Poly(Styrene-Co-Maleic Acid)-Conjugated 6-Aminofluorescein and Rhodamine Micelle as Macromolecular Fluorescent Probes for Micro-Tumors Detection and Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
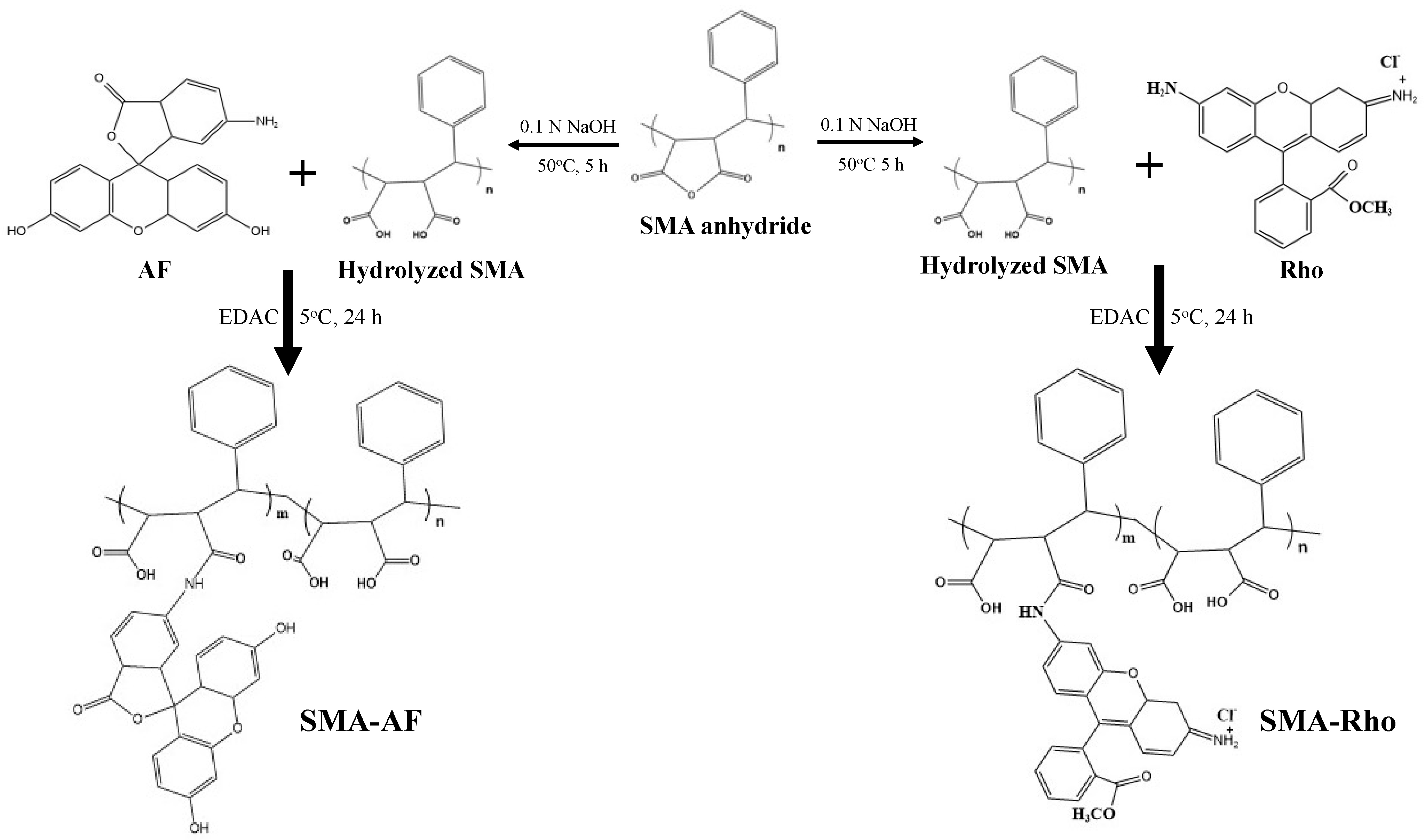
2.2. Synthesis of the SMA-6-aminofluorescein Conjugate (SMA-AF), and the SMA-rhodamine Conjugate (SMA-Rho)
2.3. Dynamic Light Scattering (DLS) and the Zeta Potential
2.4. UV-Visible Spectroscopy
2.5. Fourier Transforms Infrared (FTIR) Spectroscopy
2.6. Fluorescence Spectroscopy
2.7. In Vivo Tissue Distribution of the SMA-AF and SMA-Rho Conjugates: In Vivo Fluorescence Imaging
2.8. Statistical Analyses
3. Results and Discussion
3.1. Synthesis and Characterizations of the Polystyrene-Co-Maleic Acid-6-Aminofluorescein (SMA-AF) and the Polystyrene-co-maleic Acid-rhodamine (SMA-Rho) Conjugates
3.2. Behaviors of the SMA-AF and SMA-Rho Conjugates in Aqueous Solutions, as Analyzed Using Dynamic Light Scattering (DLS)
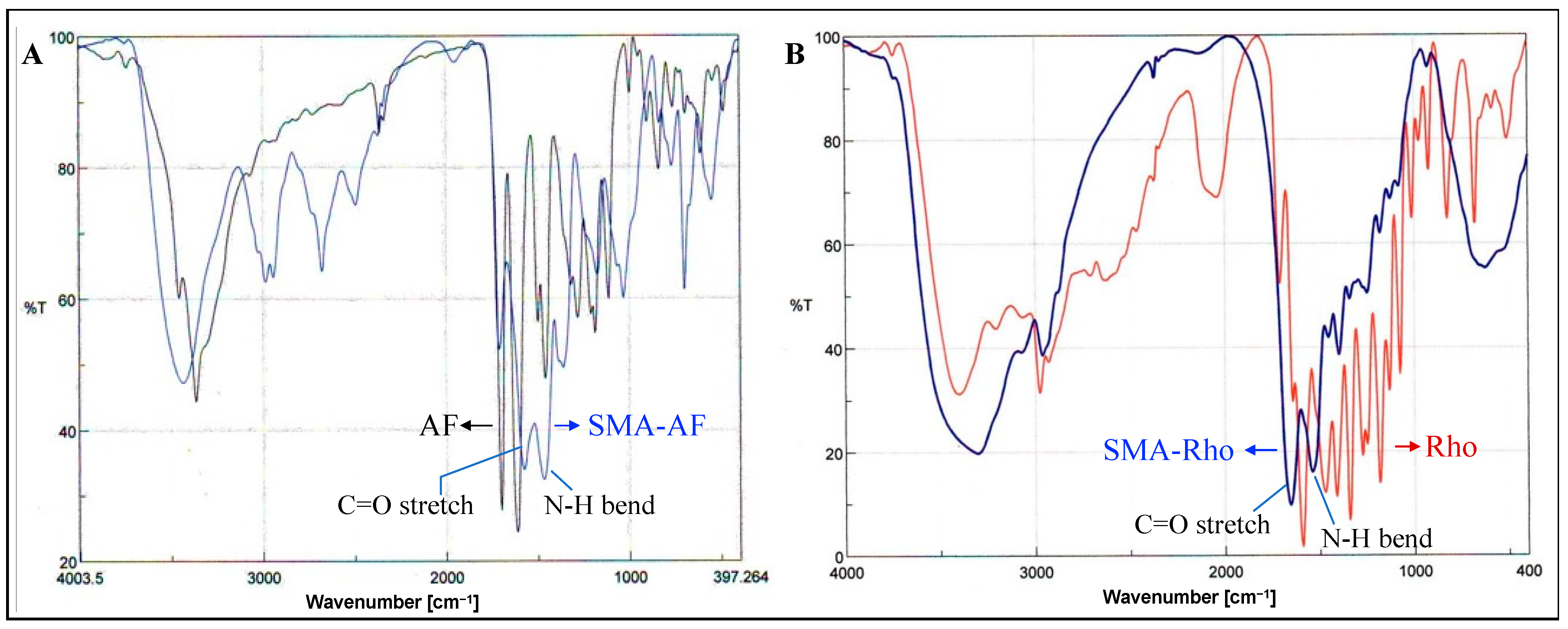
3.3. UV/vis and FTIR Characterization of the SMA-AF and SMA-Rho Conjugates
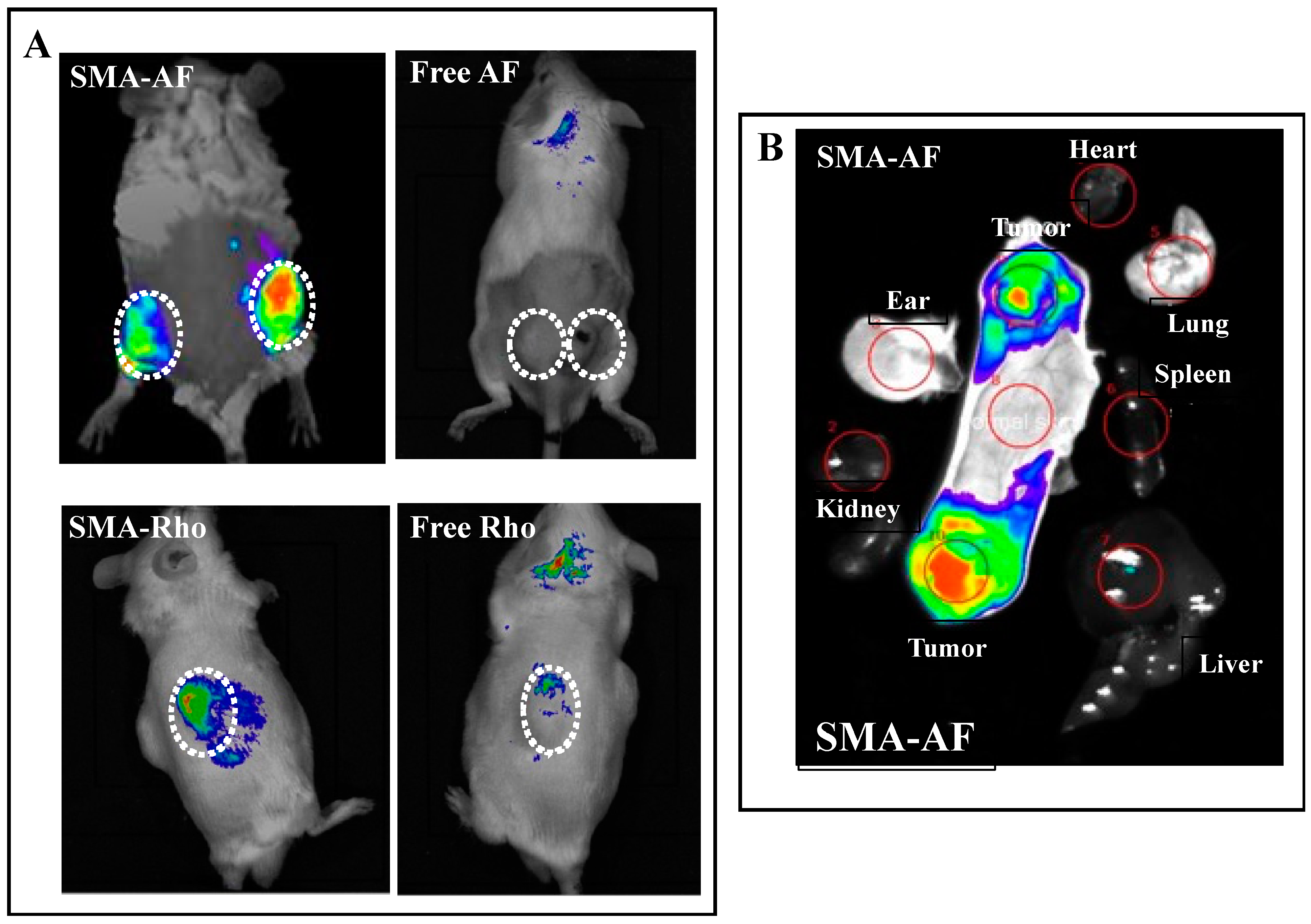
3.4. In Vivo Tissue Distribution of the SMA-AF Conjugate: In Vivo Fluorescence Imaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lauwerends, L.J.; van Driel, P.B.A.A.; Baatenburg de Jong, R.J.; Hardillo, J.A.U.; Koljenovic, S.; Puppels, G.; Mezzanotte, L.; Löwik, C.W.G.M.; Rosenthal, E.L.; Vahrmeijer, A.L.; et al. Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol. 2021, 22, 186–195. [Google Scholar] [CrossRef]
- Obara, R.; Kamiya, M.; Tanaka, Y.; Abe, A.; Kojima, R.; Kawaguchi, T.; Sugawara, M.; Takahashi, A.; Noda, T.; Urano, Y. g-Glutamyltranspeptidase (GGT)-activatable fluorescence probe for durable tumor imaging. Angew. Chem. 2020, 132, 1–6. [Google Scholar]
- Farooq, A.; Sabah, S.; Dhou, S.; Alsawaftah, N.; Husseini, G. Exogenous contrast agents in photoacoustic imaging: An in vivo review for tumor imaging. Nanomaterials 2022, 12, 393. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jin, G.; Weng, G.; Li, J.; Zhu, J.; Zhao, J. Recent advances in activatable fluorescence imaging probes for tumor imaging. Drug Discov. Today 2017, 22, 1367–1374. [Google Scholar] [CrossRef]
- Miller, K.D. Cancer treatment and survivorship statistics. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Ntziachristos, V. Advancing surgical vision with fluorescence imaging. Annu. Rev. Med. 2016, 67, 153–164. [Google Scholar] [CrossRef]
- Yang, R.Q.; Lou, K.L.; Wang, P.Y.; Gao, Y.Y.; Zhang, Y.Q.; Chen, C.; Huang, W.H.; Zhang, G.J. Surgical navigation for malignancies guided by near-infrared-II fluorescence imaging. Small Methods 2021, 5, 2001066. [Google Scholar] [CrossRef]
- Vahrmeijer, A.L. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, B.E. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef]
- Sevick-Muraca, E.M. Translation of near-infrared fluorescence imaging technologies: Emerging clinical applications. Annu. Rev. Med. 2012, 63, 217–231. [Google Scholar] [CrossRef]
- Byrd, B.K.; Marois, M.; Tichauer, K.M.; Wirth, D.J.; Hong, J.; Leonor, J.P.; Elliott, J.T.; Paulsen, K.D.; Davis, S.C. First experience imaging short-wave infrared fluorescence in a large animal: Indocyanine green angiography of a pig brain. J. Biomed. Opt. 2019, 24, 080501. [Google Scholar] [CrossRef]
- Tummers, Q.R. The value of intraoperative near-infrared fluorescence imaging based on enhanced permeability and retention of indocyanine green: Feasibility and false-positives in ovarian cancer. PLoS ONE 2015, 10, e0129766. [Google Scholar] [CrossRef]
- Hu, Z.; Fang, C.; Li, B.; Zhang, Z.; Cao, C.; Cai, M.; Su, S.; Sun, X.; Shi, X.; Li, C.; et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng. 2019, 4, 259–271. [Google Scholar] [CrossRef]
- Suo, Y.; Wu, F.; Xu, P.; Shi, H.; Wang, T.; Liu, H.; Cheng, Z. NIR-II fluorescence endoscopy for targeted imaging of colorectal cancer. Adv. Healthcare Mater. 2019, 8, 1900974. [Google Scholar] [CrossRef]
- Bharate, G.Y.; Haibo, Q.; Fang, J. Poly(styrene-co-maleic acid) micelle of photosensitizers for targeted photodynamic therapy, exhibits prolonged singlet oxygen generating capacity and superior intracellular uptake. J. Pers. Med. 2022, 12, 493. [Google Scholar] [CrossRef]
- Maeda, H.; Bharate, G.Y.; Daruwalla, J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharma. Biopharma. 2009, 71, 409–419. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced permeability and retention (EPR) Effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Chytil, P.; Kostka, L.; Etrych, T. HPMA copolymer-based nanomedicines in controlled drug delivery. J. Pers. Med. 2021, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent advances in tumor targeting via EPR effect for cancer treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- Iyer, A.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Shashni, B.; Nagasaki, Y. Newly developed self-assembling antioxidants as potential therapeutics for the cancers. J. Pers. Med. 2021, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Islam, R.; Fang, J. Tumor environment-responsive hyaluronan conjugated zinc protoporphyrin for targeted anticancer photodynamic therapy. J. Pers. Med. 2021, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Fukumitsu, N.; Ishikawa, H.; Nakai, K.; Sakurai, H. A critical review of radiation therapy: From particle beam therapy (proton, carbon, and BNCT) to beyond. J. Pers. Med. 2021, 11, 825. [Google Scholar] [CrossRef]
- Taniguchi, S. In situ delivery and production system (iDPS) of anti-cancer molecules with gene-engineered bifidobacterium. J. Pers. Med. 2021, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yokoyama, Y.; Takahashi, H.; Kouda, S.; Yamamoto, H.; Wang, J.; Morimoto, Y.; Minami, K.; Hata, T.; Shamma, A.; et al. Improved in vivo delivery of small RNA based on the calcium phosphate method. J. Pers. Med. 2021, 11, 1160. [Google Scholar] [CrossRef]
- Maeda, H.; Sawa, T.; Konno, T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J. Control. Release 2001, 74, 47–61. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Khaled, G.; Sawa, T.; Fang, J.; Akaike, T.; Maeda, H. SMA–doxorubicin, a new polymeric micellar drug for effective targeting to solid tumours. J. Control. Release 2004, 97, 219–230. [Google Scholar]
- Greish, K.; Nagamitsu, A.; Fang, J.; Maeda, H. Copoly(styrene-maleic acid)-pirarubicin micelles: High tumor-targeting e fficiency with little toxicity. Bioconjug. Chem. 2005, 16, 230–236. [Google Scholar] [CrossRef]
- Iyer, A.; Greish, K.; Fang, J.; Murakami, R.; Maeda, H. High-loading nanosized micelles of copoly (styrene-maleic acid)-zinc protoporphyrin for targeted delivery of a potent heme oxygenase inhibitor. Biomaterials 2007, 28, 1871–1881. [Google Scholar] [CrossRef]
- Saisyoa, A.; Nakamura, H.; Fang, J.; Tsukigawa, K.; Greish, K.; Furukawa, H.; Maeda, H. pH-sensitive polymeric cisplatin-ion complex with styrene-maleic acid copolymer exhibits tumor-selective drug delivery and antitumor activity as a result of the enhanced permeability and retention effect. Colloids Surf. B Biointerfaces 2016, 138, 128–137. [Google Scholar] [CrossRef]
- Andreassi, C.; Jarecki, J.; Zhou, J.; Coovert, D.D.; Monani, U.R.; Chen, X.; Whitney, M.; Pollok, B.; Zhang, M.; Androphy, E.; et al. Aclarubicin treatment restores SMN levels to cells derived from type I spinal muscular atrophy patients. Hum. Mol. Genet. 2001, 10, 2841–2849. [Google Scholar] [CrossRef]
- Nakamura, H.; Fang, J.; Bharate, G.Y.; Tsukigawa, K.; Maeda, H. Intracellular uptake and behavior of two types zinc protoporphyrin (ZnPP) micelles, SMA-ZnPP and PEG-ZnPP as anticancer agents; unique intracellular disintegration of SMA micelles. J. Control. Release 2011, 155, 367–375. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Vicent, M.J.; Duncan, R. Polymer conjugates: Nanosized medicines for treating cancer. Trends Biotechnol. 2006, 24, 39–47. [Google Scholar] [CrossRef]
- Veronese, F.M.; Schiavon, O.; Pasut, G.; Mendichi, R.; Andersson, L.; Tsirk, A.; Ford, J.; Wu, G.; Kneller, S.; Davies, J.; et al. PEG-doxorubicin conjugates: Influence of polymer structure on drug release, in vitro cytotoxicity, biodistribution, and antitumor activity. Bioconjug. Chem. 2005, 16, 775–784. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Chain length recognition: Core-shell supramolecular assembly from oppositely charged block copolymers. Science 1999, 283, 65–67. [Google Scholar] [CrossRef]
- Bharate, G.Y.; Fang, J.; Nakamura, H.; Qin, H.; Shinkai, S.; Maeda, H. 4-Amino-6-hydroxypyrazolo [3,4-d]pyrimidine (AHPP) conjugated PEG micelles: Water soluble polymeric xanthine oxidase inhibitor. J. Drug Target 2011, 19, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Iyer, A.K.; Seki, T.; Nakamura, H.; Greish, K.; Maeda, H. SMA copolymer conjugate of AHPP: A polymeric inhibitor of xanthine oxidase with potential antihypertensive effect. J. Control. Release 2009, 135, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Sawa, T.; Fang, J.; Tanaka, S.; Miyamoto, Y.; Akaike, T.; Maeda, H. Pegylated zinc protoporphyrin: A water-soluble heme oxygenase inhibitor with tumor-targeting capacity. Bioconjug. Chem. 2002, 13, 1031–1038. [Google Scholar] [CrossRef]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef]
- Kwon, G.S.; Kataoka, K. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 1995, 16, 295–309. [Google Scholar] [CrossRef]
- Lavasanifar, A.; Samuel, J.; Kwon, G.S. Poly(ethylene oxide)-blockpoly(L-amino acid) micelles for drug delivery. Adv. Drug Deliv. Rev. 2002, 54, 169–190. [Google Scholar] [CrossRef]
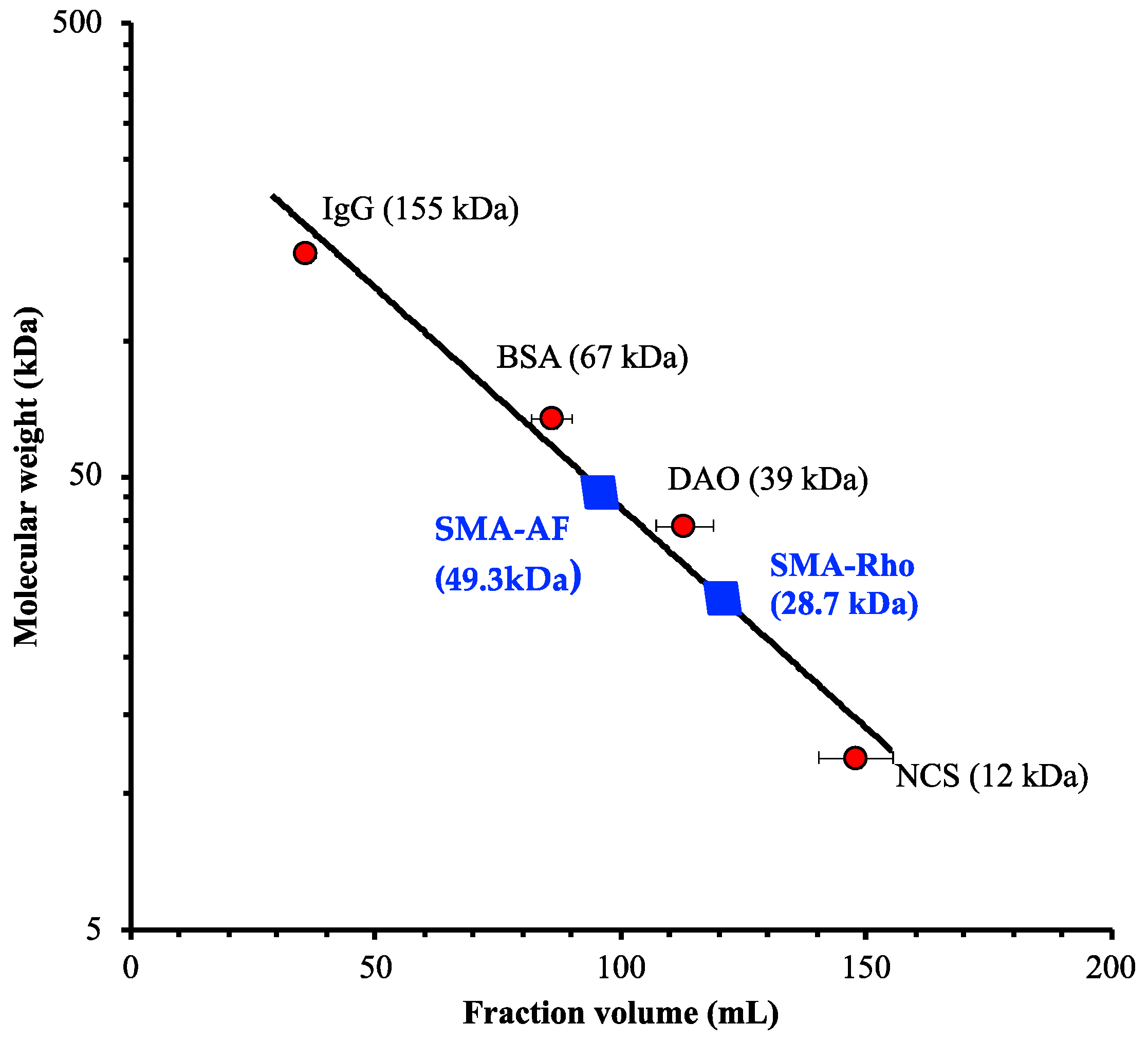
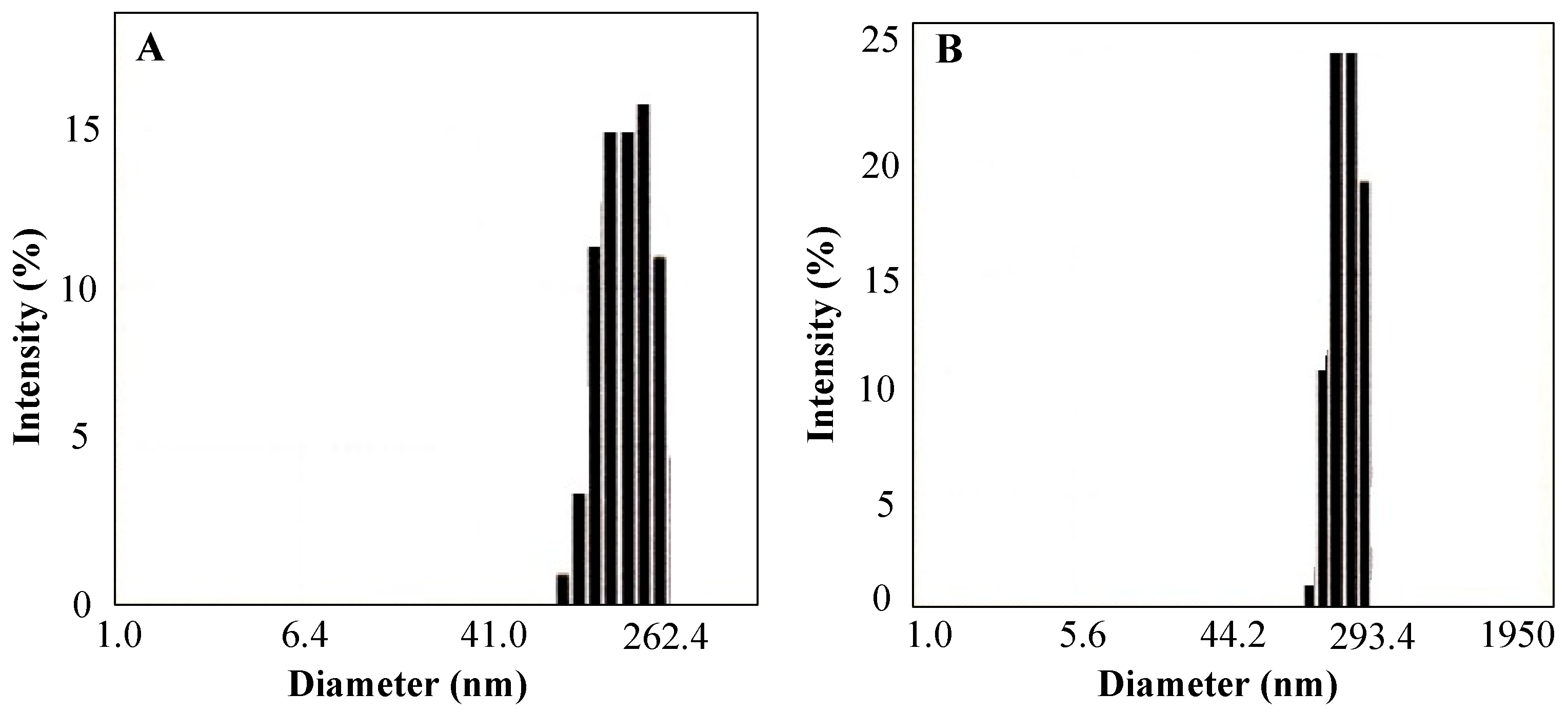
| a SMA Dyes | b Apparent Molecular Weight (kDa) | c Particle Size (nm) | d Dye Content (%W/W) | e Surface Charge (ζ, mV) |
|---|---|---|---|---|
| SMA-AF | 49.3 | 135.3 | 6.36 | −46.03 |
| SMA-Rho | 28.7 | 190.9 | 8.72 | −43.03 |
| a SMA-Dyes | b Absorption (λex, nm) | Abs. Max. (λmax, nm) | c Emission (λex, nm) | Emission Max. (λem, nm) |
|---|---|---|---|---|
| SMA-AF | 405–495 | 488.0 | 465–570 | 517.0 |
| SMA-Rho | 430–590 | 494.0 | 475–620 | 525.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharate, G.Y.; Qin, H.; Fang, J. Poly(Styrene-Co-Maleic Acid)-Conjugated 6-Aminofluorescein and Rhodamine Micelle as Macromolecular Fluorescent Probes for Micro-Tumors Detection and Imaging. J. Pers. Med. 2022, 12, 1650. https://doi.org/10.3390/jpm12101650
Bharate GY, Qin H, Fang J. Poly(Styrene-Co-Maleic Acid)-Conjugated 6-Aminofluorescein and Rhodamine Micelle as Macromolecular Fluorescent Probes for Micro-Tumors Detection and Imaging. Journal of Personalized Medicine. 2022; 12(10):1650. https://doi.org/10.3390/jpm12101650
Chicago/Turabian StyleBharate, Gahininath Y., Haibo Qin, and Jun Fang. 2022. "Poly(Styrene-Co-Maleic Acid)-Conjugated 6-Aminofluorescein and Rhodamine Micelle as Macromolecular Fluorescent Probes for Micro-Tumors Detection and Imaging" Journal of Personalized Medicine 12, no. 10: 1650. https://doi.org/10.3390/jpm12101650
APA StyleBharate, G. Y., Qin, H., & Fang, J. (2022). Poly(Styrene-Co-Maleic Acid)-Conjugated 6-Aminofluorescein and Rhodamine Micelle as Macromolecular Fluorescent Probes for Micro-Tumors Detection and Imaging. Journal of Personalized Medicine, 12(10), 1650. https://doi.org/10.3390/jpm12101650









