Metabolomic Signatures of Autism Spectrum Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Behavioral Measurements
2.3. Sample Collections and Storage
2.4. Redox Biomarkers
2.5. Mitochondrial Respiration Assay
2.6. Metabolomic Analysis
2.6.1. Sample Processing
2.6.2. Reagents
2.6.3. LC-MS/MS Method
2.7. Data Analysis
3. Results
3.1. Linear Models
3.2. Behavioral Correlations: Pathway and Network Analysis
3.3. Correlations with Targeted Metabolites: Pathway and Network Analysis
4. Discussion
4.1. Summary of Results
4.2. Energy Metabolism
4.3. Amino Acid Neurotransmitter Metabolism
4.4. Branched Chain Amino Acid Metabolism
4.5. Nicotinamide Metabolism
4.6. Aminoacyl-tRNA Biosynthesis
4.7. Histidine
4.8. Common Pathways
4.9. Limiations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Y.; Liu, B.; Chen, Q.; Xing, X.; Xu, G.; Yang, W. Prevalence of Autism Spectrum Disorder Among Children and Adolescents in the United States from 2019 to 2020. JAMA Pediatr. 2022, 176, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. A review of research trends in physiological abnormalities in autism spectrum disorders: Immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol. Psychiatry 2012, 17, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.R.; Lane, A.L.; Werner, B.A.; McLees, S.E.; Fletcher, T.S.; Frye, R.E. Modern Biomarkers for Autism Spectrum Disorder: Future Directions. Mol. Diagn. Ther. 2022, 26, 483–495. [Google Scholar] [CrossRef]
- Ginsberg, M.R.; Rubin, R.A.; Falcone, T.; Ting, A.H.; Natowicz, M.R. Brain transcriptional and epigenetic associations with autism. PLoS ONE 2012, 7, e44736. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front. Physiol. 2014, 5, 150. [Google Scholar] [CrossRef]
- Pecorelli, A.; Cervellati, C.; Hayek, J.; Valacchi, G. OxInflammation in Rett syndrome. Int. J. Biochem. Cell Biol. 2016, 81, 246–253. [Google Scholar] [CrossRef]
- Infantino, V.; Pierri, C.L.; Iacobazzi, V. Metabolic Routes in Inflammation: The Citrate Pathway and its Potential as Therapeutic Target. Curr. Med. Chem. 2019, 26, 7104–7116. [Google Scholar] [CrossRef]
- Panisi, C.; Guerini, F.R.; Abruzzo, P.M.; Balzola, F.; Biava, P.M.; Bolotta, A.; Brunero, M.; Burgio, E.; Chiara, A.; Clerici, M.; et al. Autism Spectrum Disorder from the Womb to Adulthood: Suggestions for a Paradigm Shift. J. Pers. Med. 2021, 11, 70. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 290–314. [Google Scholar] [CrossRef]
- Rose, S.; Niyazov, D.M.; Rossignol, D.A.; Goldenthal, M.; Kahler, S.G.; Frye, R.E. Clinical and Molecular Characteristics of Mitochondrial Dysfunction in Autism Spectrum Disorder. Mol. Diagn. Ther. 2018, 22, 571–593. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Alaimo, S.; Messina, M.; Pulvirenti, A.; Bastin, J.; Ferro, A.; Frye, R.E.; Rizzo, R. A Subset of Patients with Autism Spectrum Disorders Show a Distinctive Metabolic Profile by Dried Blood Spot Analyses. Front. Psychiatry 2018, 9, 636. [Google Scholar] [CrossRef]
- Frye, R.E.; Melnyk, S.; Macfabe, D.F. Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl. Psychiatry 2013, 3, e220. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Adame, M.D.; Serena, G.; Rose, D.R.; Preston, G.M.; Conrad, M.C.; Campbell, A.S.; Donabedian, D.H.; Fasano, A.; Ashwood, P.; et al. Plasma and Fecal Metabolite Profiles in Autism Spectrum Disorder. Biol. Psychiatry 2021, 89, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.F.; Turkoglu, O.; Yilmaz, A.; Ustun, I.; Ugur, Z.; Bjorndhal, T.; Han, B.; Mandal, R.; Wishart, D.; Bahado-Singh, R.O. Targeted metabolomics highlights perturbed metabolism in the brain of autism spectrum disorder sufferers. Metabolomics 2020, 16, 59. [Google Scholar] [CrossRef]
- Howsmon, D.P.; Kruger, U.; Melnyk, S.; James, S.J.; Hahn, J. Classification and adaptive behavior prediction of children with autism spectrum disorder based upon multivariate data analysis of markers of oxidative stress and DNA methylation. PLoS Comput. Biol. 2017, 13, e1005385. [Google Scholar] [CrossRef]
- Howsmon, D.P.; Vargason, T.; Rubin, R.A.; Delhey, L.; Tippett, M.; Rose, S.; Bennuri, S.C.; Slattery, J.C.; Melnyk, S.; James, S.J.; et al. Multivariate techniques enable a biochemical classification of children with autism spectrum disorder versus typically-developing peers: A comparison and validation study. Bioeng. Transl. Med. 2018, 3, 156–165. [Google Scholar] [CrossRef]
- Vargason, T.; Kruger, U.; Roth, E.; Delhey, L.M.; Tippett, M.; Rose, S.; Bennuri, S.C.; Slattery, J.C.; Melnyk, S.; James, S.J.; et al. Comparison of Three Clinical Trial Treatments for Autism Spectrum Disorder Through Multivariate Analysis of Changes in Metabolic Profiles and Adaptive Behavior. Front. Cell. Neurosci. 2018, 12, 503. [Google Scholar] [CrossRef]
- James, S.J.; Melnyk, S.; Jernigan, S.; Cleves, M.A.; Halsted, C.H.; Wong, D.H.; Cutler, P.; Bock, K.; Boris, M.; Bradstreet, J.J.; et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2006, 141b, 947–956. [Google Scholar] [CrossRef]
- Frye, R.E.; James, S.J. Metabolic pathology of autism in relation to redox metabolism. Biomark. Med. 2014, 8, 321–330. [Google Scholar] [CrossRef]
- Ming, X.; Stein, T.P.; Barnes, V.; Rhodes, N.; Guo, L. Metabolic perturbance in autism spectrum disorders: A metabolomics study. J. Proteome Res. 2012, 11, 5856–5862. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; King, J.J.; West, P.R.; Ludwig, M.A.; Donley, E.L.R.; Burrier, R.E.; Amaral, D.G. Amino Acid Dysregulation Metabotypes: Potential Biomarkers for Diagnosis and Individualized Treatment for Subtypes of Autism Spectrum Disorder. Biol. Psychiatry 2019, 85, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.; Boulin, A.; Laranjo, N.; Lee-Sarwar, K.; Chu, S.; Yadama, A.; Carey, V.; Litonjua, A.; Lasky-Su, J.; Weiss, S. Metabolomics and Communication Skills Development in Children; Evidence from the Ages and Stages Questionnaire. Metabolites 2019, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Gevi, F.; Zolla, L.; Gabriele, S.; Persico, A.M. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Mol. Autism 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Naushad, S.M.; Jain, J.M.; Prasad, C.K.; Naik, U.; Akella, R.R. Autistic children exhibit distinct plasma amino acid profile. Indian J. Biochem. Biophys. 2013, 50, 474–478. [Google Scholar] [PubMed]
- Arnold, G.L.; Hyman, S.L.; Mooney, R.A.; Kirby, R.S. Plasma amino acids profiles in children with autism: Potential risk of nutritional deficiencies. J. Autism Dev. Disord. 2003, 33, 449–454. [Google Scholar] [CrossRef]
- Gevi, F.; Belardo, A.; Zolla, L. A metabolomics approach to investigate urine levels of neurotransmitters and related metabolites in autistic children. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165859. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Cerebral Folate Deficiency, Folate Receptor Alpha Autoantibodies and Leucovorin (Folinic Acid) Treatment in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 1141. [Google Scholar] [CrossRef]
- Frye, R.E.; Huffman, L.C.; Elliott, G.R. Tetrahydrobiopterin as a novel therapeutic intervention for autism. Neurother. J. Am. Soc. Exp. NeuroTher. 2010, 7, 241–249. [Google Scholar] [CrossRef]
- Tian, Y.J.; Li, D.; Ma, Q.; Gu, X.Y.; Guo, M.; Lun, Y.Z.; Sun, W.P.; Wang, X.Y.; Cao, Y.; Zhou, S.S. Excess nicotinamide increases plasma serotonin and histamine levels. Acta Physiol. Sin. 2013, 65, 33–38. [Google Scholar]
- Tardy, A.L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ke, X.; Xiao, Z.; Zhang, Y.; Chen, Y.; Li, Y.; Wang, Z.; Lin, L.; Yao, P.; Lu, J. Untargeted Metabolomic Profiling Using UHPLC-QTOF/MS Reveals Metabolic Alterations Associated with Autism. Biomed. Res. Int. 2020, 2020, 6105608. [Google Scholar] [CrossRef]
- Yap, I.K.; Angley, M.; Veselkov, K.A.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. J. Proteome Res. 2010, 9, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Brister, D.; Werner, B.A.; Gideon, G.; McCarty, P.J.; Lane, A.; Burrows, B.T.; McLees, S.; Adelson, P.D.; Arango, J.I.; Marsh, W.; et al. Central Nervous System Metabolism in Autism, Epilepsy and Developmental Delays: A Cerebrospinal Fluid Analysis. Metabolites 2022, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Cakir, J.; Rose, S.; Delhey, L.; Bennuri, S.C.; Tippett, M.; Melnyk, S.; James, S.J.; Palmer, R.F.; Austin, C.; et al. Prenatal air pollution influences neurodevelopment and behavior in autism spectrum disorder by modulating mitochondrial physiology. Mol. Psychiatry 2021, 26, 1561–1577. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Cakir, J.; Rose, S.; Delhey, L.; Bennuri, S.C.; Tippett, M.; Palmer, R.F.; Austin, C.; Curtin, P.; Arora, M. Early life metal exposure dysregulates cellular bioenergetics in children with regressive autism spectrum disorder. Transl. Psychiatry 2020, 10, 223. [Google Scholar] [CrossRef]
- Frye, R.E.; Slattery, J.; Delhey, L.; Furgerson, B.; Strickland, T.; Tippett, M.; Sailey, A.; Wynne, R.; Rose, S.; Melnyk, S.; et al. Folinic acid improves verbal communication in children with autism and language impairment: A randomized double-blind placebo-controlled trial. Mol. Psychiatry 2018, 23, 247–256. [Google Scholar] [CrossRef]
- Delhey, L.; Kilinc, E.N.; Yin, L.; Slattery, J.; Tippett, M.; Wynne, R.; Rose, S.; Kahler, S.; Damle, S.; Legido, A.; et al. Bioenergetic variation is related to autism symptomatology. Metab. Brain. Dis. 2017, 32, 2021–2031. [Google Scholar] [CrossRef]
- Moore, M.; Evans, V.; Hanvey, G.; Johnson, C. Assessment of Sleep in Children with Autism Spectrum Disorder. Children 2017, 4, 72. [Google Scholar] [CrossRef]
- Rose, S.; Melnyk, S.; Trusty, T.A.; Pavliv, O.; Seidel, L.; Li, J.; Nick, T.; James, S.J. Intracellular and extracellular redox status and free radical generation in primary immune cells from children with autism. Autism Res. Treat. 2012, 2012, 986519. [Google Scholar] [CrossRef]
- Melnyk, S.; Pogribna, M.; Pogribny, I.; Hine, R.J.; James, S.J. A new HPLC method for the simultaneous determination of oxidized and reduced plasma aminothiols using coulometric electrochemical detection. J. Nutr. Biochem. 1999, 10, 490–497. [Google Scholar] [CrossRef]
- Perez, J.; Hill, B.G.; Benavides, G.A.; Dranka, B.P.; Darley-Usmar, V.M. Role of cellular bioenergetics in smooth muscle cell proliferation induced by platelet-derived growth factor. Biochem. J. 2010, 428, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G.; Higdon, A.N.; Dranka, B.P.; Darley-Usmar, V.M. Regulation of vascular smooth muscle cell bioenergetic function by protein glutathiolation. Biochim. Biophys. Acta 2010, 1797, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Burger, B.J.; Rose, S.; Bennuri, S.C.; Gill, P.S.; Tippett, M.L.; Delhey, L.; Melnyk, S.; Frye, R.E. Autistic Siblings with Novel Mutations in Two Different Genes: Insight for Genetic Workups of Autistic Siblings and Connection to Mitochondrial Dysfunction. Front. Pediatr. 2017, 5, 219. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Geng, L.; Rose, S.; Bennuri, S.C.; Frye, R.E. Variations in Mitochondrial Respiration Differ in IL-1ss/IL-10 Ratio Based Subgroups in Autism Spectrum Disorders. Front. Psychiatry 2019, 10, 71. [Google Scholar] [CrossRef]
- Singh, K.; Singh, I.N.; Diggins, E.; Connors, S.L.; Karim, M.A.; Lee, D.; Zimmerman, A.W.; Frye, R.E. Developmental regression and mitochondrial function in children with autism. Ann. Clin. Transl. Neurol. 2020, 7, 683–694. [Google Scholar] [CrossRef]
- Gu, H.; Zhang, P.; Zhu, J.; Raftery, D. Globally Optimized Targeted Mass Spectrometry: Reliable Metabolomics Analysis with Broad Coverage. Anal. Chem. 2015, 87, 12355–12362. [Google Scholar] [CrossRef]
- Gu, H.; Carroll, P.A.; Du, J.; Zhu, J.; Neto, F.C.; Eisenman, R.N.; Raftery, D. Quantitative Method to Investigate the Balance between Metabolism and Proteome Biomass: Starting from Glycine. Angew. Chem. 2016, 55, 15646–15650. [Google Scholar] [CrossRef]
- Shi, X.; Wang, S.; Jasbi, P.; Turner, C.; Hrovat, J.; Wei, Y.; Liu, J.; Gu, H. Database-Assisted Globally Optimized Targeted Mass Spectrometry (dGOT-MS): Broad and Reliable Metabolomics Analysis with Enhanced Identification. Anal. Chem. 2019, 91, 13737–13745. [Google Scholar] [CrossRef]
- Jasbi, P.; Mitchell, N.M.; Shi, X.; Grys, T.E.; Wei, Y.; Liu, L.; Lake, D.F.; Gu, H. Coccidioidomycosis Detection Using Targeted Plasma and Urine Metabolic Profiling. J. Proteome Res. 2019, 18, 2791–2802. [Google Scholar] [CrossRef]
- Eghlimi, R.; Shi, X.; Hrovat, J.; Xi, B.; Gu, H. Triple Negative Breast Cancer Detection Using LC-MS/MS Lipidomic Profiling. J. Proteome Res. 2020, 19, 2367–2378. [Google Scholar] [CrossRef] [PubMed]
- Jasbi, P.; Shi, X.; Chu, P.; Elliott, N.; Hudson, H.; Jones, D.; Serrano, G.; Chow, B.; Beach, T.G.; Liu, L.; et al. Metabolic Profiling of Neocortical Tissue Discriminates Alzheimer’s Disease from Mild Cognitive Impairment, High Pathology Controls, and Normal Controls. J. Proteome Res. 2021, 20, 4303–4317. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Chen, B.; Zhan, Y.; Kessi, M.; Chen, S.; Xiong, J.; Deng, X.; Yang, L.; Peng, J.; Yin, F.; He, F. Urine Organic Acids as Metabolic Indicators for Global Developmental Delay/Intellectual Disability in Chinese Children. Front. Mol. Biosci. 2021, 8, 792319. [Google Scholar] [CrossRef]
- Orozco, J.S.; Hertz-Picciotto, I.; Abbeduto, L.; Slupsky, C.M. Metabolomics analysis of children with autism, idiopathic-developmental delays, and Down syndrome. Transl. Psychiatry 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sotelo-Orozco, J.; Abbeduto, L.; Hertz-Picciotto, I.; Slupsky, C.M. Association Between Plasma Metabolites and Psychometric Scores Among Children with Developmental Disabilities: Investigating Sex-Differences. Front. Psychiatry 2020, 11, 579538. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Rose, S.; Slattery, J.; MacFabe, D.F. Gastrointestinal dysfunction in autism spectrum disorder: The role of the mitochondria and the enteric microbiome. Microb. Ecol. Health Dis. 2015, 26, 27458. [Google Scholar] [CrossRef]
- Zheng, H.F.; Wang, W.Q.; Li, X.M.; Rauw, G.; Baker, G.B. Body fluid levels of neuroactive amino acids in autism spectrum disorders: A review of the literature. Amino Acids 2017, 49, 57–65. [Google Scholar] [CrossRef]
- Frye, R.E.; DeLatorre, R.; Taylor, H.B.; Slattery, J.; Melnyk, S.; Chowdhury, N.; James, S.J. Metabolic effects of sapropterin treatment in autism spectrum disorder: A preliminary study. Transl. Psychiatry 2013, 3, e237. [Google Scholar] [CrossRef]
- Frye, R.E.; Casanova, M.F.; Fatemi, S.H.; Folsom, T.D.; Reutiman, T.J.; Brown, G.L.; Edelson, S.M.; Slattery, J.C.; Adams, J.B. Neuropathological Mechanisms of Seizures in Autism Spectrum Disorder. Front. Neurosci. 2016, 10, 192. [Google Scholar] [CrossRef]
- Gundersen, R.Y.; Vaagenes, P.; Breivik, T.; Fonnum, F.; Opstad, P.K. Glycine--an important neurotransmitter and cytoprotective agent. Acta Anaesthesiol. Scand. 2005, 49, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Novarino, G.; El-Fishawy, P.; Kayserili, H.; Meguid, N.A.; Scott, E.M.; Schroth, J.; Silhavy, J.L.; Kara, M.; Khalil, R.O.; Ben-Omran, T.; et al. Mutations in BCKD-kinase lead to a potentially treatable form of autism with epilepsy. Science 2012, 338, 394–397. [Google Scholar] [CrossRef]
- Monirujjaman, M.; Ferdouse, A. Metabolic and Physiological Roles of Branched-Chain Amino Acids. Adv. Mol. Biol. 2014, 2014, 364976. [Google Scholar] [CrossRef]
- Koju, N.; Qin, Z.H.; Sheng, R. Reduced nicotinamide adenine dinucleotide phosphate in redox balance and diseases: A friend or foe? Acta Pharmacol. Sin. 2022, 43, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- Riddick, D.S.; Ding, X.; Wolf, C.R.; Porter, T.D.; Pandey, A.V.; Zhang, Q.-Y.; Gu, J.; Finn, R.D.; Ronseaux, S.; McLaughlin, L.A.; et al. NADPH-cytochrome P450 oxidoreductase: Roles in physiology, pharmacology, and toxicology. Drug Metab. Dispos. 2013, 41, 12–23. [Google Scholar] [CrossRef]
- Arroyo, A.; Kagan, V.E.; Tyurin, V.A.; Burgess, J.R.; de Cabo, R.; Navas, P.; Villalba, J.M. NADH and NADPH-dependent reduction of coenzyme Q at the plasma membrane. Antioxid. Redox Signal. 2000, 2, 251–262. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free. Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Zhang, R. MNADK, a Long-Awaited Human Mitochondrion-Localized NAD Kinase. J. Cell. Physiol. 2015, 230, 1697–1701. [Google Scholar] [CrossRef]
- Ji, J.; Damschroder, D.; Bessert, D.; Lazcano, P.; Wessells, R.; Reynolds, C.A.; Greenberg, M.L. NAD supplementation improves mitochondrial performance of cardiolipin mutants. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159094. [Google Scholar] [CrossRef]
- Ibba, M.; Soll, D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 2000, 69, 617–650. [Google Scholar] [CrossRef]
- Golovina, E.; Fadason, T.; Lints, T.J.; Walker, C.; Vickers, M.H.; O’Sullivan, J.M. Understanding the impact of SNPs associated with autism spectrum disorder on biological pathways in the human fetal and adult cortex. Sci. Rep. 2021, 11, 15867. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, M.E.; Brosnan, J.T. Histidine Metabolism and Function. J. Nutr. 2020, 150, 2570s–2575s. [Google Scholar] [CrossRef]
- Meltzer, A.; Van de Water, J. The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology 2017, 42, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A. Biochemical, Biomedical and Metabolic Aspects of Imidazole-Containing Dipeptides with the Inherent Complexity to Neurodegenerative Diseases and Various States of Mental Well-Being: A Challenging Correction and Neurotherapeutic Pharmaceutical Biotechnology for Treating Cognitive Deficits, Depression and Intellectual Disabilities. Curr. Pharm. Biotechnol. 2014, 15, 738–778. [Google Scholar] [CrossRef]
- Hajizadeh-Zaker, R.; Ghajar, A.; Mesgarpour, B.; Afarideh, M.; Mohammadi, M.R.; Akhondzadeh, S. l-Carnosine as an Adjunctive Therapy to Risperidone in Children with Autistic Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Child. Adolesc. Psychopharmacol. 2018, 28, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Mehrazad-Saber, Z.; Kheirouri, S.; Noorazar, S.G. Effects of l-Carnosine Supplementation on Sleep Disorders and Disease Severity in Autistic Children: A Randomized, Controlled Clinical Trial. Basic Clin. Pharmacol. Toxicol. 2018, 123, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Chez, M.G.; Buchanan, C.P.; Aimonovitch, M.C.; Becker, M.; Schaefer, K.; Black, C.; Komen, J. Double-blind, placebo-controlled study of L-carnosine supplementation in children with autistic spectrum disorders. J. Child Neurol. 2002, 17, 833–837. [Google Scholar] [CrossRef]
- Neville, B.G.; Bentovim, A.; Clayton, B.E.; Shepherd, J. Histidinaemia. Study of relation between clinical and biological findings in 7 subjects. Arch. Dis. Child 1972, 47, 190–200. [Google Scholar] [CrossRef][Green Version]
- Kotsopoulos, S.; Kutty, K.M. Histidinemia and infantile autism. J. Autism Dev. Disord. 1979, 9, 55–60. [Google Scholar] [CrossRef]
- Ishikawa, M. Developmental disorders in histidinemia—Follow-Up study of language development in histidinemia. Acta Paediatr. Jpn. 1987, 29, 224–228. [Google Scholar] [CrossRef]
- Lott, I.T.; Wheelden, J.A.; Levy, H.L. Speech and histidinemia: Methodology and evaluation of four cases. Dev. Med. Child Neurol. 1970, 12, 596–603. [Google Scholar] [CrossRef] [PubMed]

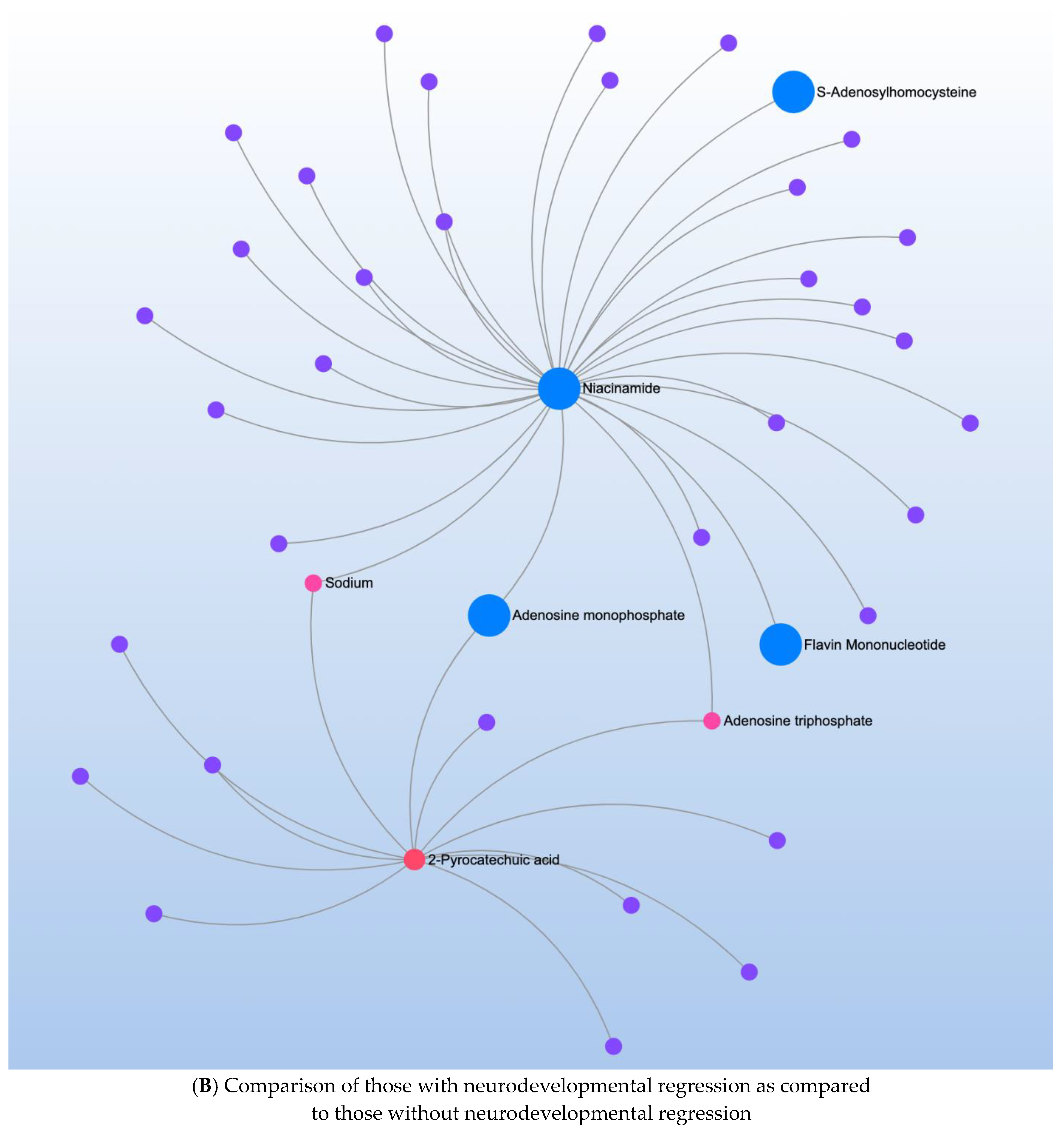


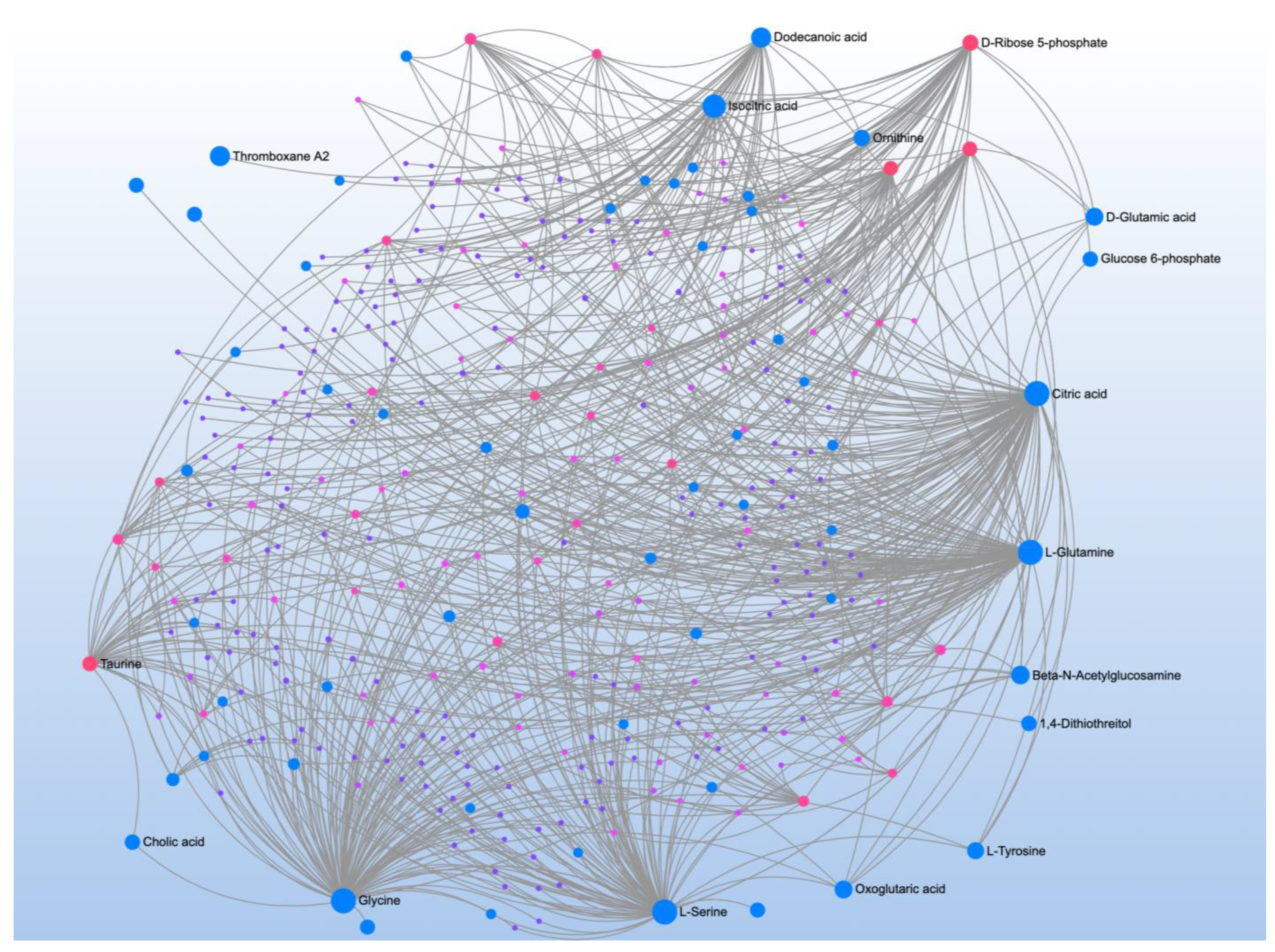

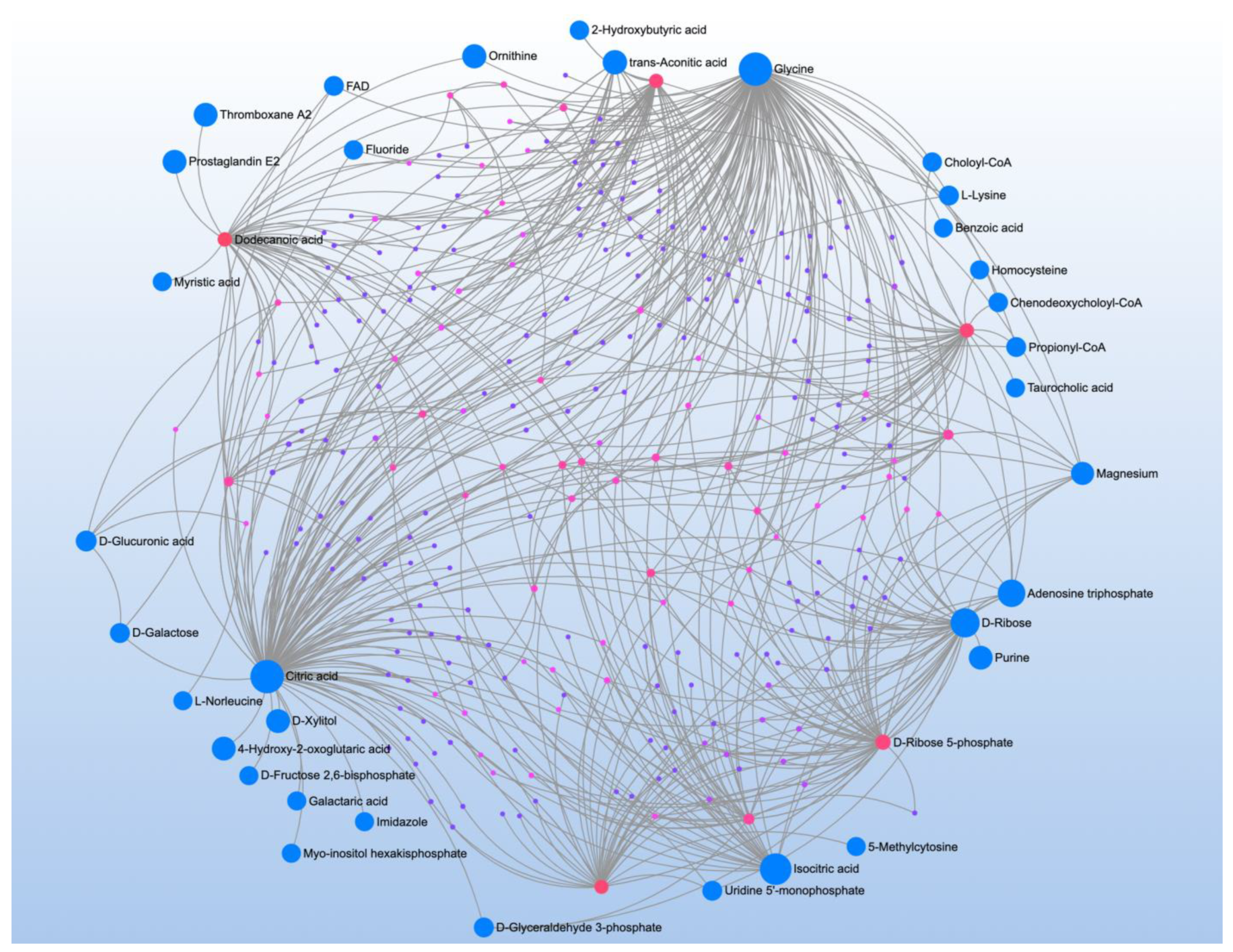

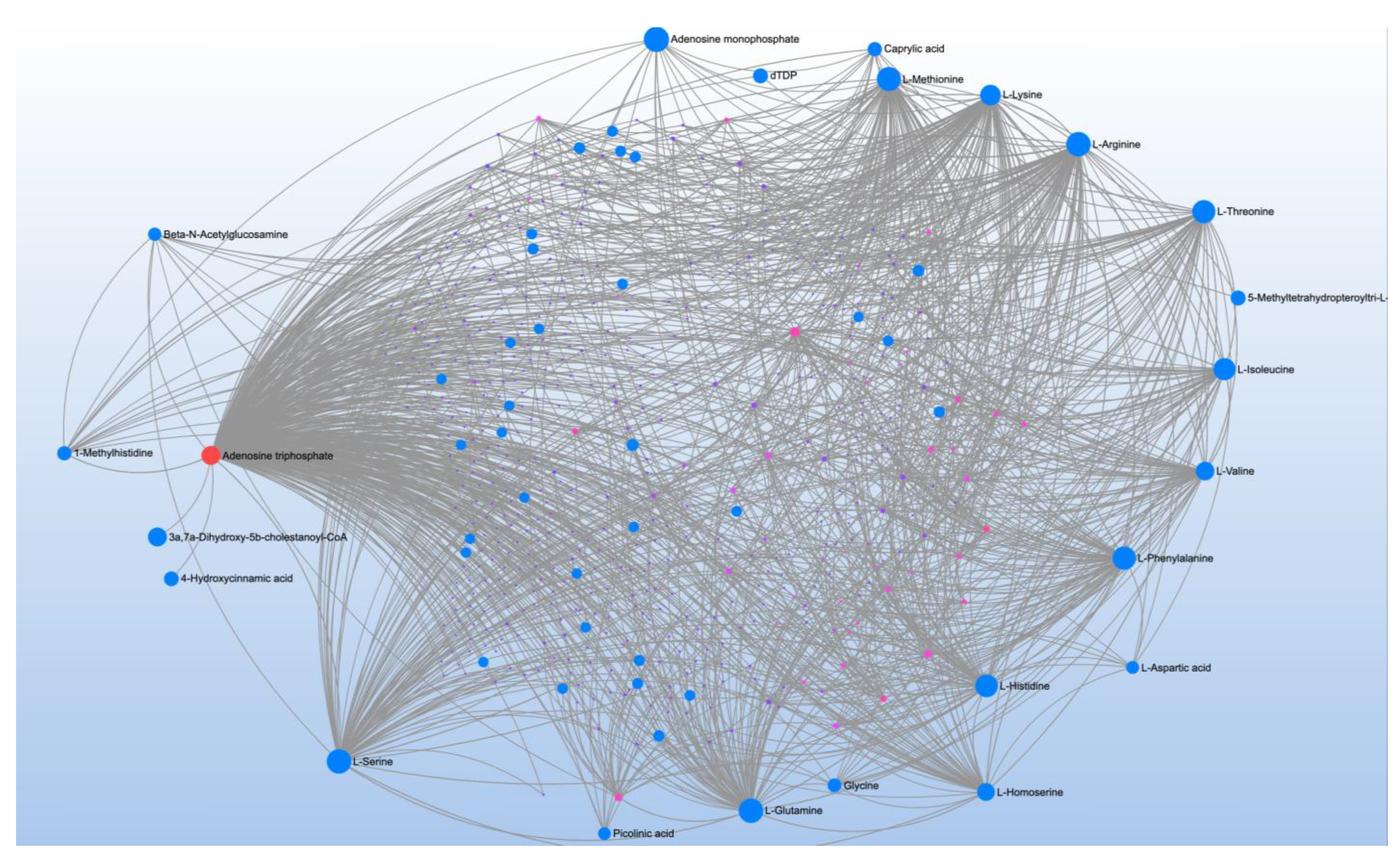
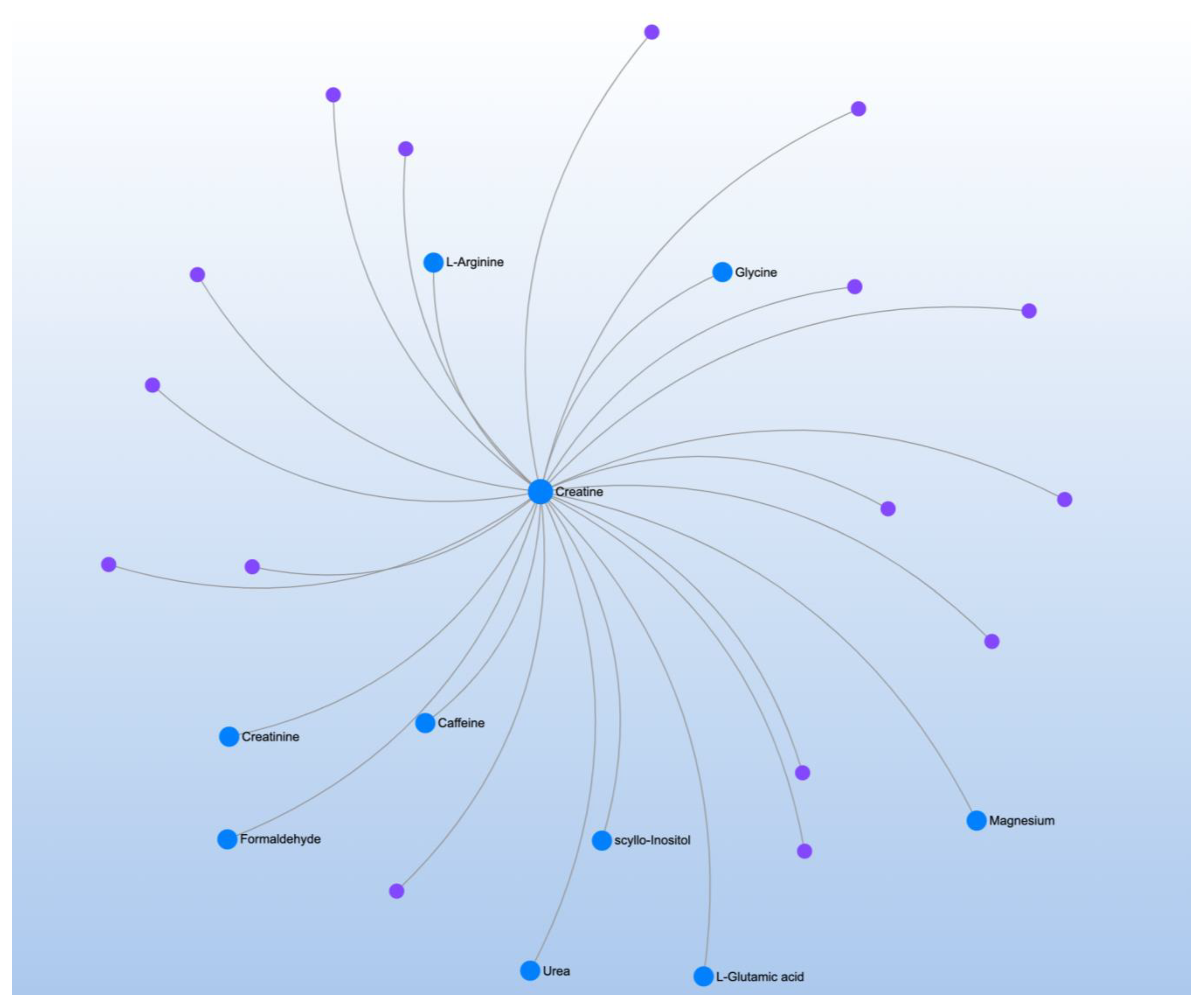

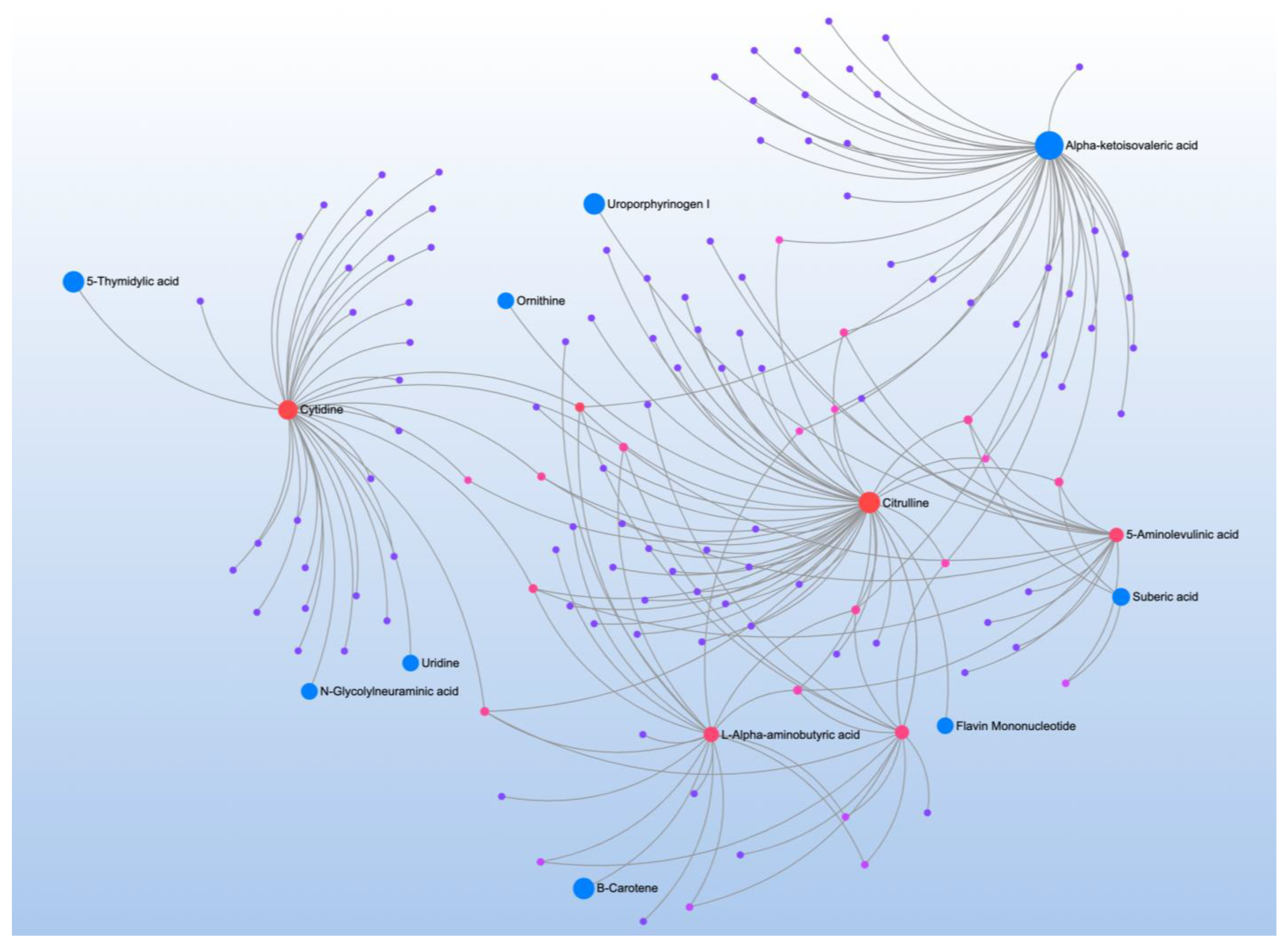
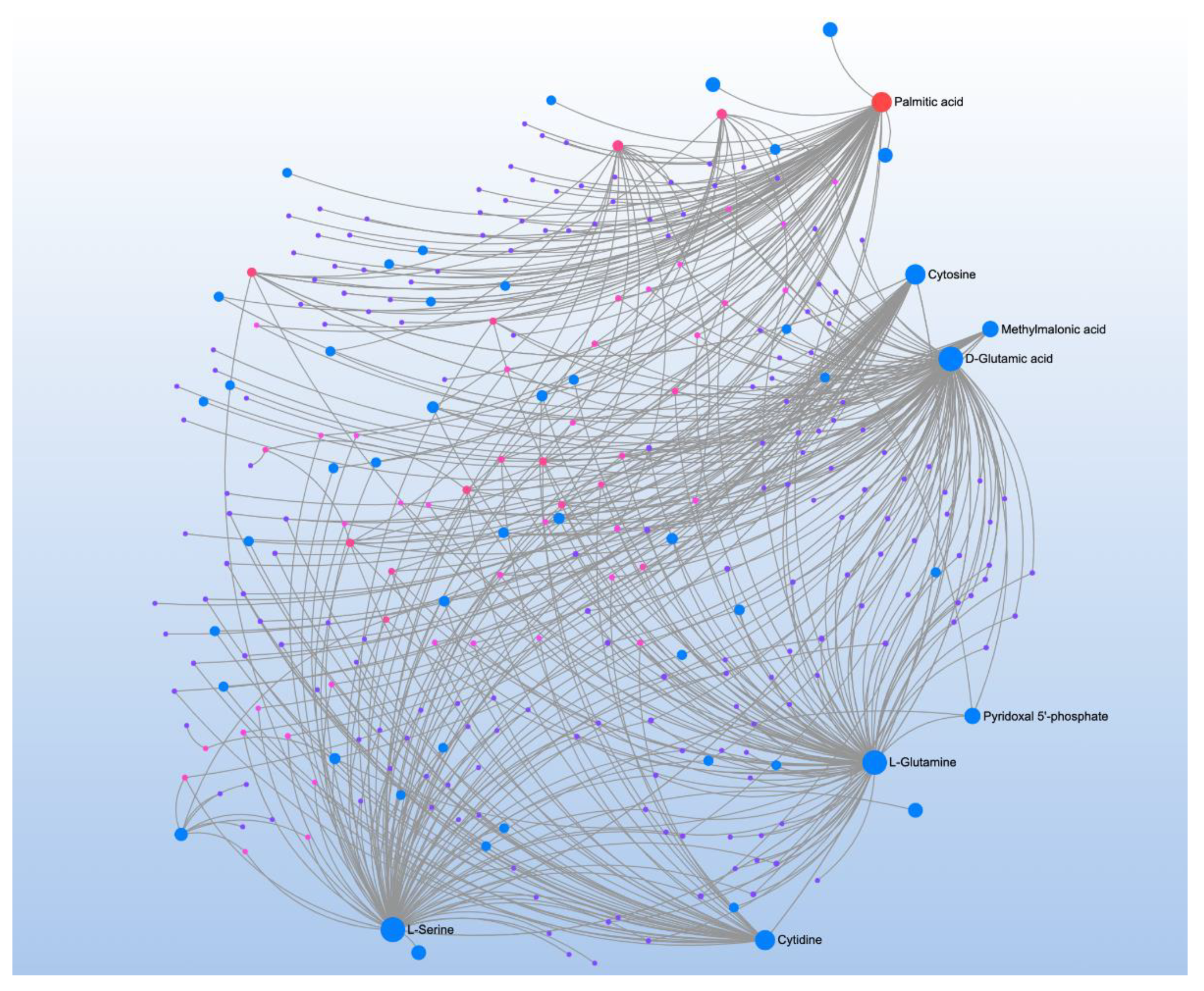
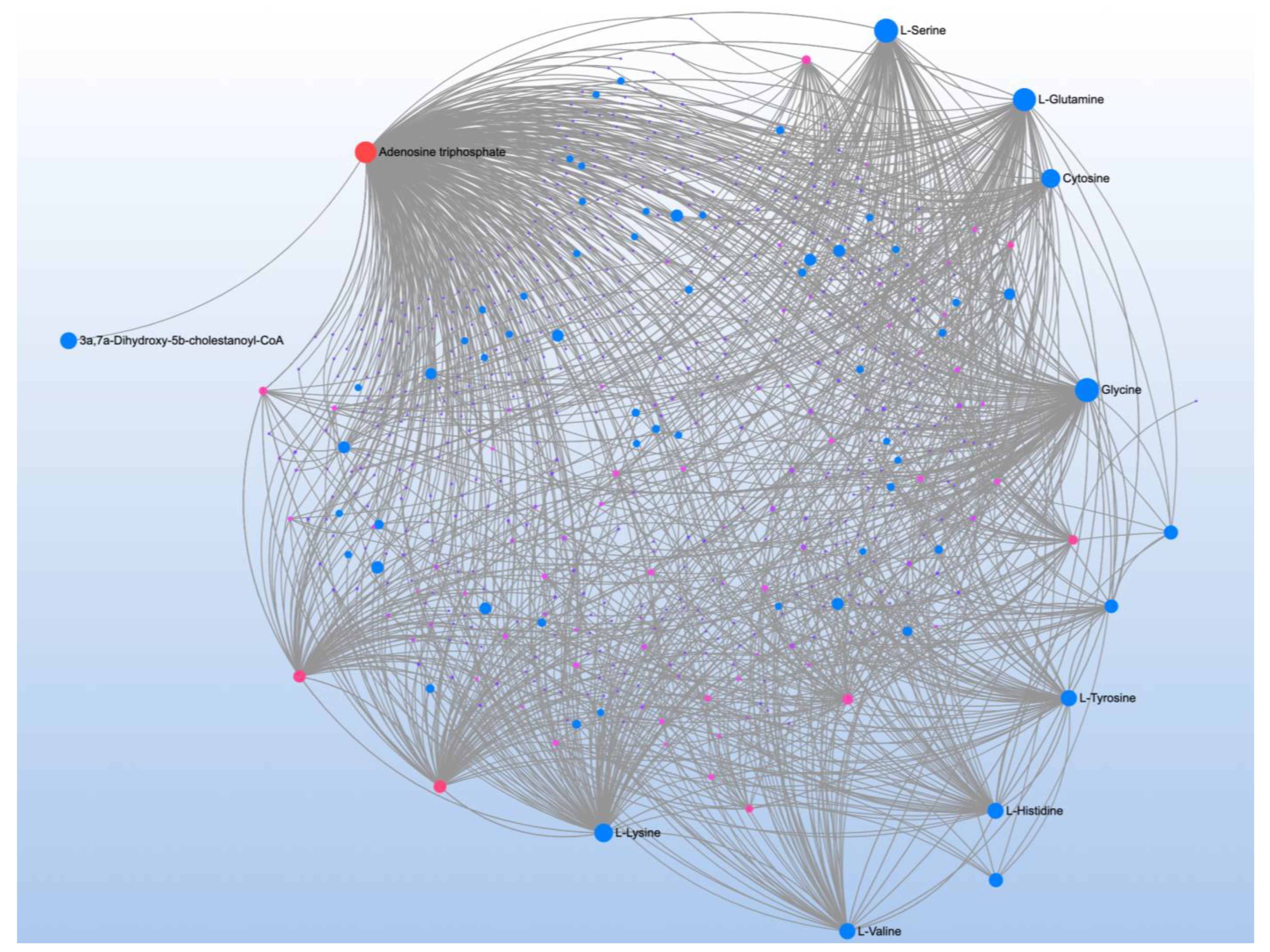
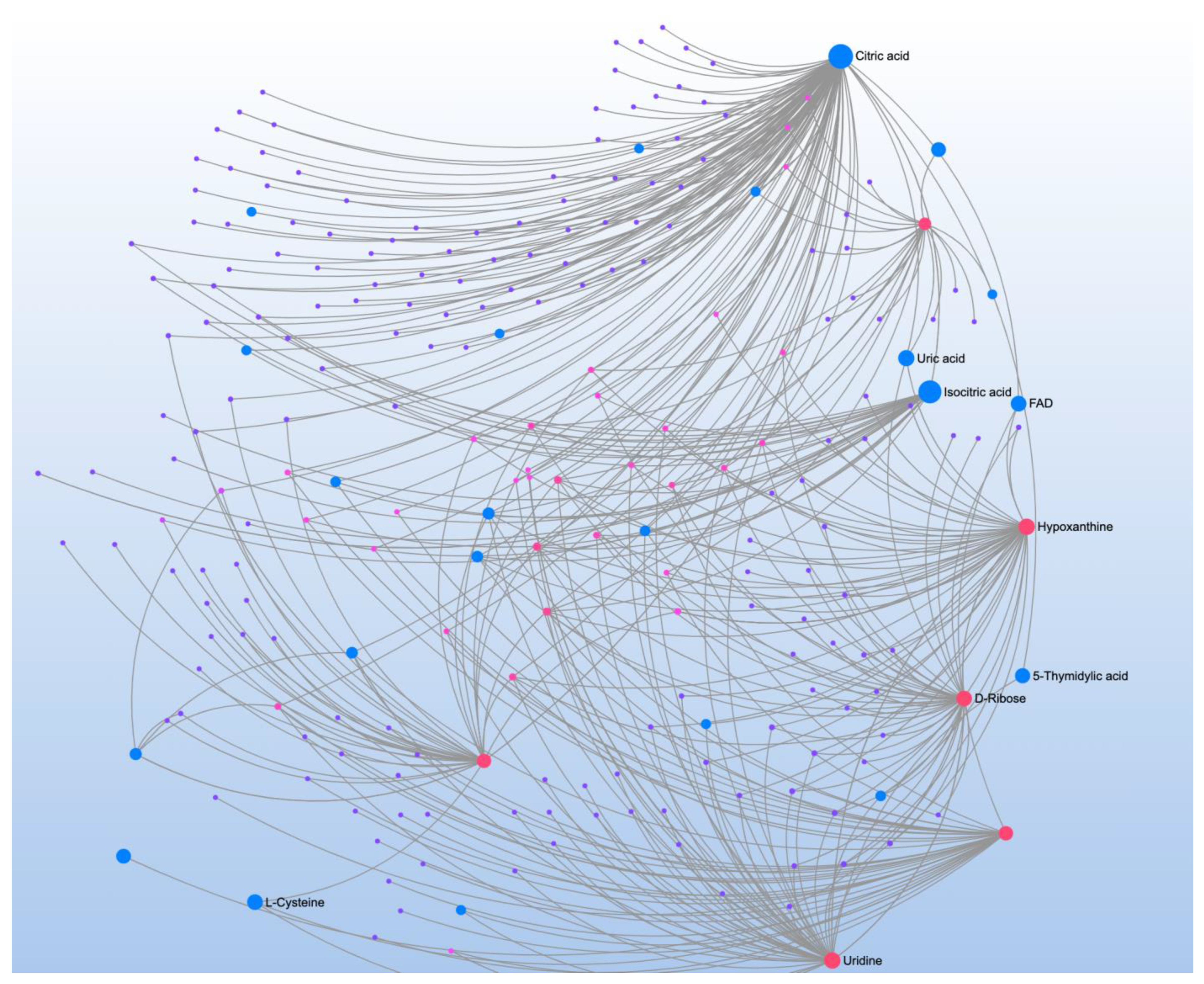
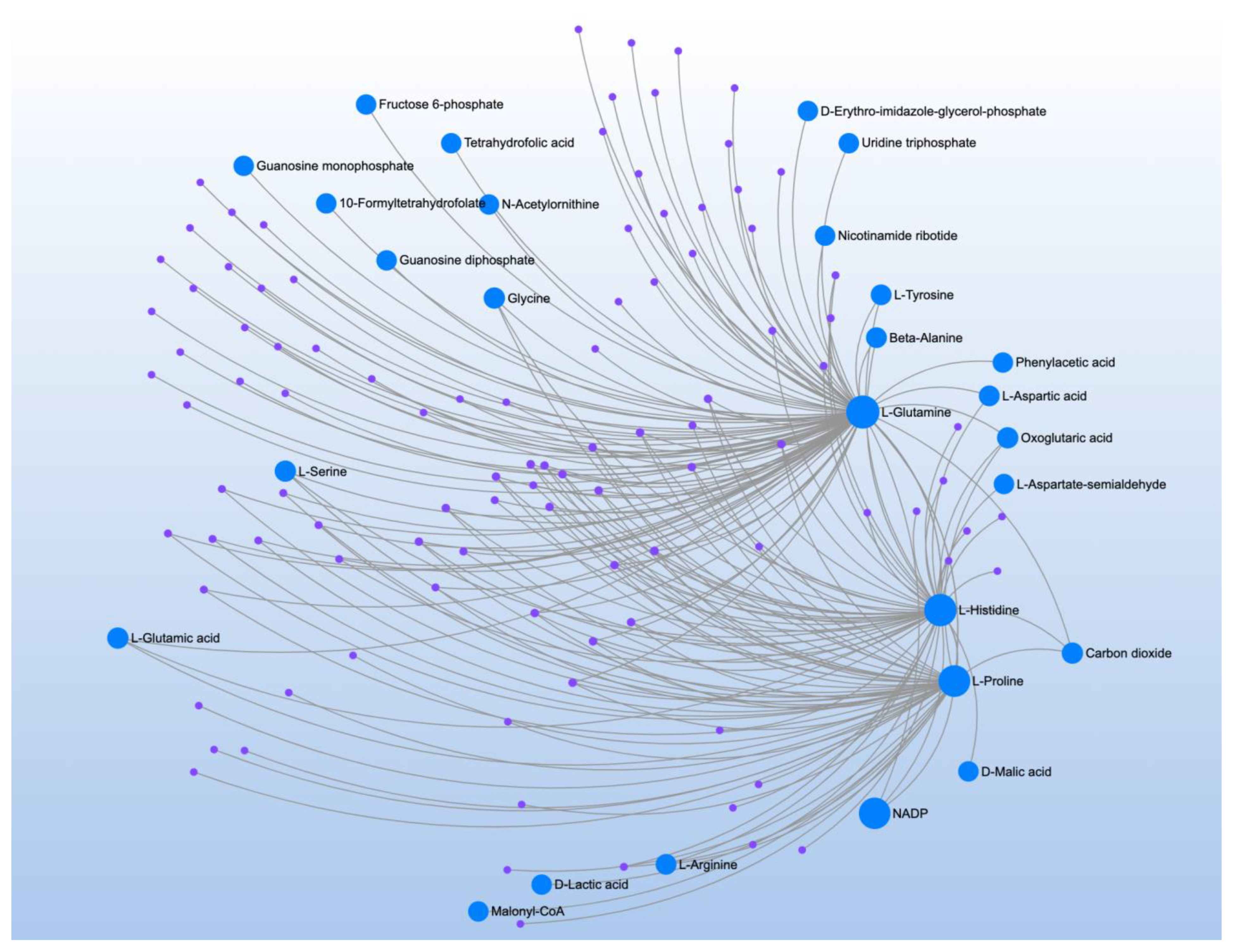
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | Fold ∆ | Pathway | Matched Metabolites | Impact |
| Cytidine *** | 7.17 | Histidine metabolism * | L-Histidine *, 1-Methylhistamine ** | 0.31 |
| Taurine ** | 4.52 | Phenylalanine, tyrosine and tryptophan biosynthesis | Phenylpyruvate | 0.05 |
| 5-Aminolevulinic acid ** | 0.22 | |||
| Dodecanoic acid ** | 0.26 | Glutathione metabolism | Glycine **, Pyroglutamic acid * | 0.10 |
| Glycine ** | 3.77 | |||
| 1-Methylhistamine ** | 3.72 | |||
| Adenosine triphosphate ** | 3.65 | |||
| 2,3,4,5-Tetrahydroxypentanoic acid ** | 3.63 | |||
| 4-Hydroxyproline ** | 0.28 | |||
| Dihydroxyacetone ** | 3.27 | |||
| 4-Pyridoxic acid ** | 3.26 | |||
| Phenylpyruvic acid ** | 0.31 | |||
| 2-Aminoisobutyric acid ** | 3.22 | |||
| Xylitol ** | 3.21 | |||
| Isobutyric acid * | 3.20 | |||
| Capric acid * | 0.31 | |||
| D-Leucic acid * | 0.32 | |||
| L-Histidine * | 3.17 | |||
| DL-Acetylcarnitine * | 3.10 | |||
| Ribitol * | 3.05 | |||
| L-3-Phenyllactic acid * | 0.33 | |||
| Pyroglutamic acid * | 2.83 | |||
| 4-Hydroxybenzaldehyde * | 0.37 | |||
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | Fold ∆ | Pathway | Matched Metabolites | Impact |
| 2-Pyrocatechuic acid * | 0.26 | Nicotinate/nicotinamide metabolism * | Niacinamide * | 0.19 |
| Niacinamide * | 3.92 | |||
| Acetamide * | 3.74 | |||
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | Fold ∆ | Pathway | Matched Metabolites | Impact |
| 5-Aminolevulinic acid *** | 0.15 | Phenylalanine, tyrosine and tryptophan metabolism * | Phenylpyruvic acid * | 0.00 |
| Cytidine *** | 6.12 | |||
| 4-Hydroxyproline ** | 0.17 | |||
| 4-Pyridoxic acid ** | 4.50 | |||
| 2,3,4,5-Tetrahydroxypentanoic acid ** | 4.31 | |||
| D-Leucic acid * | 0.26 | |||
| L-3-Phenyllactic acid * | 0.27 | |||
| Ribitol * | 3.66 | |||
| Isobutyric acid * | 3.49 | |||
| Xylitol * | 3.48 | |||
| 4-Hydroxybenzaldehyde * | 0.29 | |||
| Taurine * | 3.27 | |||
| 1-Methylhistamine * | 3.23 | |||
| 2-Aminoisobutyric acid * | 3.22 | |||
| 4-Hydroxybutyric acid * | 0.31 | |||
| Capric acid * | 0.32 | |||
| Phenylpyruvic acid * | 0.33 | |||
| Dodecanoic acid * | 0.33 | |||
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | Fold ∆ | Pathway | Matched Metabolites | Impact |
| Cytidine *** | 9.66 | Aminoacyl-tRNA biosynthesis *** | L-Asparagine *, L-Histidine *, Glycine **, L-Serine ** | 0.17 |
| Taurine *** | 7.54 | |||
| Adenosine triphosphate *** | 6.86 | Histidine metabolism ** | L-Histidine*, 1-Methylhistamine * | 0.31 |
| Glycine ** | 6.49 | Glyoxylate and dicarboxylate metabolism * | L-Serine **, Glycine ** | 0.15 |
| Dodecanoic acid ** | 0.20 | Glycine, serine and threonine metabolism * | L-Serine **, Glycine ** | 0.46 |
| D,L-Acetyl-carnitine ** | 4.84 | |||
| Dihydroxyacetone ** | 4.76 | |||
| Acetamide ** | 4.69 | |||
| L-Histidine ** | 4.60 | |||
| Hydroxykynurenine ** | 4.54 | |||
| L-Serine ** | 4.49 | |||
| 1-Methylhistamine * | 4.16 | |||
| L-Asparagine * | 3.94 | |||
| trans-Aconitic acid * | 0.28 | |||
| 2,3,4,5-Tetrahydroxypentanoic acid * | 3.49 | |||
| Capric acid * | 0.30 | |||
| Heptadecanoic acid * | 3.33 | |||
| 2-Aminoisobutyric acid * | 3.31 | |||
| Isobutyric acid * | 3.27 | |||
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | r | Pathway | Matched Metabolites | Impact |
| Isocitric acid *** | 0.37 | Citrate cycle (TCA cycle) ** | Isocitrate ***, Citrate **, Fumarate * | 0.17 |
| Glucuronic acid *** | 0.36 | Glyoxylate and dicarboxylate metabolism * | Citrate **, Glycine *, Isocitrate *** | 0.14 |
| Citric acid ** | 0.35 | Pentose phosphate pathway * | D-Ribose 5-phosphate **, D-Ribose * | 0.17 |
| Phenylpyruvic acid ** | 0.33 | |||
| 3S-methyl-2-oxo-pentanoic acid ** | 0.32 | |||
| Phenylbutazone ** | −0.32 | |||
| trans-Aconitic acid ** | 0.31 | |||
| 2-Hydroxybutyric acid ** | −0.30 | |||
| Cytidine ** | −0.29 | |||
| Amiloride ** | −0.29 | |||
| D-Ribose 5-phosphate ** | −0.28 | |||
| 4-Hydroxyproline * | 0.27 | |||
| Glycine * | −0.26 | |||
| Citraconic acid * | 0.26 | |||
| D-Ribose * | 0.26 | |||
| Ketoleucine * | 0.26 | |||
| 1-Methylhistamine * | −0.25 | |||
| Dodecanoic acid * | 0.25 | |||
| Pregnenolone sulfate * | −0.25 | |||
| 5-Aminolevulinic acid * | 0.24 | |||
| 2-Methylglutaric acid * | −0.24 | |||
| Taurine * | −0.24 | |||
| L-3-Phenyllactic acid * | 0.24 | |||
| Fumaric acid * | 0.23 | |||
| N-Acetylglutamine * | −0.22 | |||
| Dihydroxyacetone * | −0.22 | |||
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | r | Pathway | Matched Metabolites | Impact |
| Phenylpyruvic acid ** | 0.35 | Glyoxylate and dicarboxylate metabolism *** | Citrate *, L-Serine **, Glycine **, Isocitrate *, L-Glutamine * | 0.18 |
| Cytidine ** | −0.35 | |||
| Glycine ** | −0.33 | Glycine, serine and threonine metabolism * | L-Serine **, Glycine **, 5-Aminolevulinate | 0.46 |
| Taurine ** | −0.33 | |||
| Phenylbutazone ** | −0.32 | Citrate cycle (TCA Cycle) * | Isocitrate *, Citrate * | 0.14 |
| Amiloride ** | −0.31 | Aminoacyl-tRNA biosynthesis * | L-Glutamine *, Glycine **, L-Serine ** | 0.17 |
| Glucuronic acid ** | 0.31 | |||
| L-Serine ** | −0.30 | |||
| 3S-methyl-2-oxo-pentanoic acid ** | 0.30 | |||
| DL-Acetylcarnitine ** | −0.30 | |||
| 1-Methylhistamine ** | −0.30 | |||
| L-3-Phenyllactic acid ** | 0.29 | |||
| Dihydroxyacetone * | −0.29 | |||
| L-Glutamine * | −0.28 | |||
| Dodecanoic acid * | 0.27 | |||
| Citraconic acid * | 0.27 | |||
| Isocitric acid * | 0.27 | |||
| 4-Pyridoxic acid * | −0.26 | |||
| Capric acid * | 0.26 | |||
| Ketoleucine * | 0.26 | |||
| Pregnenolone sulfate * | −0.26 | |||
| 5-Aminolevulinic acid * | 0.25 | |||
| Isovaleric acid * | −0.25 | |||
| 2-Aminoisobutyric acid * | −0.24 | |||
| Citric acid * | 0.24 | |||
| 4-Hydroxyproline * | 0.24 | |||
| D-Leucic acid * | 0.23 | |||
| D-Ribose 5-phosphate * | −0.22 | |||
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | r | Pathway | Matched Metabolites | Impact |
| Phenylpyruvic acid *** | −0.44 | Glyoxylate and dicarboxylate Metabolism *** | Citrate *, L-Serine **, Glycine *, Isocitrate *, L-Glutamine * | 0.18 |
| Cytidine *** | 0.42 | |||
| 5-Aminolevulinic acid *** | −0.39 | Valine, leucine and isoleucine Biosynthesis ** | 3-Methyl-2-oxobutanoic acid *, 4-Methyl-2-oxopentanoate *** | 0 |
| 3S-methyl-2-oxo-pentanoic acid *** | −0.38 | |||
| 4-Hydroxyproline ** | −0.36 | Aminoacyl-tRNA biosynthesis ** | L-Glutamine *, Glycine *, L-Serine **, L-Alanine * | 0.17 |
| L-3-Phenyllactic acid ** | −0.36 | |||
| Taurine ** | 0.35 | Alanine, aspartate and glutamate Metabolism * | L-Alanine *, L-Glutamine *, Citrate * | 0.11 |
| Citraconic acid ** | −0.35 | |||
| Ketoleucine ** | −0.35 | Glycine, serine and threonine Metabolism * | L-Serine **, Glycine *, 5-Aminolevulinate *** | 0.46 |
| 1-Methylhistamine ** | 0.33 | |||
| D-Leucic acid ** | −0.32 | Pentose and glucuronate Interconversions * | Xylitol *, D-Glucuronate ** | 0.3 |
| Glucuronic acid ** | −0.32 | |||
| Amiloride ** | 0.30 | Citrate cycle (TCA Cycle) * | Isocitrate *, Citrate * | 0.14 |
| L-Serine ** | 0.29 | |||
| Xylitol * | 0.29 | |||
| Isocitric acid * | −0.28 | |||
| Glycine * | 0.27 | |||
| DL-Acetylcarnitine * | 0.25 | |||
| L-Glutamine * | 0.25 | |||
| Dihydroxyacetone * | 0.25 | |||
| trans-Aconitic acid * | −0.25 | |||
| Citric acid * | −0.25 | |||
| Capric acid * | −0.24 | |||
| L-Alanine * | −0.24 | |||
| 2-Aminoisobutyric acid * | 0.24 | |||
| Isobutyric acid * | 0.23 | |||
| 2,3,4,5-Tetrahydroxypentanoic acid * | 0.23 | |||
| 4-Pyridoxic acid * | 0.23 | |||
| D-Ribose 5-phosphate * | 0.23 | |||
| Alpha-ketoisovaleric acid * | −0.22 | |||
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | r | Pathway | Matched Metabolites | Impact |
| Creatinine ** | −0.40 | Pentose and glucuronate interconversions | L-Arabitol *, Xylitol * | 0.17 |
| Ribitol * | 0.33 | |||
| Galactitol * | 0.32 | Galactose metabolism | Galactitol *, D-Sorbitol * | 0 |
| Sorbitol * | 0.31 | |||
| L-Arabitol * | 0.30 | |||
| Xylitol * | 0.30 | |||
| Mannitol * | 0.30 | |||
| Phenylbutazone * | −0.30 | |||
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | r | Pathway | Matched Metabolites | Impact |
| L-3-Phenyllactic acid *** | −0.39 | Phenylalanine metabolism ** | Phenylpyruvate **, 2-Hydroxyphenylacetate * | 0.26 |
| Phenylpyruvic acid ** | −0.35 | |||
| D-Leucic acid ** | −0.34 | Phenylalanine, tyrosine and tryptophan biosynthesis * | Phenylpyruvate ** | 0 |
| Guanine * | −0.26 | |||
| 3-Hexenedioic acid * | −0.25 | |||
| L-Arabinose * | 0.25 | |||
| Ortho-Hydroxyphenylacetic acid * | −0.25 | |||
| Amiloride * | 0.24 | |||
| Glucuronic acid * | −0.24 | |||
| 3S-methyl-2-oxo-pentanoic acid * | −0.23 | |||
| Cytidine * | 0.23 | |||
| L-Alpha-aminobutyric acid * | −0.23 | |||
| 3-Hydroxyisovaleric acid * | −0.22 | |||
| Caprylic acid * | −0.22 | |||
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | r | Pathway | Matched Metabolites | Impact |
| Methylguanidine *** | 0.41 | Aminoacyl-tRNA biosynthesis *** | L-Asparagine *, L-Histidine *, L-Phenylalanine **, L-Arginine **, L-Glutamine *, L-Serine *, L-Methionine **, L-Valine *, L-Lysine ***, L-Isoleucine **, L-Threonine ***, L-Proline ** | 0.17 |
| L-Threonine *** | 0.41 | |||
| L-Homoserine *** | 0.41 | |||
| L-Lysine *** | 0.36 | |||
| L-Isoleucine ** | 0.33 | |||
| L-Arginine ** | 0.31 | |||
| Caprylic acid ** | 0.29 | Valine, leucine and isoleucine biosynthesis *** | L-Threonine ***, L-Isoleucine ***, L-Valine * | 0 |
| Adenosine triphosphate ** | 0.29 | |||
| L-Methionine ** | 0.29 | Cysteine and methionine metabolism * | L-Serine *, L-Methionine **, (S)-2-Aminobutanoate* | 0.17 |
| Indolelactic acid ** | 0.29 | |||
| 2-hydroxyglutaric acid ** | 0.28 | Arginine biosynthesis * | L-Arginine ***, L-Glutamine * | 0.08 |
| L-Proline ** | 0.27 | Nicotinate and nicotinamide metabolism * | Quinolinate *, Nicotinamide * | 0.19 |
| L-Phenylalanine ** | 0.27 | Histidine metabolism * | L-Histidine *, N(pi)-Methyl-L-histidine * | 0.22 |
| L-Alloisoleucine ** | 0.27 | |||
| L-Kynurenine * | 0.26 | |||
| L-Asparagine * | 0.26 | |||
| L-Alpha-aminobutyric acid * | 0.26 | |||
| 1-Methylhistidine * | 0.25 | |||
| 5-Hydroxyindoleacetic acid * | 0.25 | |||
| Quinolinic acid * | 0.25 | |||
| L-Valine * | 0.24 | |||
| L-Glutamine * | 0.24 | |||
| L-Serine * | 0.23 | |||
| L-Histidine * | 0.22 | |||
| Capric acid * | 0.22 | |||
| Niacinamide * | 0.21 | |||
| L-3-Phenyllactic acid * | 0.21 | |||
| Picolinic acid * | 0.20 | |||
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | r | Pathway | Matched Metabolites | Impact |
| Creatine * | −0.26 | Glycine, serine and threonine metabolism * | Creatine * | 0 |
| Nonadecanoic acid * | 0.21 | Arginine and proline metabolism * | Creatine * | 0.01 |
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | r | Pathway | Matched Metabolites | Impact |
| Nonadecanoic acid * | 0.24 | Phenylalanine, tyrosine and tryptophan biosynthesis ** | Phenylpyruvate * | 0 |
| Phenylpyruvic acid * | 0.22 | |||
| Phenylalanine metabolism * | Phenylpyruvate * | 0.26 | ||
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | r | Pathway | Matched Metabolites | Impact |
| Suberic acid ** | 0.31 | Valine, leucine and isoleucine * | 3-Methyl-2-oxobutanoic acid | 0 |
| Cytidine * | −0.26 | |||
| 5-Aminolevulinic acid * | 0.25 | |||
| L-Alpha-aminobutyric acid * | 0.24 | |||
| Citrulline * | 0.24 | |||
| 4-Hydroxyproline * | 0.23 | |||
| Alpha-ketoisovaleric acid * | −0.21 | |||
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | r | Pathway | Matched Metabolites | Impact |
| Stearic acid *** | 0.34 | D-Glutamine and D-glutamate metabolism ** | D-Glutamate **, L-Glutamine * | 0.5 |
| Palmitic acid ** | 0.30 | |||
| Isobutyric acid ** | 0.29 | Glyoxylate and dicarboxylate metabolism * | L-Serine **, L-Glutamine * | 0.04 |
| Cytidine ** | 0.28 | Glycine, serine and threonine metabolism * | L-Serine **, 5-Aminolevulinate * | 0.22 |
| D-Leucic acid ** | −0.27 | Biosynthesis of unsaturated fatty acids * | Palmitic acid **, Octadecanoic acid | 0 |
| L-Serine ** | 0.27 | |||
| D-Glutamic acid ** | 0.27 | |||
| 4-Pyridoxic acid * | 0.26 | |||
| 5-Aminolevulinic acid * | −0.24 | |||
| L-Glutamine * | 0.22 | |||
| 4-Hydroxyproline * | −0.22 | |||
| Cytosine * | 0.22 | |||
| Methylmalonic acid * | −0.22 | |||
| Acetamide * | 0.21 | |||
| N-Acetylethanolamine * | 0.21 | |||
| 2-Methylglutaric acid * | −0.20 | |||
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | r | Pathway | Matched Metabolites | Impact |
| L-Glutamine *** | 0.47 | Aminoacyl-tRNA biosynthesis*** | L-Asparagine ***, L-Histidine ***, L-Glutamine ***, Glycine **, L-Serine ***, L-Valine *, L-Lysine *, L-Tyrosine **, L-Proline *** | 0.17 |
| L-Serine *** | 0.42 | |||
| L-Proline *** | 0.39 | |||
| L-Histidine *** | 0.39 | |||
| DL-Acetylcarnitine *** | 0.38 | Glyoxylate and dicarboxylate metabolism* | L-Serine ***, Glycine **, L-Glutamine *** | 0.15 |
| Palmitic acid *** | 0.37 | |||
| Acetamide *** | 0.37 | Glycine, serine and threonine metabolism* | L-Serine ***, Glycine **, 5-Aminolevulinate * | 0.46 |
| L-Asparagine *** | 0.34 | |||
| Stearic acid ** | 0.33 | Histidine metabolism* | L-Histidine ***, Imidazole-4-acetate * | 0.22 |
| D-Leucic acid ** | −0.33 | |||
| Epinephrine ** | 0.32 | |||
| Taurine ** | 0.30 | |||
| 6-Methyl-DL-Tryptophan ** | 0.29 | |||
| Cytidine ** | 0.28 | |||
| 3-Hexenedioic acid ** | −0.27 | |||
| 5-Hydroxyindoleacetic acid ** | 0.27 | |||
| L-Tyrosine ** | 0.27 | |||
| Glycine ** | 0.27 | |||
| Adenosine triphosphate ** | 0.27 | |||
| Imidazoleacetic acid * | 0.26 | |||
| Dihydroxyacetone * | 0.26 | |||
| Isobutyric acid * | 0.25 | |||
| Cytosine * | 0.23 | |||
| L-Alloisoleucine * | 0.23 | |||
| 5-Aminolevulinic acid * | −0.23 | |||
| Methylmalonic acid * | −0.22 | |||
| Picolinic acid * | 0.22 | |||
| 2-hydroxyglutaric acid * | 0.22 | |||
| L-Valine * | 0.21 | |||
| L-Lysine * | 0.21 | |||
| N-Acetylglutamine * | 0.21 | |||
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | r | Pathway | Matched Metabolites | Impact |
| Hypoxanthine *** | −0.34 | Citrate cycle (TCA cycle) ** | Isocitrate *, Citrate * | 0.14 |
| 3-Hexenedioic acid ** | 0.31 | Glyoxylate and dicarboxylate metabolism * | Citrate *, Isocitrate * | 0.03 |
| Pyroglutamic acid ** | −0.27 | |||
| D-Ribose ** | 0.27 | |||
| Uridine ** | −0.27 | |||
| L-Arabinose * | −0.26 | |||
| Citric acid * | 0.25 | |||
| Isocitric acid * | 0.24 | |||
| Glucuronic acid * | 0.23 | |||
| Salicylic acid * | 0.22 | |||
| 2-Methylglutaric acid * | −0.22 | |||
| Creatine * | −0.21 | |||
| Nonadecanoic acid * | 0.20 | |||
| N-Acetylglutamine * | −0.20 | |||
| Significant Metabolites | Significant Pathways | |||
|---|---|---|---|---|
| Metabolite | r | Pathway | Matched Metabolites | Impact |
| L-Proline ** | 0.28 | Aminoacyl-tRNA biosynthesis *** | L-Histidine *, L-Glutamine *, L-Proline ** | 0 |
| Acetamide * | 0.26 | |||
| 3-Hydroxyisovaleric acid * | 0.26 | D-Glutamine and D-glutamate metabolism * | L-Glutamine * | 0 |
| L-Glutamine * | 0.25 | |||
| Ribitol * | 0.22 | Nitrogen metabolism * | L-Glutamine * | 0 |
| L-Histidine * | 0.21 | |||
| DL-Acetylcarnitine * | 0.21 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brister, D.; Rose, S.; Delhey, L.; Tippett, M.; Jin, Y.; Gu, H.; Frye, R.E. Metabolomic Signatures of Autism Spectrum Disorder. J. Pers. Med. 2022, 12, 1727. https://doi.org/10.3390/jpm12101727
Brister D, Rose S, Delhey L, Tippett M, Jin Y, Gu H, Frye RE. Metabolomic Signatures of Autism Spectrum Disorder. Journal of Personalized Medicine. 2022; 12(10):1727. https://doi.org/10.3390/jpm12101727
Chicago/Turabian StyleBrister, Danielle, Shannon Rose, Leanna Delhey, Marie Tippett, Yan Jin, Haiwei Gu, and Richard E. Frye. 2022. "Metabolomic Signatures of Autism Spectrum Disorder" Journal of Personalized Medicine 12, no. 10: 1727. https://doi.org/10.3390/jpm12101727
APA StyleBrister, D., Rose, S., Delhey, L., Tippett, M., Jin, Y., Gu, H., & Frye, R. E. (2022). Metabolomic Signatures of Autism Spectrum Disorder. Journal of Personalized Medicine, 12(10), 1727. https://doi.org/10.3390/jpm12101727








