keese, Christel Weiss, Hanna-Sophie Krücken, Katarina A. P. Pecher, Augusto Ministro, Nuh N. Rahbari, Christoph Reissfelder, Ulrich Rother, Babak Yazdani, and et al. 2022. "Investigation of Different Methods of Intraoperative Graft Perfusion Assessment during Kidney Transplantation for the Prediction of Delayed Graft Function: A Prospective Pilot Trial" Journal of Personalized Medicine 12, no. 10: 1749. https://doi.org/10.3390/jpm12101749
APA StyleGerken, A. L. H., Keese, M., Weiss, C., Krücken, H.-S., Pecher, K. A. P., Ministro, A., Rahbari, N. N., Reissfelder, C., Rother, U., Yazdani, B., Kälsch, A.-I., Krämer, B. K., & Schwenke, K. (2022). Investigation of Different Methods of Intraoperative Graft Perfusion Assessment during Kidney Transplantation for the Prediction of Delayed Graft Function: A Prospective Pilot Trial. Journal of Personalized Medicine, 12(10), 1749. https://doi.org/10.3390/jpm12101749






