Negative Pressure Wound Therapy for the Treatment of Fournier’s Gangrene: A Rare Case with Rectal Fistula and Systematic Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Selection Process
2.4. Data Collection Process and Data Items
2.5. Risk of Bias Assessment
3. Results
Characteristics of the Included Studies
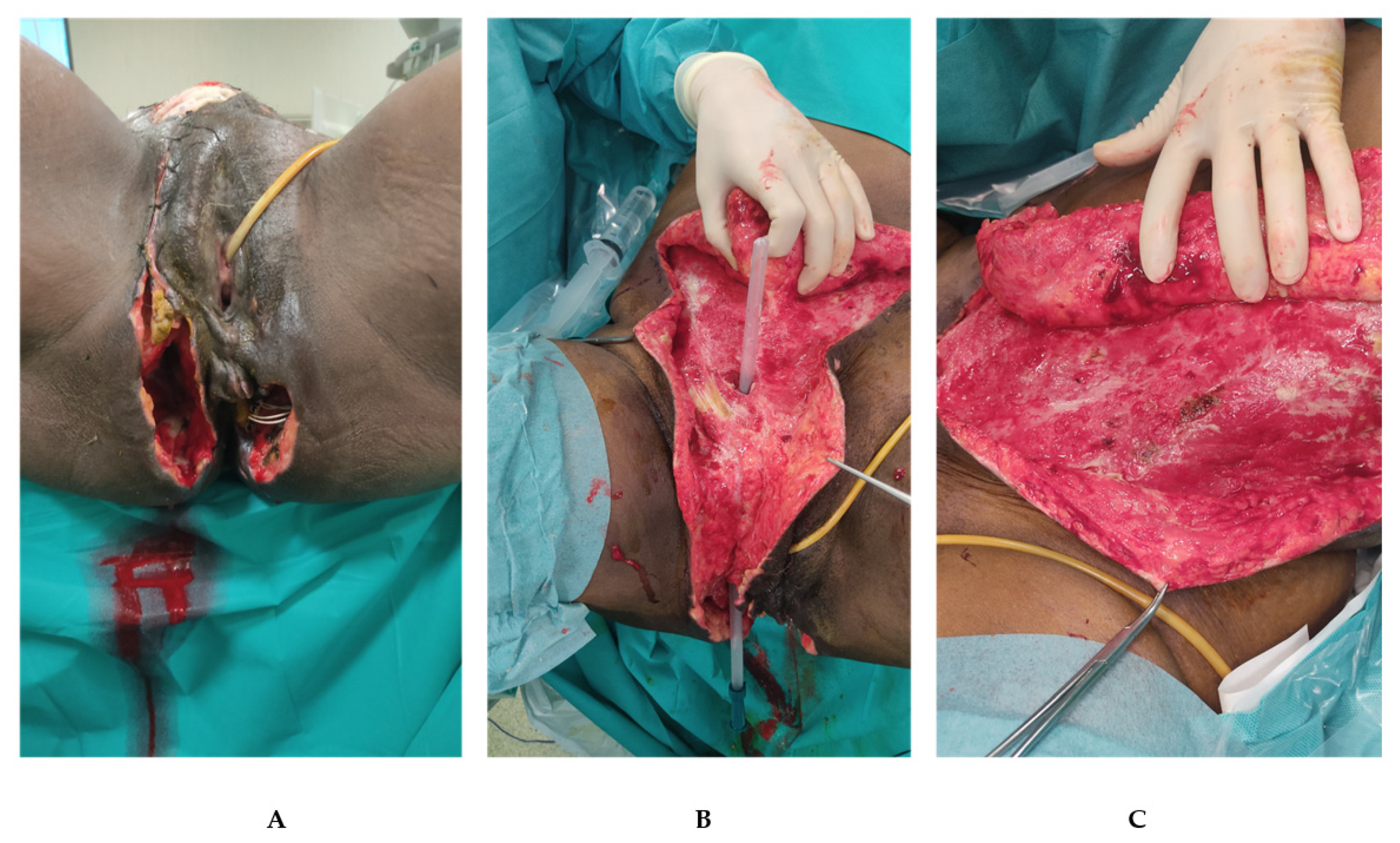
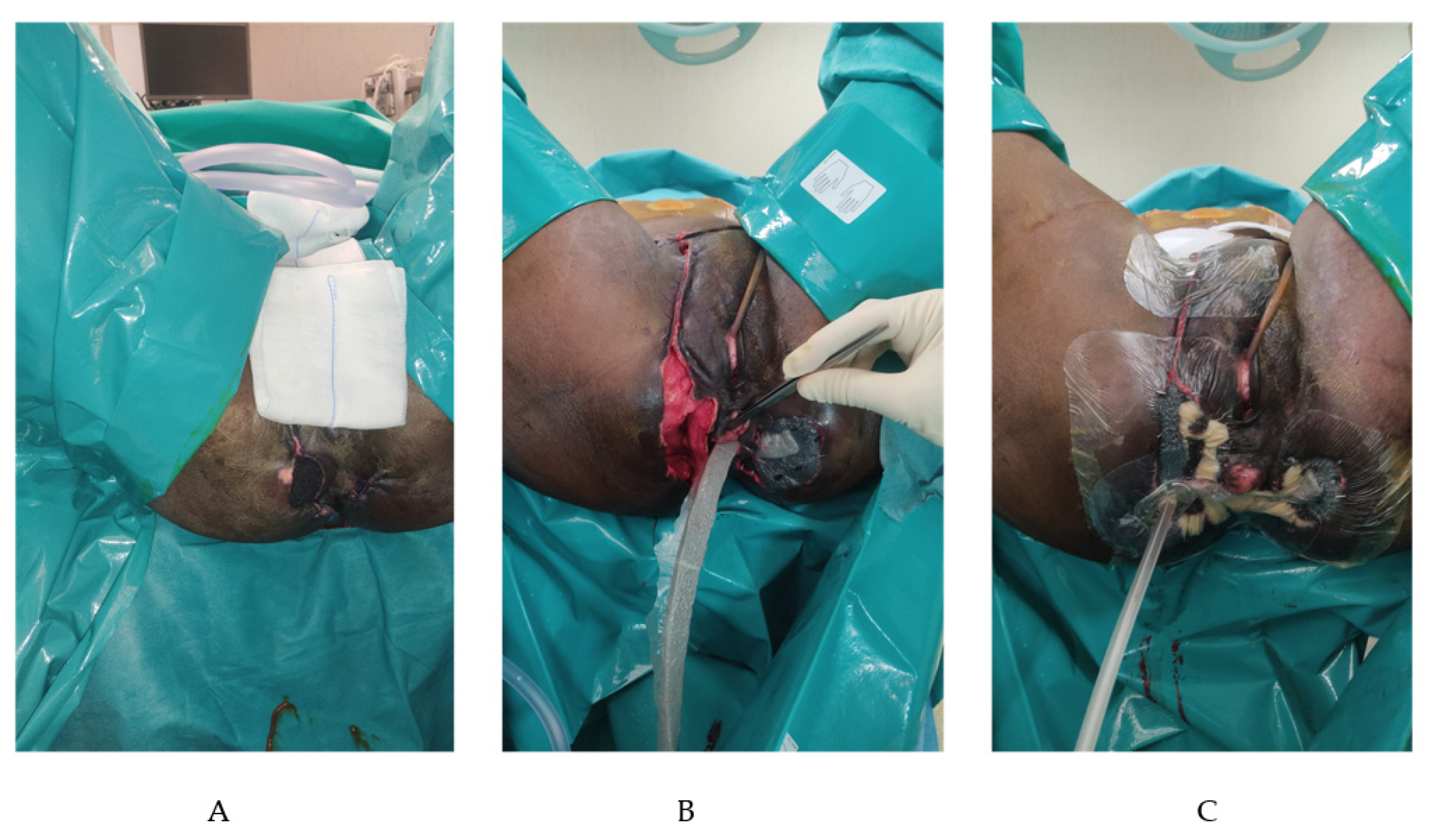
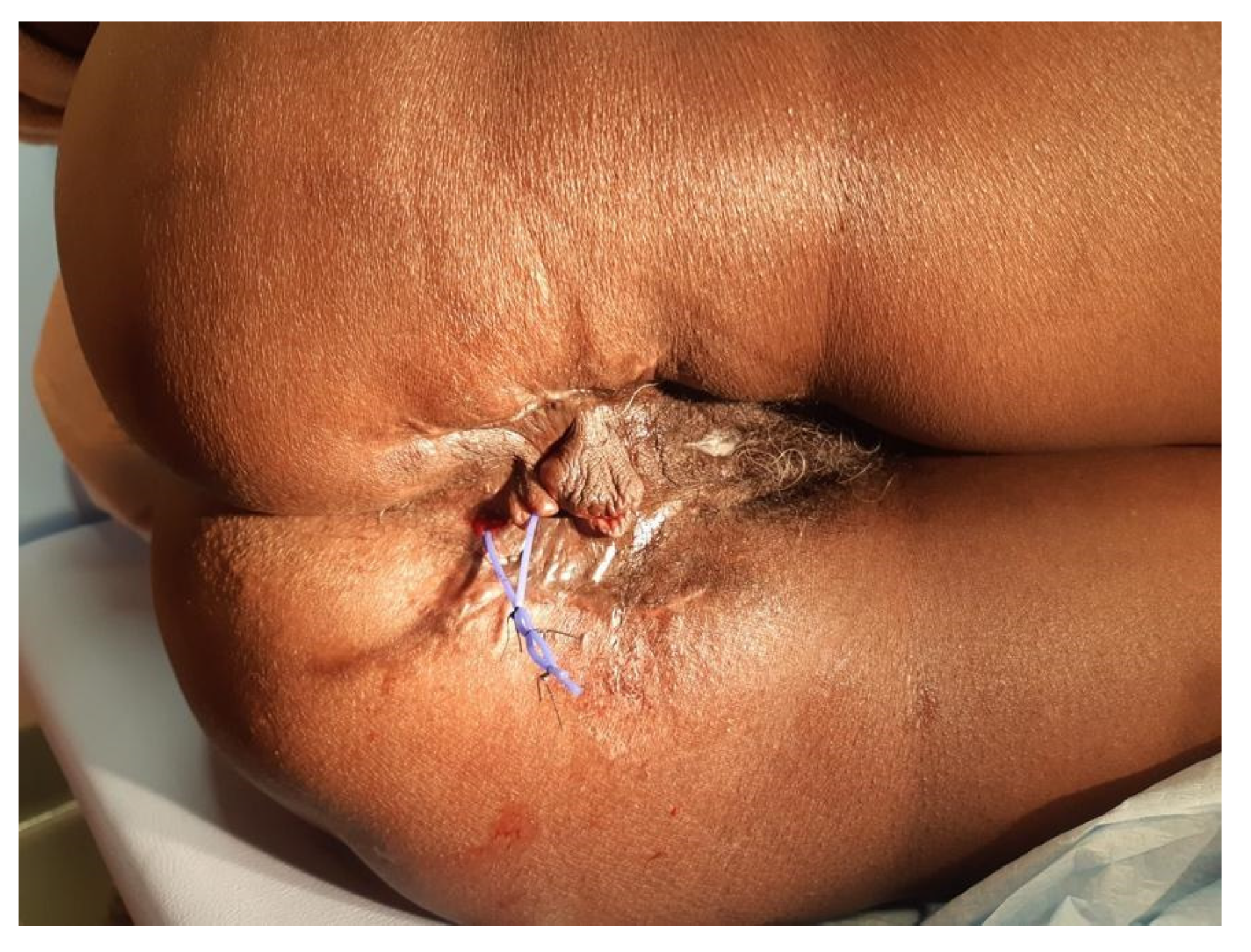
4. Case Presentation
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
| Study | Type of Study | Year | Gender (M:F) | N° of Patients | Origin of FG | Enterostomy Y/N (%) | Antibiotic Therapy (Y/N) | Mono/Polymicrobial (%) | Site of FG | FSGI (median) | NLR | HBO (Y/N) | NPWT (%) | RF (Y/N) | In-Hospital LOS NPWT/no-NPWT (Days/mean) | Timee to INitial Debridement to Wound Closure NPWT/no-NPWT (Days-Mean) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anorectal | Uro-genital | Unclear/Other | P | A-P | |||||||||||||||
| Ozturk et al. [14] | Case series | 2009 | 7:3 | 10 | 6 | 4 | 0 | Y (60) | Y | Poly (80) | 6 | 4 | n/s | n/s | N | 50 | N | 14/13 | 10/9 |
| Cuccia et al. [15] | Case series | 2009 | n/S | 6 | n/s | n/s | n/s | N | Y | n/s | 6 | 0 | 10.5 | n/s | Y | 100 | N | 41.8 | 180 |
| Tucci et al. [16] | Case series | 2009 | 0:2 | 2 | 2 | 0 | 0 | N | Y | n/s | 2 | 0 | n/s | n/s | N | 100 | N | n/s | 67 |
| Czymek et al. [4] | Cohort study | 2010 | 26:12 | 38 | 16 | 8 | 14 | Y(66) | Y | Poly (88) | n/s | n/s | n/s | n/s | N | 50 | Y (29) | 62.7 | n/s |
| Wagner et al. [17] | Case series | 2011 | 38:3 | 41 | 23 | 18 | 0 | N | Y | Poly (95) | 41 | 0 | 2.3 | n/s | Y | 100 | N | 23.4 | 30 |
| Pour et al. [18] | Case report | 2011 | 1:0 | 1 | 0 | 0 | 1 | Y (100) | Y | Poly | 0 | 1 | n/s | n/s | N | 100 | Y (50) | n/s | 60 |
| Zagli et al. [19] | Case report | 2011 | 2:0 | 2 | 2 | 0 | 0 | Y (100) | Y | n/S | 1 | 1 | n/s | n/s | Y | 100 | N | n/s | n/s |
| Jones et al. [20] | Case report | 2012 | 3:0 | 3 | 0 | 3 | 0 | N | Y | Poly | 3 | 0 | n/s | n/s | N | 100 | N | 16 | 81 |
| Pastore et al. [21] | Case report | 2013 | 1:0 | 1 | 1 | 0 | 0 | N | Y | Mono | 0 | 1 | n/s | n/s | Y | 100 | N | 34 | 34 |
| Agostini et al. [22] | Case report | 2014 | 1:0 | 1 | 1 | 0 | 0 | Y (100) | Y | Poly | 0 | 1 | n/s | n/s | Y | 100 | N | 58 | 90 |
| Ludolph et al. [23] | Case series | 2014 | 3:0 | 3 | 0 | 3 | 0 | N | Y | n/s | 3 | 0 | n/s | n/s | N | 100 | N | n/s | 35 |
| Lee et al. [24] | Case series | 2014 | 7:1 | 8 | 3 | 0 | 5 | N | Y | Poly | 3 | 0 | n/s | n/s | N | 100 | N | n/s | n/s |
| Ye et al. [25] | Case report | 2014 | 1:0 | 1 | 1 | 0 | 0 | N | Y | Mono | 1 | 0 | n/s | 0.87 | N | 100 | N | 21 | 73 |
| Oymaci et al. [26] | Case series | 2014 | 10:6 | 16 | 12 | 2 | 2 | Y (75) | Y | n/s | 16 | 0 | 4.56 | n/s | N | 62.5 | N | 25.8 | n/s |
| Oguz et al. [27] | Case series | 2015 | 34:9 | 43 | n/s | n/s | n/s | Y(28) | Y | Poly | 43 | 0 | 5.8 | n/s | N | 18.6 | N | 22.6 | n/s |
| Ozkan et al. [28] | Case series | 2016 | 7:5 | 12 | 8 | 3 | 1 | Y (59) | Y | Poly | 9 | 3 | n/s | n/s | N | 33 | N | 18/20 | n/s |
| Emre et al. [29] | Case report | 2016 | 1:0 | 1 | 1 | 0 | 0 | Y | Y | Poly (50) | 0 | 1 | n/s | n/s | N | Y | N | 80 | 50 |
| Yanaral et al. [30] | Cohort Study | 2017 | n/s | 54 | 23 | 31 | 0 | N | Y | Poly | n/s | n/s | n/s | n/s | N | 43 | N | 17/14 | 13/12 |
| Misiakos et al. [31] | Cohort study | 2017 | 47:15 | 62 | 29 | 0 | 33 | N | Y | n/s | 29 | 9 | n/s | n/s | n/s | 6 | N | 20 | n/s |
| Hong et al. [32] | Case series | 2017 | 18:2 | 20 | 14 | 2 | 4 | Y (55) | Y | Poly (30) | n/s | n/s | 6.8 | n/s | Y | 20 | N | 36.9 | n/s |
| Yucel et al. [33] | Case series | 2017 | n/s | 25 | 13 | 4 | 8 | Y (4) | Y | n/s | 25 | 0 | n/s | n/s | N | 64 | N | 26.4/12.6 | n/s |
| Chang et al. [34] | Case series | 2018 | 11:2 | 13 | n/s | n/s | n/s | N | Y | Poly | n/s | n/s | 4.3 | n/s | N | Y | N | 26.5 | n/s |
| Tian et al. [35] | Case report | 2018 | 1:0 | 1 | 1 | 0 | 0 | N | Y | Poly | 1 | 0 | n/s | 0.87 | N | Y | N | n/s | 30 |
| Kostovski et al. [38] | Case report | 2020 | 0:1 | 1 | 1 | 0 | 0 | N | Y | Poly | 0 | 1 | n/s | n/s | N | Y | N | 35 | n/s |
| Iacovelli et al. [40] | Cohort Study | 2020 | 92:0 | 92 | 10 | 45 | 37 | Y | Y | Poly | 62 | 30 | 3 | n/s | N | Y | N | 28/18 | 40/23 |
| Gul et al. [39] | Cohort study | 2020 | 13:9 | 22 | n/s | n/s | n/s | Y (36) | Y | Poly | n/s | n/s | Y | n/s | Y | 54 | N | 31/21 | n/S |
| Syllaios et al. [36] | Case report | 2020 | 1:0 | 1 | 1 | 0 | 0 | Y | Y | Poly | 1 | 0 | n/s | 0.91 | N | Y | N | 25 | 13 |
| Zhang et al. [37] | Case series | 2020 | 10:2 | 12 | n/s | n/s | n/s | Y (25) | Y | Poly (25) | 7 | 5 | n/s | n/s | N | 83 | 25 | n/s | n/s |
References
- Sartelli, M.; Guirao, X.; Hardcastle, T.C.; Kluger, Y.; Boermeester, M.A.; Raşa, K.; Ansaloni, L.; Coccolini, F.; Montravers, P.; Abu-Zidan, F.M.; et al. 2018 WSES/SIS-E consensus conference: Recommendations for the management of skin and soft-tissue infections. World J. Emerg. Surg. 2018, 13, 58. [Google Scholar] [CrossRef]
- Tang, L.-M.; Su, Y.-J.; Lai, Y.-C. The evaluation of microbiology and prognosis of fournier’s gangrene in past five years. SpringerPlus 2015, 4, 14. [Google Scholar] [CrossRef]
- Malik, A.M.; Sheikh, S.; Pathan, R.; Khan, A.; Sheikh, U. The spectrum of presentation and management of Fournier’s gangrene—An experience of 73 cases. J. Pak. Med. Assoc. 2010, 60, 617–619. [Google Scholar]
- Czymek, R.; Frank, P.; Limmer, S.; Schmidt, A.; Jungbluth, T.; Roblick, U.; Bürk, C.; Bruch, H.-P.; Kujath, P. Fournier’s gangrene: Is the female gender a risk factor? Langenbeck’s Arch. Surg. 2010, 395, 173–180. [Google Scholar] [CrossRef]
- Morgan, M. Diagnosis and management of necrotising fasciitis: A multiparametric approach. J. Hosp. Infect. 2010, 75, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-H.; Khin, L.-W.; Heng, K.-S.; Tan, K.-C.; Low, C.-O. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: A tool for distinguishing necrotizing fasciitis from other soft tissue infections*. Crit. Care Med. 2004, 32, 1535–1541. [Google Scholar] [CrossRef]
- McGillicuddy, E.A.; Lischuk, A.W.; Schuster, K.M.; Kaplan, L.J.; Maung, A.; Lui, F.Y.; Bokhari, S.A.J.; Davis, K.A. Development of a Computed Tomography-Based Scoring System for Necrotizing Soft-Tissue Infections. J. Trauma Inj. Infect. Crit. Care 2011, 70, 894–899. [Google Scholar] [CrossRef]
- Paz Maya, S.; Dualde Beltrán, D.; Lemercier, P.; Leiva-Salinas, C. Necrotizing fasciitis: An urgent diagnosis. Skelet. Radiol. 2014, 43, 577–589. [Google Scholar] [CrossRef]
- Laor, E.; Palmer, L.S.; Tolia, B.M.; Reid, R.E.; Winter, H.I. Outcome Prediction in Patients with Fournier’s Gangrene. J. Urol. 1995, 154, 89–92. [Google Scholar] [CrossRef]
- Peinemann, F.; Sauerland, S. Negative-Pressure Wound Therapy. Dtsch. Arztebl. Int. 2011, 108, 22. [Google Scholar] [CrossRef] [PubMed]
- Ubbink, D.T.; Westerbos, S.J.; Nelson, E.A.; Vermeulen, H. A systematic review of topical negative pressure therapy for acute and chronic wounds. Br. J. Surg. 2008, 95, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Strobe Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Ozturk, E.; Ozguc, H.; Yilmazlar, T. The use of vacuum assisted closure therapy in the management of Fournier’s gangrene. Am. J. Surg. 2009, 197, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, G.; Mucciardi, G.; Morgia, G.; D’Alcontres, F.S.; Galì, A.; Cotrufo, S.; Romeo, M.; Magno, C. Vacuum-Assisted Closure for the Treatment of Fournier’s Gangrene. Urol. Int. 2009, 82, 426–431. [Google Scholar] [CrossRef]
- Tucci, G.; Amabile, D.; Cadeddu, F.; Milito, G. Fournier’s gangrene wound therapy: Our experience using VAC device. Langenbeck’s Arch. Surg. 2009, 394, 759–760. [Google Scholar] [CrossRef]
- Wagner, S.; Greco, F.; Hoda, M.R.; Kawan, F.; Heynemann, H.; Fornara, P. Is Intensive Multimodality Therapy the Best Treatment for Fournier Gangrene? Evaluation of Clinical Outcome and Survival Rate of 41 Patients. Surg. Infect. 2011, 12, 379–383. [Google Scholar] [CrossRef]
- Pour, S.M. Use of Negative Pressure Wound Therapy with Silver Base Dressing for Necrotizing Fasciitis. J. Wound Ostomy Cont. Nurs. 2011, 38, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Zagli, G.; Cianchi, G.; Degl’Innocenti, S.; Parodo, J.; Bonetti, L.; Prosperi, P.; Peris, A. Treatment of Fournier’s Gangrene with Combination of Vacuum-Assisted Closure Therapy, Hyperbaric Oxygen Therapy, and Protective Colostomy. Case Rep. Anesthesiol. 2011, 2011, 430983. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.G.; El-Zawahry, A.M. Curative Treatment without Surgical Reconstruction after Perineal Debridement of Fournier’s Gangrene. J. Wound Ostomy Cont. Nurs. 2012, 39, 98–102. [Google Scholar] [CrossRef]
- Pastore, A.L.; Palleschi, G.; Ripoli, A.; Silvestri, L.; Leto, A.; Autieri, D.; Maggioni, C.; Moschese, D.; Petrozza, V.; Carbone, A. A multistep approach to manage Fournier’s gangrene in a patient with unknown type II diabetes: Surgery, hyperbaric oxygen, and vacuum-assisted closure therapy: A case report. J. Med. Case Rep 2013, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Agostini, T.; Mori, F.; Perello, R.; Dini, M.; Russo, G.L. Successful combined approach to a severe fournier’s gangrene. Indian J. Plast. Surg. 2014, 47, 132–136. [Google Scholar] [PubMed]
- Ludolph, I.; Titel, T.; Beier, J.P.; Dragu, A.; Schmitz, M.; Wullich, B.; Horch, R.E. Penile reconstruction with dermal template and vacuum therapy in severe skin and soft tissue defects caused by Fournier’s gangrene and hidradenitis suppurativa. Int. Wound J. 2016, 13, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Jung, H.; Kwon, H.; Jung, S.-N. Extended negative pressure wound therapy-assisted dermatotraction for the closure of large open fasciotomy wounds in necrotizing fasciitis patients. World J. Emerg. Surg. 2014, 9, 29. [Google Scholar] [CrossRef]
- Ye, J.; Xie, T.; Wu, M.; Ni, P.; Lu, S. Negative Pressure Wound Therapy Applied Before and after Split-Thickness Skin Graft Helps Healing of Fournier Gangrene. Medicine 2015, 94, e426. [Google Scholar] [CrossRef]
- Oymaci, E.; Coskun, A.; Yakan, S.; Erkan, N.; Ucar, A.D.; Yildirim, M. Evaluation of factors affecting mortality in Fournier’s Gangrene: Retrospective clinical study of sixteen cases. Turk. J. Surg. 2014, 30, 85–89. [Google Scholar] [CrossRef]
- Oguz, A.; Gümüş, M.; Turkoglu, A.; Bozdağ, Z.; Ülger, B.V.; Agaçayak, E.; Böyük, A. Fournier’s Gangrene: A Summary of 10 Years of Clinical Experience. Int. Surg. 2015, 100, 934–941. [Google Scholar] [CrossRef]
- Ozkan, O.F.; Koksal, N.; Altinli, E.; Celik, A.; A Uzun, M.; Cıkman, O.; Akbas, A.; Ergun, E.; A Kiraz, H.; Karaayvaz, M. Fournier’s gangrene current approaches. Int. Wound J. 2016, 13, 713–716. [Google Scholar] [CrossRef]
- Emre, A.; Sertkaya, M.; Akbulut, S.; Duman, Y.; Kale, I.T. Neglected Fournier’s Gangrene Caused by Acinetobacter baumannii: A Rare Case Report. Case Rep. Surg. 2016, 2016, 8461354. [Google Scholar]
- Yanaral, F.; Balci, C.; Simsek, A.; Aydin, M.; Ozgor, F.; Onuk, O.; Nuhoglu, B. Comparison of conventional dressings and vacuum-assisted closure in the wound therapy of Fournier’s gangrene. Arch. Ital. Urol. Androl. 2017, 89, 208. [Google Scholar] [CrossRef]
- Misiakos, E.P.; Bagias, G.; Papadopoulos, I.; Danias, N.; Patapis, P.; Machairas, N.; Karatzas, T.; Arkadopoulos, N.; Toutouzas, K.; Alexakis, N.; et al. Early Diagnosis and Surgical Treatment for Necrotizing Fasciitis: A Multicenter Study. Front. Surg. 2017, 4, 5. [Google Scholar] [CrossRef]
- Hong, K.S.; Yi, H.J.; Lee, R.-A.; Kim, K.H.; Chung, S.S. Prognostic factors and treatment outcomes for patients with Fournier’s gangrene: A retrospective study. Int. Wound J. 2017, 14, 1352–1358. [Google Scholar] [CrossRef]
- Yucel, M. Fournier’s gangrene: A retrospective analysis of 25 patients. Turk. J. Trauma Emerg. Surg. 2017, 23, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.-S.; Chou, C.; Hu, C.-Y.; Huang, S.-H. Suture Technique to Prevent Air Leakage during Negative-Pressure Wound Therapy in Fournier Gangrene. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1650. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, T.; Zhao, C.-Q.; Lei, Z.-Y.; Fan, D.-L.; Mao, T.-C. Negative pressure wound therapy and split thickness skin graft aided in the healing of extensive perineum necrotizing fasciitis without faecal diversion: A case report. BMC Surg. 2018, 18, 77. [Google Scholar] [CrossRef]
- Syllaios, A.; Davakis, S.; Karydakis, L.; Vailas, M.; Garmpis, N.; Mpaili, E.; Kyros, E.; Felekouras, E.; Papalampros, A. Treatment of Fournier’s Gangrene with Vacuum-assisted Closure Therapy as Enhanced Recovery Treatment Modality. In Vivo 2020, 34, 1499–1502. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, X.; Zhang, K.; Liu, T. A retrospective case series of Fournier’s gangrene: Necrotizing fasciitis in perineum and perianal region. BMC Surg. 2020, 20, 259. [Google Scholar] [CrossRef]
- Kostovski, O.; Spasovska, O.; Trajkovski, G.; Antovic, S.; Kostovska, I.; Tosheska-Trajkovska, K.; Kuzmanovska, B.; Pejkova, S.; Jankulovski, N. Challenging Treatment of a Female Patient with Extensive Fournier’s Gangrene—Case Report. Prague Med. Rep. 2021, 122, 39–44. [Google Scholar] [CrossRef]
- Gul, M.O.; Sunamak, O.; Kina, U.; Gunay, E.; Akyuz, C. Fournier’s Gangrene: Our Five-Year Series and the Role of Vacuum-Assisted Closure in the Treatment. Niger J. Clin. Pract. 2021, 24, 1277–1282. [Google Scholar]
- Iacovelli, V.; Cipriani, C.; Sandri, M.; Filippone, R.; Ferracci, A.; Micali, S.; Rocco, B.; Puliatti, S.; Ferrarese, P.; Benedetto, G.; et al. The role of vacuum-assisted closure (VAC) therapy in the management of FOURNIER’S gangrene: A retrospective multi-institutional cohort study. World J. Urol. 2021, 39, 121–128. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altomare, M.; Benuzzi, L.; Molteni, M.; Virdis, F.; Spota, A.; Cioffi, S.P.B.; Reitano, E.; Renzi, F.; Chiara, O.; Sesana, G.; et al. Negative Pressure Wound Therapy for the Treatment of Fournier’s Gangrene: A Rare Case with Rectal Fistula and Systematic Review of the Literature. J. Pers. Med. 2022, 12, 1695. https://doi.org/10.3390/jpm12101695
Altomare M, Benuzzi L, Molteni M, Virdis F, Spota A, Cioffi SPB, Reitano E, Renzi F, Chiara O, Sesana G, et al. Negative Pressure Wound Therapy for the Treatment of Fournier’s Gangrene: A Rare Case with Rectal Fistula and Systematic Review of the Literature. Journal of Personalized Medicine. 2022; 12(10):1695. https://doi.org/10.3390/jpm12101695
Chicago/Turabian StyleAltomare, Michele, Laura Benuzzi, Mattia Molteni, Francesco Virdis, Andrea Spota, Stefano Piero Bernardo Cioffi, Elisa Reitano, Federica Renzi, Osvaldo Chiara, Giovanni Sesana, and et al. 2022. "Negative Pressure Wound Therapy for the Treatment of Fournier’s Gangrene: A Rare Case with Rectal Fistula and Systematic Review of the Literature" Journal of Personalized Medicine 12, no. 10: 1695. https://doi.org/10.3390/jpm12101695
APA StyleAltomare, M., Benuzzi, L., Molteni, M., Virdis, F., Spota, A., Cioffi, S. P. B., Reitano, E., Renzi, F., Chiara, O., Sesana, G., & Cimbanassi, S. (2022). Negative Pressure Wound Therapy for the Treatment of Fournier’s Gangrene: A Rare Case with Rectal Fistula and Systematic Review of the Literature. Journal of Personalized Medicine, 12(10), 1695. https://doi.org/10.3390/jpm12101695







