Vitamin D Levels in Patients with Overlap Syndrome, Is It Associated with Disease Severity?
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Study Variables
2.3. Polysomnography
2.4. Pulmonary Function Testing
2.5. Blood Samples and Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adeloye, D.; Chua, S.; Lee, C.; Basquill, C.; Papana, A.; Theodoratou, E.; Nair, H.; Gasevic, D.; Sridhar, D.; Campbell, H.; et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J. Glob. Health 2015, 5, 020415. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Owens, R.L.; Malhotra, A. Sleep-disordered breathing and COPD: The overlap syndrome. Respir. Care 2010, 55, 1333–1344, discussion 1344–1336. [Google Scholar] [PubMed]
- McNicholas, W.T. Chronic obstructive pulmonary disease and obstructive sleep apnea: Overlaps in pathophysiology, systemic inflammation, and cardiovascular disease. Am. J. Respir. Crit. Care Med. 2009, 180, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Weitzenblum, E.; Chaouat, A.; Kessler, R.; Canuet, M. Overlap syndrome: Obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2008, 5, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Voulgaris, A.; Archontogeorgis, K.; Papanas, N.; Pilitsi, E.; Nena, E.; Xanthoudaki, M.; Mikhailidis, D.P.; Froudarakis, M.E.; Steiropoulos, P. Increased risk for cardiovascular disease in patients with obstructive sleep apnoea syndrome-chronic obstructive pulmonary disease (overlap syndrome). Clin. Respir. J. 2019, 13, 708–715. [Google Scholar] [CrossRef]
- Ruchala., M.; Brominska, B.; Cyranska-Chyrek, E.; Kuznar-Kaminska, B.; Kostrzewska, M.; Batura-Gabryel, H. Obstructive sleep apnea and hormones—A novel insight. Arch. Med. Sci. 2017, 13, 875–884. [Google Scholar] [CrossRef]
- Archontogeorgis, K.; Papanas, N.; Nena, E.; Tzouvelekis, A.; Tsigalou, C.; Voulgaris, A.; Xanthoudaki, M.; Mouemin, T.; Froudarakis, M.; Steiropoulos, P. Insulin Sensitivity and Insulin Resistance in Non-Diabetic Middle-Aged Patients with Obstructive Sleep Apnoea Syndrome. Open Cardiovasc. Med. J. 2017, 11, 159–168. [Google Scholar] [CrossRef]
- Laghi, F.; Adiguzel, N.; Tobin, M.J. Endocrinological derangements in COPD. Eur. Respir. J. 2009, 34, 975–996. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Yun, J. The association between serum vitamin D and obstructive sleep apnea: An updated meta-analysis. Respir. Res. 2020, 21, 294. [Google Scholar] [CrossRef]
- Burkes, R.M.; Ceppe, A.S.; Doerschuk, C.M.; Couper, D.; Hoffman, E.A.; Comellas, A.P.; Barr, R.G.; Krishnan, J.A.; Cooper, C.; Labaki, W.W.; et al. Associations Among 25-Hydroxyvitamin D Levels, Lung Function, and Exacerbation Outcomes in COPD: An Analysis of the SPIROMICS Cohort. Chest 2020, 157, 856–865. [Google Scholar] [CrossRef]
- Bouloukaki, I.; Tsiligianni, I.; Mermigkis, C.; Bonsignore, M.R.; Markakis, M.; Pataka, A.; Steiropoulos, P.; Ermidou, C.; Alexaki, I.; Tzanakis, N.; et al. Vitamin D deficiency in patients evaluated for obstructive sleep apnea: Is it associated with disease severity? Sleep Breath 2021, 25, 1109–1117. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.S.; Chouchane, A.I. Role of Vitamin D Beyond the Skeletal Function: A Review of the Molecular and Clinical Studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Skrabakova, Z.; Gonzalez-Gross, M.; Valtuena, J.; de Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Molgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Siachpazidou, D.I.; Stavrou, V.; Zouridis, S.; Gogou, E.; Economou, N.T.; Pastaka, C.; Hatzoglou, C.; Gourgoulianis, K.I. 25-hydroxyvitamin D levels in patients with obstructive sleep apnea and continuous positive airway pressure treatment: A brief review. Sleep Sci. 2020, 13, 78–83. [Google Scholar]
- Martineau, A.R.; James, W.Y.; Hooper, R.L.; Barnes, N.C.; Jolliffe, D.A.; Greiller, C.L.; Islam, K.; McLaughlin, D.; Bhowmik, A.; Timms, P.M.; et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): A multicentre, double-blind, randomised controlled trial. Lancet Respir. Med. 2015, 3, 120–130. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Tsara, V.; Serasli, E.; Amfilochiou, A.; Constantinidis, T.; Christaki, P. Greek version of the Epworth Sleepiness Scale. Sleep Breath 2004, 8, 91–95. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Gamaldo, C.E.; Harding, S.M.; Lloyd, R.M.; Marcus, C.L.; Vaughn, B.V.; American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; Version 2.3; American Academy of Sleep Medicine: Darien, IL, USA, 2016. [Google Scholar]
- McNicholas, W.T.; Strohl, K.P.; White, D.P.; Levy, P.; Schmidt, W.; Wheatley, J.R.; Redline, S.; Carley, D.; Buysse, J.D.; Young, T.; et al. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999, 22, 667–689. [Google Scholar]
- Mirza, S.; Clay, R.D.; Koslow, M.A.; Scanlon, P.D. COPD Guidelines: A Review of the 2018 GOLD Report. Mayo Clin. Proc. 2018, 93, 1488–1502. [Google Scholar] [CrossRef]
- Archontogeorgis, K.; Nena, E.; Papanas, N.; Zissimopoulos, A.; Voulgaris, A.; Xanthoudaki, M.; Manolopoulos, V.; Froudarakis, M.; Steiropoulos, P. Vitamin D Levels in Middle-Aged Patients with Obstructive Sleep Apnoea Syndrome. Curr. Vasc. Pharmacol. 2018, 16, 289–297. [Google Scholar] [CrossRef]
- Salepci, B.; Caglayan, B.; Nahid, P.; Parmaksiz, E.T.; Kiral, N.; Fidan, A.; Comert, S.S.; Dogan, C.; Gungor, G.A. Vitamin D Deficiency in Patients Referred for Evaluation of Obstructive Sleep Apnea. J. Clin. Sleep Med. 2017, 13, 607–612. [Google Scholar] [CrossRef]
- Siachpazidou, D.I.; Kotsiou, O.S.; Stavrou, V.; Pastaka, C.; Gogou, E.; Kechagia, M.; Varsamas, C.; Economou, N.T.; Zouridis, S.; Patrikioy, E.; et al. Serum vitamin D levels in patients with obstructive sleep apnea syndrome and level changes after continuous positive airway pressure therapy. Sleep Breath 2021, 25, 657–668. [Google Scholar] [CrossRef]
- Ma, D.; Zheng, X.; Dong, L.; Zheng, C.; Chen, Y.; Chen, Z.; Lin, M.; Li, X.; Li, Z.; Liu, C. The Relationship of Serum 25-Hydroxyvitamin-D Level with Severity of Obstructive Sleep Apnea in Patients with Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes 2020, 13, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Neighbors, C.L.P.; Noller, M.W.; Song, S.A.; Zaghi, S.; Neighbors, J.; Feldman, D.; Kushida, C.A.; Camacho, M. Vitamin D and obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. 2018, 43, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Romigi, A.; Izzi, F.; Mercuri, N.B.; Cordella, A.; Tarquini, E.; Giambrone, M.P.; Marciani, M.G.; Placidi, F. Continuous Positive Airway Pressure Treatment Increases Serum Vitamin D Levels in Male Patients with Obstructive Sleep Apnea. J. Clin. Sleep Med. 2015, 11, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Izzi, F.; Mercuri, N.B.; Romigi, A.; Cordella, A.; Tarantino, U.; Placidi, F. Vitamin D status of male OSAS patients improved after long-term CPAP treatment mainly in obese subjects. Sleep Med. 2017, 29, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Theorell-Haglow, J.; Hoyos, C.M.; Phillips, C.L.; Yee, B.J.; Herrmann, M.; Brennan-Speranza, T.C.; Grunstein, R.R.; Liu, P.Y. Changes of vitamin D levels and bone turnover markers after CPAP therapy: A randomized sham-controlled trial. J. Sleep Res. 2018, 27, e12606. [Google Scholar] [CrossRef]
- Kerley, C.P.; Hutchinson, K.; Bolger, K.; McGowan, A.; Faul, J.; Cormican, L. Serum Vitamin D Is Significantly Inversely Associated with Disease Severity in Caucasian Adults with Obstructive Sleep Apnea Syndrome. Sleep 2016, 39, 293–300. [Google Scholar] [CrossRef]
- Forli, L.; Halse, J.; Haug, E.; Bjortuft, O.; Vatn, M.; Kofstad, J.; Boe, J. Vitamin D deficiency, bone mineral density and weight in patients with advanced pulmonary disease. J. Intern. Med. 2004, 256, 56–62. [Google Scholar] [CrossRef]
- Janssens, W.; Bouillon, R.; Claes, B.; Carremans, C.; Lehouck, A.; Buysschaert, I.; Coolen, J.; Mathieu, C.; Decramer, M.; Lambrechts, D. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 2010, 65, 215–220. [Google Scholar] [CrossRef]
- Persson, L.J.; Aanerud, M.; Hiemstra, P.S.; Hardie, J.A.; Bakke, P.S.; Eagan, T.M. Chronic obstructive pulmonary disease is associated with low levels of vitamin D. PLoS ONE 2012, 7, e38934. [Google Scholar] [CrossRef]
- Chen, F.Y.; Xiao, M.; Ling, B.; Liu, L.; Chen, L. Vitamin D does not improve lung function decline in COPD: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8637–8644. [Google Scholar]
- Li, X.; He, J.; Yu, M.; Sun, J. The efficacy of vitamin D therapy for patients with COPD: A meta-analysis of randomized controlled trials. Ann. Palliat. Med. 2020, 9, 286–297. [Google Scholar] [CrossRef]
- Janssens, W.; Mathieu, C.; Boonen, S.; Decramer, M. Vitamin D deficiency and chronic obstructive pulmonary disease: A vicious circle. Vitam. Horm. 2011, 86, 379–399. [Google Scholar]
- Archontogeorgis, K.; Nena, E.; Papanas, N.; Steiropoulos, P. The role of vitamin D in obstructive sleep apnoea syndrome. Breathe 2018, 14, 206–215. [Google Scholar] [CrossRef]
- Ragia, G.; Archontogeorgis, K.; Simmaco, M.; Gentile, G.; Borro, M.; Zissimopoulos, A.; Froudarakis, M.; Manolopoulos, V.G.; Steiropoulos, P. Genetics of Obstructive Sleep Apnea: Vitamin D Receptor Gene Variation Affects Both Vitamin D Serum Concentration and Disease Susceptibility. OMICS 2019, 23, 45–53. [Google Scholar] [CrossRef]
- Du, W.; Liu, J.; Zhou, J.; Ye, D.; OuYang, Y.; Deng, Q. Obstructive sleep apnea, COPD, the overlap syndrome, and mortality: Results from the 2005-2008 National Health and Nutrition Examination Survey. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 665–674. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, T.; Wang, C.; Ji, Y. The association between vitamin D and COPD risk, severity, and exacerbation: An updated systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 2597–2607. [Google Scholar] [CrossRef]
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A., Jr. Vitamin D, respiratory infections, and asthma. Curr. Allergy Asthma Rep. 2009, 9, 81–87. [Google Scholar] [CrossRef]
- Ayyildiz, F.; Yildiran, H.; Afandiyeva, N.; Gulbahar, O.; Kokturk, O. The effects of vitamin D supplemantation on prognosis in patients with mild obstructive sleep apnea syndrome. Turk. J. Med. Sci. 2021, 51, 2524–2533. [Google Scholar] [CrossRef]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Dhillon, H.; Swaid, B.; Yelangi, A.; Sundus, S.; Bachuwa, G.; Alkotob, M.L.; et al. Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83,000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis. JAMA Cardiol. 2019, 4, 765–776. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, M.; Sun, J.Y.; Cheng, C.; Shen, H.; Sun, W.; Kong, X.Q. The Association Between Vitamin D Levels and the 10-Year Risk of Atherosclerotic Cardiovascular Disease: A Population-Based Study. J. Cardiovasc. Nurs. 2022. [Google Scholar] [CrossRef]
- Pascale, A.V.; Finelli, R.; Giannotti, R.; Visco, V.; Fabbricatore, D.; Matula, I.; Mazzeo, P.; Ragosa, N.; Massari, A.; Izzo, R.; et al. Vitamin D, parathyroid hormone and cardiovascular risk: The good, the bad and the ugly. J. Cardiovasc. Med. 2018, 19, 62–66. [Google Scholar] [CrossRef]
- Ntritsos, G.; Franek, J.; Belbasis, L.; Christou, M.A.; Markozannes, G.; Altman, P.; Fogel, R.; Sayre, T.; Ntzani, E.E.; Evangelou, E. Gender-specific estimates of COPD prevalence: A systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 1507–1514. [Google Scholar] [CrossRef]
- Wimms, A.; Woehrle, H.; Ketheeswaran, S.; Ramanan, D.; Armitstead, J. Obstructive Sleep Apnea in Women: Specific Issues and Interventions. BioMed Res. Int. 2016, 2016, 1764837. [Google Scholar] [CrossRef]
| Control Group (n = 30) | Patients with OSA (n = 30) | Patients with OVS (n = 30) | p | |
|---|---|---|---|---|
| Gender (males/females) | 27/3 | 26/4 | 26/4 | 0.902 |
| Age (years) | 56 (48.8–64.3) | 56 (52.8–65) | 60 (54.8–67.3) | 0.386 |
| Neck circumference (cm) | 41 (38–45.5) | 46 (43.3–48.8) * | 45 (44–50) * | 0.005 |
| Waist circumference (cm) | 119 (104–131.5) | 122 (119–129) | 130 (119–135.5) * | 0.023 |
| Hip circumference (cm) | 119 (107.5–125.5) | 117 (112.3–122.8) | 119 (110–123.5) | 0.554 |
| WHR | 1.01 (0.94–1.05) | 1.03 (1–1.08) | 1.08 (1.04–1.11) * | 0.040 |
| BMI (kg/m2) | 33.6 (29.9–40.2) | 36.9 (34.5–41.6) | 36.5 (32–42) | 0.105 |
| Tobacco smoking | ||||
| Non-smokers | 36.7% | 30% | 0% **, # | 0.001 |
| Ex-smokers | 36.7% | 13.3% * | 40% # | 0.049 |
| Current smokers | 26.7% | 56.7% * | 60% * | 0.017 |
| Control Group (n = 30) | Patients with OSA (n = 30) | Patients with OVS (n = 30) | p | |
|---|---|---|---|---|
| TST (min) | 326.3 (280.8–358.5) | 310.1 (260.8–346.3) | 320.5 (278.3–360.8) | 0.843 |
| N1 (%) | 10 (5.6–17.2) | 11.4 (6.6–23.4) | 11 (4.8–18.2) | 0.191 |
| N2 (%) | 62.2 (56.9–71) | 66.7 (56.2–77.3) | 66.4 (60.7–71.4) | 0.676 |
| N3 (%) | 11.7 (4.4–20.5) | 7 (2.1–15.8) | 9.9 (6.6–19.1) | 0.391 |
| REM (%) | 13.5 (4.9–15.1) | 6.2 (1.2–13.7) * | 4.1 (0.3–12.3) * | 0.011 |
| Sleep efficiency (%) | 84.6 (75–91.9) | 86.4 (74.2–91.9) | 83.2 (72.4–92.2) | 0.871 |
| Arousal index | 14.4 (9.1–18.2 | 36.5 (21.5–47.4) ** | 26.1 (10.3–44.9) * | <0.001 |
| AHI (events/h) | 2.9 (1.5–4) | 40.2 (22.1–70) ** | 37.1 (18–62.6) ** | <0.001 |
| Aver SaO2 (%) | 94.3 (93.8–95.3) | 92(89–93) ** | 90.8 (84.9–92.1) **,# | <0.001 |
| Min SaO2 (%) | 88 (84–89) | 74.5 (71.8–83.8) ** | 74.5 (63.5–81.3) **,# | <0.001 |
| T < 90% (%) | 0.1 (0–0.4) | 19.7 (3.8–39.4) * | 27.3 (7.7–83.1) **,# | <0.001 |
| ESS score | 8 (4.8–12.3) | 11.5 (7.8–15.3) | 9 (6–13.8) | 0.106 |
| Control Group (n = 30) | Patients with OSA (n = 30) | Patients with OVS (n = 30) | p | |
|---|---|---|---|---|
| FEV1 (% predicted) | 105.5 (91.3–113.8) | 89.9 (74.6–100.3) | 64.7 (48.8–76) **, ## | <0.001 |
| FVC (% predicted) | 102.5 (87.5–111.4) | 85.6 (71–97.3) * | 77.9 (62.4–89.1) ** | <0.001 |
| FEV1/FVC (%) | 83 (80.4–87.8) | 84.6 (79.1–90.3) | 67.1 (62.5–69.2) **, ## | <0.001 |
| pO2 (mmHg) | 79 (73.5–84.3) | 75 (66.8–79.5) * | 67 (60.8–76) ** | <0.001 |
| pCO2 (mmHg) | 40.5 (37–43) | 41 (37–44.5) | 46.5 (42–52) **, # | <0.001 |
| Glucose (mg/dL) | 95 (81–112.5) | 112.5 (96,5–151.3) | 112.5 (95.3–129.3) | 0.080 |
| Creatinine (mg/dL) | 0.8 (0.75–1) | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) | 0.707 |
| SGOT (U/L) | 20 (17.5–22.3) | 22 (17.3–27) | 20 (17–24) | 0.296 |
| SGPT (U/L) | 19.5 (16.8–28) | 24 (18–30.5) | 21.5 (16.8–26.3) | 0.624 |
| Cholesterol(mg/dL) | 207 (182–258) | 210.5 (162.5–242.8) | 185.5 (149–214.5) | 0.154 |
| Triglycerides (mg/dL) | 158 (117.5–214.3) | 158 (120.5–190) | 156.5 (117–244.8) | 0.166 |
| LDL-C (mg/dL) | 128.2 (101.9–159.4) | 124.2 (95.4–156.1) | 96.1 (77.3–126.2) * | 0.030 |
| HDL-C (mg/dL) | 46 (41.5–56) | 44.5 (35.8–52.5) | 50 (43–56.3) | 0.411 |
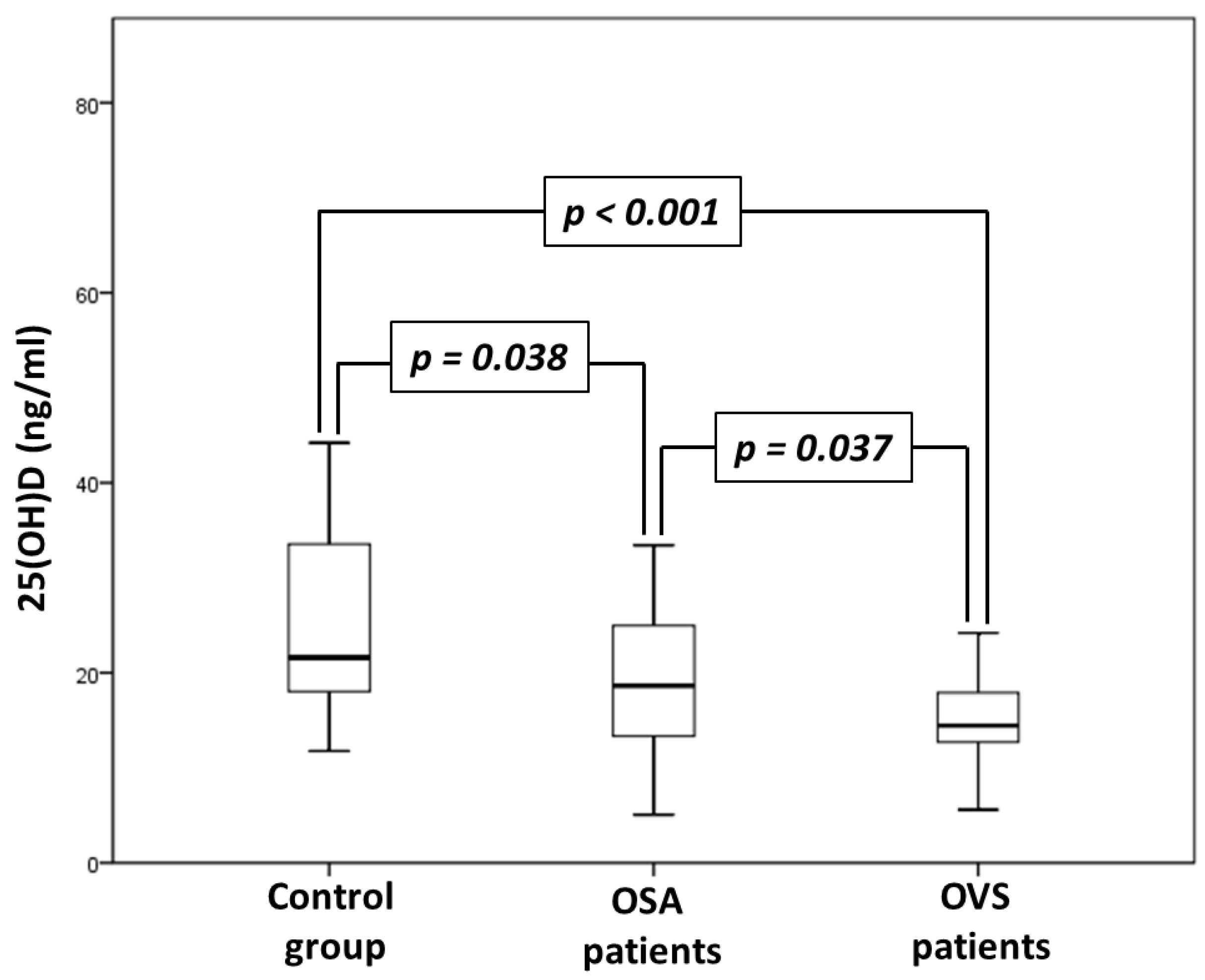
| 25(OH)D (ng/mL) | 21.6 (17.8–33.6) | 18.6 (13.2–25.2) * | 14.5 (12.3–17.9) **, # | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Archontogeorgis, K.; Voulgaris, A.; Nena, E.; Zissimopoulos, A.; Bouloukaki, I.; Schiza, S.E.; Steiropoulos, P. Vitamin D Levels in Patients with Overlap Syndrome, Is It Associated with Disease Severity? J. Pers. Med. 2022, 12, 1693. https://doi.org/10.3390/jpm12101693
Archontogeorgis K, Voulgaris A, Nena E, Zissimopoulos A, Bouloukaki I, Schiza SE, Steiropoulos P. Vitamin D Levels in Patients with Overlap Syndrome, Is It Associated with Disease Severity? Journal of Personalized Medicine. 2022; 12(10):1693. https://doi.org/10.3390/jpm12101693
Chicago/Turabian StyleArchontogeorgis, Kostas, Athanasios Voulgaris, Evangelia Nena, Athanasios Zissimopoulos, Izolde Bouloukaki, Sophia E. Schiza, and Paschalis Steiropoulos. 2022. "Vitamin D Levels in Patients with Overlap Syndrome, Is It Associated with Disease Severity?" Journal of Personalized Medicine 12, no. 10: 1693. https://doi.org/10.3390/jpm12101693
APA StyleArchontogeorgis, K., Voulgaris, A., Nena, E., Zissimopoulos, A., Bouloukaki, I., Schiza, S. E., & Steiropoulos, P. (2022). Vitamin D Levels in Patients with Overlap Syndrome, Is It Associated with Disease Severity? Journal of Personalized Medicine, 12(10), 1693. https://doi.org/10.3390/jpm12101693









