Determinants of Treatment Benefit and Post-Treatment Survival for Patients with Hepatocellular Carcinoma Enrolled in Second-Line Trials after the Failure of Sorafenib Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Outcome Measures
2.3. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
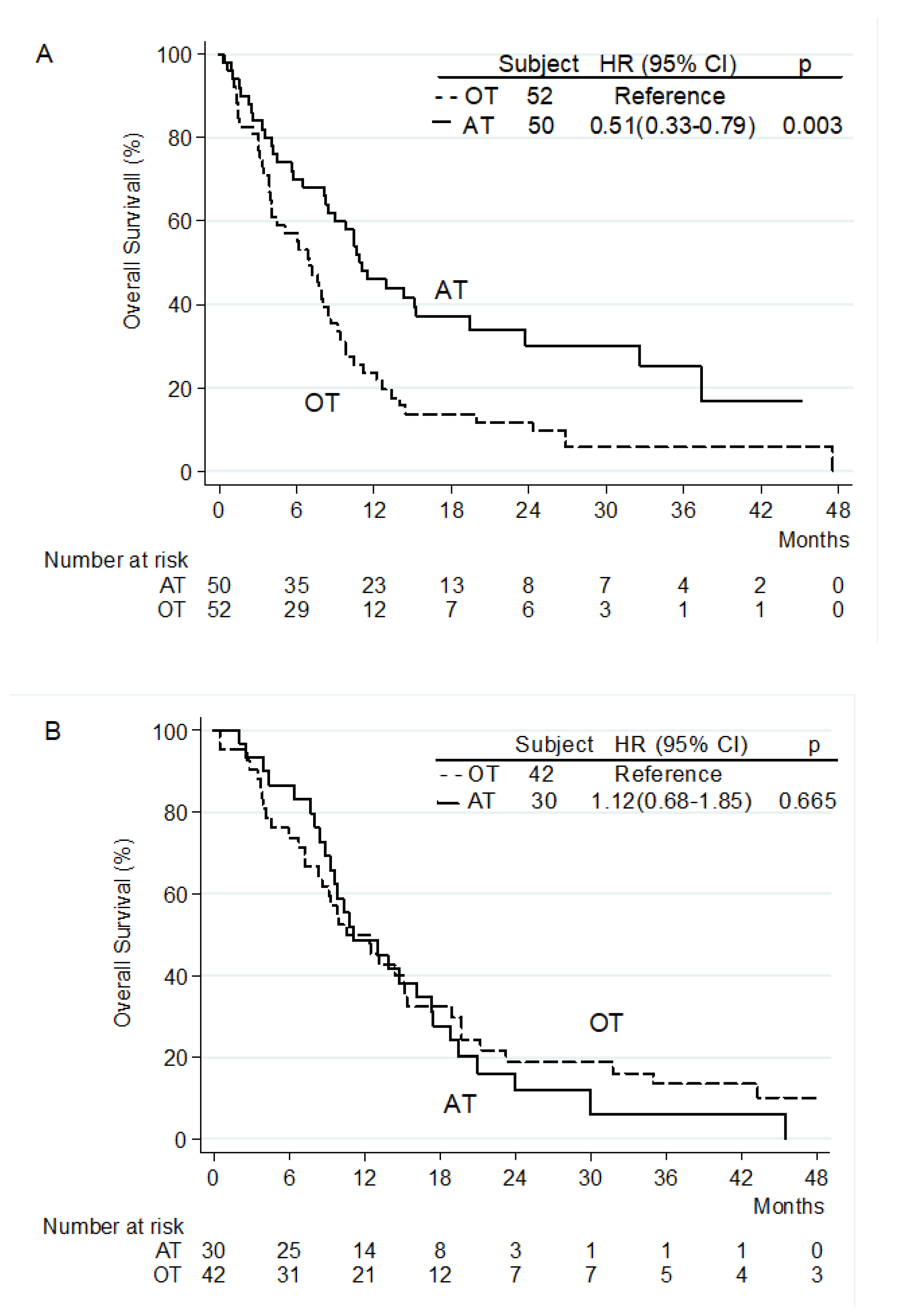
3.2. Overall Survival from the Beginning of Second-Line Treatment
3.3. Overall Survival from Start of Sorafenib
3.4. Time to Treatment Failure from Start of Second-Line Treatment
3.5. Post-Second-Line Treatment Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogel, A.; Meyer, T.; Sapisochin, G. Hepatocellular carcinoma. Lancet 2022. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Xu, J.; Bai, Y. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2-3 study. Lancet Oncol. 2021, 22, 977–990. [Google Scholar] [CrossRef]
- Qin, S.; Chan, L.S.; Gu, S. Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): A randomized, phase III trial. Ann. Oncol. 2022, 33 (Suppl. 7), S808–S869. [Google Scholar] [CrossRef]
- Qin, S.; Kudo, M.; Meyer, T. Final analysis of RATIONALE-301: Randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann. Oncol. 2022, 33 (Suppl. 7), S808–S869. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022. [Google Scholar] [CrossRef]
- Kelley, R.K.; Rimassa, L.; Cheng, A.L. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.K.; Kim, T.Y. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.Y.; Merle, P. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Chen, Z.; Fang, W. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study. J. Clin. Oncol. 2022, 40 (Suppl. 4). [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Hepatobiliary Cancers. Version 1.2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (accessed on 1 September 2022).
- Johnson, P.J.; Berhane, S.; Kagebayashi, C. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Alsina, A.; Kudo, M.; Vogel, A. Effects of Subsequent Systemic Anticancer Medication Following First-Line Lenvatinib: A Post Hoc Responder Analysis from the Phase 3 REFLECT Study in Unresectable Hepatocellular Carcinoma. Liver Cancer. 2020, 9, 93–104. [Google Scholar] [CrossRef]
- Iavarone, M.; Cabibbo, G.; Biolato, M. Predictors of survival in patients with advanced hepatocellular carcinoma who permanently discontinued sorafenib. Hepatology 2015, 62, 784–791. [Google Scholar] [CrossRef]
- Facciorusso, A.; Abd El Aziz, M.A.; Sacco, R. Efficacy of Regorafenib in Hepatocellular Carcinoma Patients: A Systematic Review and Meta-Analysis. Cancers (Basel) 2019, 12, 36. [Google Scholar] [CrossRef]
- Pfister, D.; Núñez, N.G.; Pinyol, R. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Kirstein, M.M.; Scheiner, B.; Marwede, T. Sequential systemic treatment in patients with hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2020, 52, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.Y.; Wu, C.H.; Lu, L.C. Prognosis of patients with advanced hepatocellular carcinoma who failed first-line systemic therapy. J. Hepatol. 2014, 60, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Personeni, N.; Giordano, L.; Abbadessa, G. Prognostic value of the neutrophil-to-lymphocyte ratio in the ARQ 197-215 second-line study for advanced hepatocellular carcinoma. Oncotarget 2017, 8, 14408–14415. [Google Scholar] [CrossRef]
- Bruix, J.; Cheng, A.L.; Meinhardt, G. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J. Hepatol. 2017, 67, 999–1008. [Google Scholar] [CrossRef]
- Liu, L.; Gong, Y.; Zhang, Q. Prognostic Roles of Blood Inflammatory Markers in Hepatocellular Carcinoma Patients Taking Sorafenib. A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 9, 1557. [Google Scholar] [CrossRef]
- Choi, W.M.; Kim, J.Y.; Choi, J. Kinetics of the neutrophil-lymphocyte ratio during PD-1 inhibition as a prognostic factor in advanced hepatocellular carcinoma. Liver Int. 2021, 41, 2189–2199. [Google Scholar] [CrossRef]
- Song, W.; Tian, C.; Wang, K. The pretreatment lymphocyte to monocyte ratio predicts clinical outcome for patients with hepatocellular carcinoma: A meta-analysis. Sci. Rep. 2017, 7, 46601. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhu, J.; Zhao, L. Lymphocyte to monocyte ratio and neutrophil to lymphocyte ratio are superior inflammation-based predictors of recurrence in patients with hepatocellular carcinoma after hepatic resection. J. Surg. Oncol. 2017, 115, 718–728. [Google Scholar] [CrossRef]
- Wang, W.; Tsuchiya, K.; Kurosaki, M. Sorafenib-regorafenib sequential therapy in Japanese patients with unresectable hepatocellular carcinoma—Relative dose intensity and post-regorafenib therapies in real world practice. Cancers (Basel) 2019, 11, 1517. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Merle, P.; Granito, A. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J. Hepatol. 2018, 69, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Ryoo, B.Y.; Merle, P. Second-line cabozantinib after sorafenib treatment for advanced hepatocellular carcinoma: A subgroup analysis of the phase 3 CELESTIAL trial. ESMO Open. 2020, 5, e000714. [Google Scholar] [CrossRef]
- Llovet, J.M.; Decaens, T.; Raoul, J.L. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: Results from the randomized phase III BRISK-PS study. J. Clin. Oncol. 2013, 31, 3509–3516. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Kudo, M.; Assenat, E. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: The EVOLVE-1 randomized clinical trial. JAMA 2014, 312, 57–67. [Google Scholar] [CrossRef]
- Zhu, A.X.; Park, J.O.; Ryoo, B.Y. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 859–870. [Google Scholar] [CrossRef]
- Rimassa, L.; Assenat, E.; Peck-Radosavljevic, M. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): A final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018, 19, 682–693. [Google Scholar] [CrossRef]
- Reig, M.; Galle, P.R.; Kudo, M. Pattern of progression in advanced hepatocellular carcinoma treated with ramucirumab. Liver Int. 2021, 41, 598–607. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | n = 174 (%) |
|---|---|
| Median Age | |
| Years (range) | 69 (24–85) |
| Gender | |
| Male | 157 (90.2) |
| Female | 17 (9.8) |
| Diagnosis | |
| Histology | 117 (67.2) |
| AASLD criteria | 57 (32.8) |
| ECOG PS † | |
| 0 | 103 (59.5) |
| 1 | 70 (40.5) |
| Barcelona Clinic Liver Cancer stage | |
| B | 37 (21.3) |
| C | 137 (78.7) |
| Etiology | |
| Hepatitis C Infection | 70 (40.2) |
| Hepatitis B Infection | 23 (13.2) |
| Alcohol | 34 (19.5) |
| Non-alcoholic fatty liver disease | 15 (9.0) |
| Others | 32 (18.3) |
| Child–Pugh Class | |
| A | 172 (98.9) |
| B7 | 2 (1.1) |
| Prior surgery | |
| Yes | 71 (40.9) |
| No | 103 (59.1) |
| Prior locoregional treatments | |
| Yes | 112 (64.4) |
| No | 62 (35.6) |
| Reason for sorafenib discontinuation | |
| Disease progression | 141 (81.0) |
| Adverse events | 33 (19.0) |
| Disease Extent | |
| EHS | 102 (58.6) |
| Intrahepatic only | 72 (41.4) |
| Portal Vein Thrombosis | |
| Yes | 50 (28.8) |
| No | 124 (71.2) |
| Median AFP | |
| ng/dL (range) | 86 (1–436300) |
| Median neutrophils to lymphocyte ratio (range) | 3 (0–17) |
| Second-line treatment | |
| Targeted agents | 40 (23.0) |
| ICI +/– targeted agents | 40 (23.0) |
| Other treatments not approved for HCC | 52 (29.9) |
| Placebo | 42 (24.1) |
| Reasons for second-line treatment discontinuation ‡ | |
| Disease progression | 125 (73.1) |
| Adverse events | 14 (8.1) |
| Liver failure or ECOG PS worsening | 32 (18.8) |
| Albumin-Bilirubin grade after second-line treatment § | |
| 1 | 16 (22.9) |
| 2 | 41 (58.5) |
| 3 | 13 (18.6) |
| Class of Drug | Median OS (Months) | HR (95% CI) | p-Value |
|---|---|---|---|
| Placebo | 8.9 | Reference | |
| Other treatments not approved for HCC | 8.4 | 1.27 (0.83–1.96) | 0.27 |
| Targeted agents approved for HCC | 10.9 | 0.89 (0.55–1.43) | 0.63 |
| Anti-PD1 antibodies +/– anti-CTLA4 antibodies | 11.3 | 0.83 (0.45–1.51) | 0.53 |
| Anti-PD1 antibodies + targeted agents +/– anti-CTLA4 antibodies | 14.7 | 0.68 (0.36–1.29) | 0.24 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age (continuous trait) | 1.00 (0.98–1.01) | 0.58 | ||
| Sex | ||||
| Male/Female | 1.55 (0.86–2.80) | 0.15 | ||
| ECOG PS | ||||
| 1/0 | 1.48 (1.06–2.07) | 0.020 | ||
| Barcelona Clinic Liver Cancer stage | ||||
| B/C | 0.76 (0.51–1.13) | 0.17 | ||
| HCC etiology | ||||
| Non-viral/Viral (HBV or HCV-related) | 0.88 (0.63–1.22) | 0.42 | ||
| Previous surgery | ||||
| Yes/No | 0.60 (0.43–0.84) | 0.03 | ||
| Sorafenib duration (continuous trait) | 1.00 (0.97–1.02) | 0.67 | ||
| Reason for sorafenib discontinuation | ||||
| AEs/Disease Progression | 0.69 (0.46–1.05) | 0.08 | ||
| Pattern of progression during first-line sorafenib | ||||
| EHS/Intrahepatic | 1.21 (0.84–1.74) | 0.30 | ||
| Time from sorafenib discontinuation to second-line start (continuous trait) | 1.01 (0.98–1.04) | 0.56 | ||
| Disease extent at the start of second-line treatment | ||||
| EHS/Intrahepatic | 1.21 (0.87–1.68) | 0.26 | 2.14 (1.36–3.37) | 0.001 |
| Portal vein thrombosis | ||||
| Yes/No | 1.91 (1.35–2.72) | <0.001 | 1.85 (1.28–2.69) | 0.001 |
| AFP levels at start of second-line treatment (ng/dL) | ||||
| ≥400/<400 | 1.19 (0.82–1.73) | 0.36 | ||
| NLR (continuous trait) | ||||
| High vs. low | 1.51 (1.08–1.23) | <0.001 | ||
| Second line treatment | 0.72 (0.52–1.00) | 0.048 | 0.24 (0.12–0.47) | <0.001 |
| AT/OT |
| Survival Rate | Sorafenib → AT (n = 94) | Sorafenib → OT (n = 80) |
|---|---|---|
| 6 months | 100% | 97% |
| 12 months | 81% | 79% |
| 24 months | 46% | 42% |
| 36 months | 29% | 17% |
| 48 months | 19% | 11% |
| 60 months | 12% | 6% |
| Type of Treatment | |||
|---|---|---|---|
| Targeted Agents (n = 40) | Anti-PD1 Antibodies, Alone or in Combination (n = 40) | OT (n = 94) | |
| Disease progression | 25 (62%) | 28 (70%) | 72 (77%) |
| AEs | 5 (13%) | 2 (5%) | 7 (7%) |
| Liver failure or ECOG PS worsening | 10 (25%) | 7 (18%) | 15 (16%) |
| Ongoing treatment | - | 3 (7%) | - |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Prior second-line treatment | 0.80 (0.58–1.12) | 0.20 | ||
| AT/OT | ||||
| Reason for second-line treatment discontinuation | ||||
| Disease Progression/Liver Failure or PS worsening | 0.38 (0.25–0.57) | <0.001 | ||
| AEs/Liver Failure or ECOG PS worsening | 0.45 (0.23–0.87) | 0.02 | ||
| Enrolment onto third-line trial | ||||
| Yes/No | 0.31 (0.19–0.51) | <0.001 | 0.34 (0.15–0.78) | 0.01 |
| AFP levels at second-line treatment discontinuation (ng/dL) | ||||
| ≥400/<400 | 1.67 (1.13–2.47) | 0.010 | 2.01 (1.08–3.74) | 0.029 |
| NLR at second-line treatment discontinuation (continuous trait) | ||||
| 1.15 (1.08–1.22) | 0.034 | |||
| Radiological response (RECIST v 1.1 criteria) during prior second-line treatment | ||||
| PD (or NA)/PR | 2.67 (1.22–5.84) | 0.014 | ||
| SD (or NA)/PR | 1.52 (0.70–3.28) | 0.293 | ||
| ALBI grade at second-line treatment discontinuation | ||||
| 2/1 | 2.99 (1.36–6.54) | 0.006 | 1.85 (0.81–4.21) | 0.14 |
| 3/1 | 20.1 (7.4–54.55) | <0.001 | 7.53 (2.48–22.90) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Personeni, N.; Pressiani, T.; Zanuso, V.; Casadei-Gardini, A.; D’Alessio, A.; Valgiusti, M.; Dadduzio, V.; Bergamo, F.; Soldà, C.; Rizzato, M.D.; et al. Determinants of Treatment Benefit and Post-Treatment Survival for Patients with Hepatocellular Carcinoma Enrolled in Second-Line Trials after the Failure of Sorafenib Treatment. J. Pers. Med. 2022, 12, 1726. https://doi.org/10.3390/jpm12101726
Personeni N, Pressiani T, Zanuso V, Casadei-Gardini A, D’Alessio A, Valgiusti M, Dadduzio V, Bergamo F, Soldà C, Rizzato MD, et al. Determinants of Treatment Benefit and Post-Treatment Survival for Patients with Hepatocellular Carcinoma Enrolled in Second-Line Trials after the Failure of Sorafenib Treatment. Journal of Personalized Medicine. 2022; 12(10):1726. https://doi.org/10.3390/jpm12101726
Chicago/Turabian StylePersoneni, Nicola, Tiziana Pressiani, Valentina Zanuso, Andrea Casadei-Gardini, Antonio D’Alessio, Martina Valgiusti, Vincenzo Dadduzio, Francesca Bergamo, Caterina Soldà, Mario Domenico Rizzato, and et al. 2022. "Determinants of Treatment Benefit and Post-Treatment Survival for Patients with Hepatocellular Carcinoma Enrolled in Second-Line Trials after the Failure of Sorafenib Treatment" Journal of Personalized Medicine 12, no. 10: 1726. https://doi.org/10.3390/jpm12101726
APA StylePersoneni, N., Pressiani, T., Zanuso, V., Casadei-Gardini, A., D’Alessio, A., Valgiusti, M., Dadduzio, V., Bergamo, F., Soldà, C., Rizzato, M. D., Giordano, L., Santoro, A., & Rimassa, L. (2022). Determinants of Treatment Benefit and Post-Treatment Survival for Patients with Hepatocellular Carcinoma Enrolled in Second-Line Trials after the Failure of Sorafenib Treatment. Journal of Personalized Medicine, 12(10), 1726. https://doi.org/10.3390/jpm12101726








