Personalized Prescription of Chemotherapy Based on Assessment of mRNA Expression of BRCA1, RRM1, ERCC1, TOP1, TOP2α, TUBβ3, TYMS, and GSTP1 Genes in Tumors Compared to Standard Chemotherapy in the Treatment of Non-Small-Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Group
2.2. RNA Extraction
2.3. Expression Profiling of the Chemosensitivity Genes
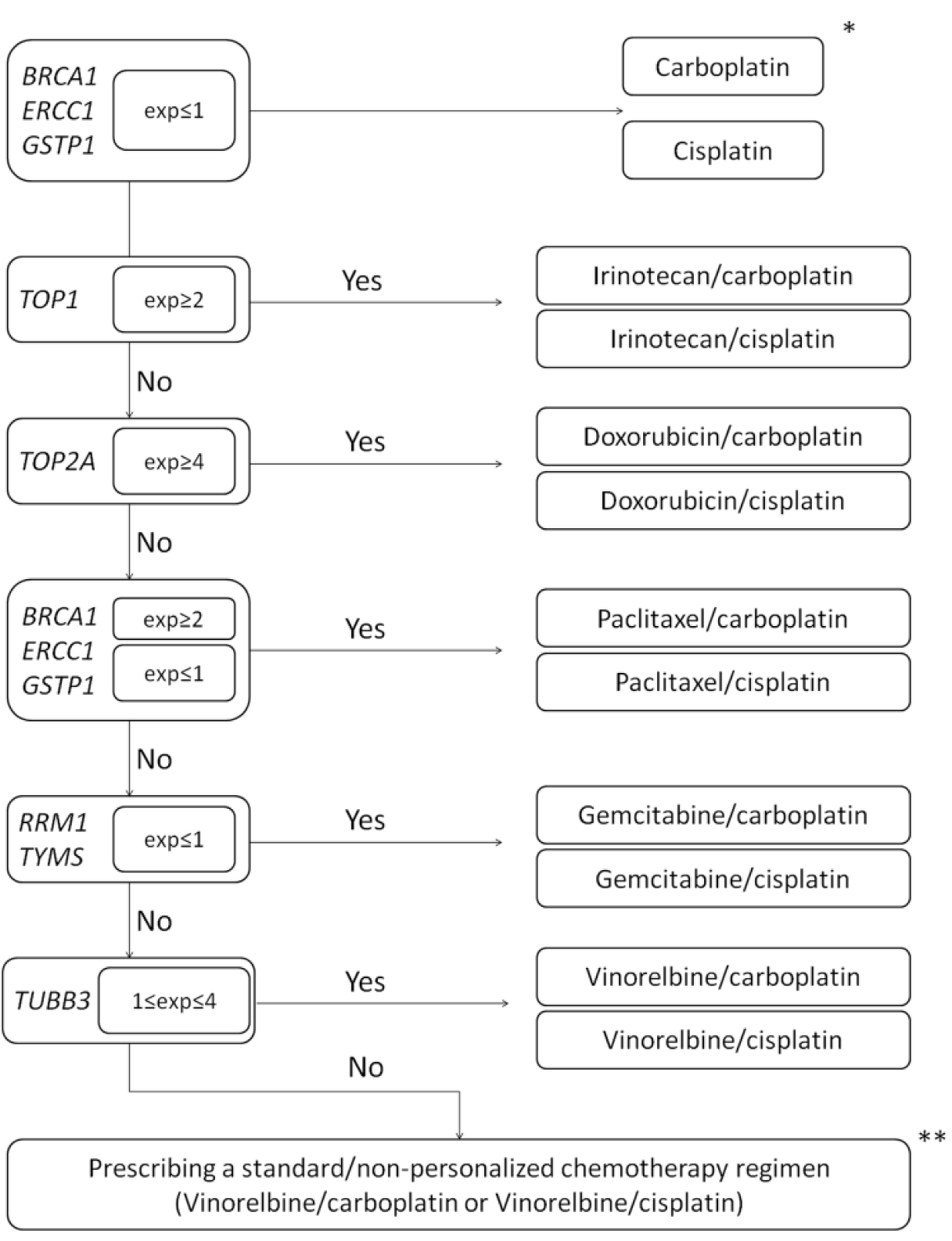
2.4. Selection and Implementation of Chemotherapy Schemes
2.5. Statistical Analysis
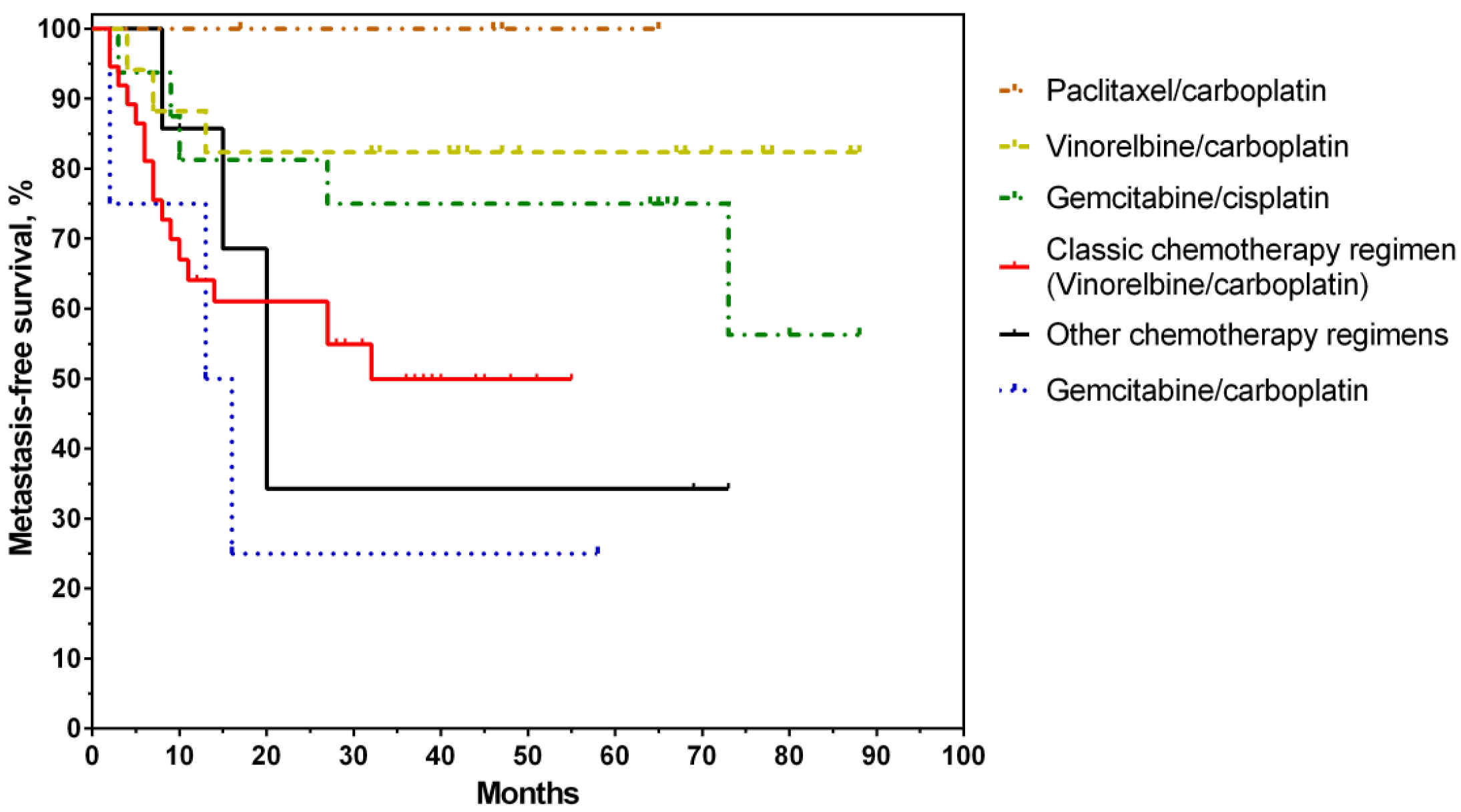
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pignon, J.-P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.-Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef] [PubMed]
- Devarakonda, S.; Rotolo, F.; Tsao, M.-S.; Lanc, I.; Brambilla, E.; Masood, A.; Olaussen, K.A.; Fulton, R.; Sakashita, S.; McLeer-Florin, A. Tumor mutation burden as a biomarker in resected non–small-cell lung cancer. J. Clin. Oncol. 2018, 36, 2995. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.A.; Ding, K.; Seymour, L.; Twumasi-Ankrah, P.; Graham, B.; Gandara, D.; Johnson, D.H.; Kesler, K.A.; Green, M.; Vincent, M. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non–small-cell lung cancer: Updated survival analysis of JBR-10. J. Clin. Oncol. 2010, 28, 29. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Cho, S.; Kim, K.; Jheon, S.; Yang, H.C.; Kim, D.K. Effect of adjuvant chemotherapy after complete resection for pathologic stage IB Lung adenocarcinoma in high-risk patients as defined by a new recurrence risk scoring model. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2017, 49, 898. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Crino, L.; Weder, W.; Van Meerbeeck, J.; Felip, E. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21, v103–v115. [Google Scholar] [CrossRef] [PubMed]
- Tsyganov, M.M.; Rodionov, E.O.; Pevzner, A.M.; Ibragimova, M.K.; Miller, S.V.; Cheremisina, O.V.; Frolova, I.G.; Tuzikov, S.A.; Litviakov, N.V. Prognostic significance of ERCC1, RRM1, TOP1, TOP2A, TYMS, TUBB3, GSTP1 and BRCA1 mRNA expressions in patients with non-small-cell lung cancer receiving a platinum-based chemotherapy. J. Balk. Union Oncol. 2020, 25, 1728–1736. [Google Scholar]
- El Baiomy, M.A.; El Kashef, W.F. ERCC1 expression in metastatic triple negative breast cancer patients treated with platinum-based chemotherapy. Asian Pac. J. Cancer Prev. 2017, 18, 507–513. [Google Scholar] [CrossRef]
- Wang, S.; Liu, F.; Zhu, J.; Chen, P.; Liu, H.; Liu, Q.; Han, J. DNA repair genes ERCC1 and BRCA1 expression in non-small cell lung cancer chemotherapy drug resistance. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 1999. [Google Scholar] [CrossRef]
- Deng, X.; Hou, J.; Deng, Q.; Zhong, Z. Predictive value of clinical toxicities of chemotherapy with fluoropyrimidines and oxaliplatin in colorectal cancer by DPYD and GSTP1 gene polymorphisms. World J. Surg. Oncol. 2020, 18, 1–10. [Google Scholar] [CrossRef]
- Khrunin, A.; Moisseev, A.; Gorbunova, V.; Limborska, S. Genetic polymorphisms and the efficacy and toxicity of cisplatin-based chemotherapy in ovarian cancer patients. Pharm. J. 2010, 10, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Bepler, G.; Williams, C.; Schell, M.J.; Chen, W.; Zheng, Z.; Simon, G.; Gadgeel, S.; Zhao, X.; Schreiber, F.; Brahmer, J. Randomized International Phase III Trial of ERCC1 and RRM1 Expression–Based Chemotherapy Versus Gemcitabine/Carboplatin in Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2013, 31, 2404. [Google Scholar] [CrossRef] [PubMed]
- Person, F.; Wilczak, W.; Hube-Magg, C.; Burdelski, C.; Möller-Koop, C.; Simon, R.; Noriega, M.; Sauter, G.; Steurer, S.; Burdak-Rothkamm, S. Prevalence of βIII-tubulin (TUBB3) expression in human normal tissues and cancers. Tumor Biol. 2017, 39, 1010428317712166. [Google Scholar] [CrossRef] [PubMed]
- Narvi, E.; Jaakkola, K.; Winsel, S.; Oetken-Lindholm, C.; Halonen, P.; Kallio, L.; Kallio, M. Altered TUBB3 expression contributes to the epothilone response of mitotic cells. Br. J. Cancer 2013, 108, 82–90. [Google Scholar] [CrossRef]
- Shan, F.; Liu, Y.L.; Wang, Q.; Shi, Y.L. Thymidylate synthase predicts poor response to pemetrexed chemotherapy in patients with advanced breast cancer. Oncol. Lett. 2018, 16, 3274–3280. [Google Scholar] [CrossRef]
- Ma, W.; Wang, B.; Zhang, Y.; Wang, Z.; Niu, D.; Chen, S.; Zhang, Z.; Shen, N.; Han, W.; Zhang, X. Prognostic significance of TOP2A in non-small cell lung cancer revealed by bioinformatic analysis. Cancer Cell Int. 2019, 19, 239. [Google Scholar] [CrossRef]
- K Kathiravan, M.; N Kale, A.; Nilewar, S. Discovery and development of topoisomerase inhibitors as anticancer agents. Mini Rev. Med. Chem. 2016, 16, 1219–1229. [Google Scholar] [CrossRef]
- Wasim, L.; Chopra, M. Synergistic anticancer effect of panobinostat and topoisomerase inhibitors through ROS generation and intrinsic apoptotic pathway induction in cervical cancer cells. Cell. Oncol. 2018, 41, 201–212. [Google Scholar] [CrossRef]
- Schwartz, G.F.; Hortobagyi, G.N. Proceedings of the consensus conference on neoadjuvant chemotherapy in carcinoma of the breast, April 26–28, 2003, Philadelphia, Pennsylvania. Breast J. 2004, 10, 273–294. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; Crino, L.; Dooms, C.; Douillard, J.-Y.; Faivre-Finn, C.; Lim, E.; Rocco, G.; Senan, S.; Van Schil, P.; Veronesi, G. 2nd ESMO Consensus Conference on Lung Cancer: Early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, F.; Chia, S.; Tu, D.; Shepherd, L.; Levine, M.; Huntsman, D.; Bramwell, V.; Andrulis, I.; Pritchard, K. Topoisomerase II alpha protein and responsiveness of breast cancer to adjuvant chemotherapy with CEF compared to CMF in the NCIC CTG randomized MA. 5 adjuvant trial. Breast Cancer Res. Treat. 2011, 128, 401. [Google Scholar] [CrossRef] [PubMed]
- Tsyganov, M.; Rodionov, E.; Miller, S.; Litvyakov, N. Substantiation of Expressive Markers Use to Personalize Lung Cancer Chemotherapy. Antibiot. Khimioterapiia 2014, 60, 38–45. [Google Scholar]
- Tutt, A.; Robson, M.; Garber, J.E.; Domchek, S.M.; Audeh, M.W.; Weitzel, J.N.; Friedlander, M.; Arun, B.; Loman, N.; Schmutzler, R.K. Oral poly (ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet 2010, 376, 235–244. [Google Scholar] [CrossRef]
- De Luca, P.; De Siervi, A. Critical role for BRCA1 expression as a marker of chemosensitivity response and prognosis. Front. Biosci. (Elite Ed.) 2016, 8, 72–83. [Google Scholar] [PubMed]
- Yu, Y.; Ding, S.; Liang, Y.; Zheng, Y.; Li, W.; Yang, L.; Zheng, X.; Jiang, J. Expression of ERCC1, TYMS, TUBB3, RRM1 and TOP2A in patients with esophageal squamous cell carcinoma: A hierarchical clustering analysis. Exp. Ther. Med. 2014, 7, 1578–1582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Azuma, K.; Sasada, T.; Kawahara, A.; Takamori, S.; Hattori, S.; Ikeda, J.; Itoh, K.; Yamada, A.; Kage, M.; Kuwano, M. Expression of ERCC1 and class III β-tubulin in non-small cell lung cancer patients treated with carboplatin and paclitaxel. Lung Cancer 2009, 64, 326–333. [Google Scholar] [CrossRef]
- Vulsteke, C.; Lambrechts, D.; Dieudonné, A.; Hatse, S.; Brouwers, B.; Van Brussel, T.; Neven, P.; Belmans, A.; Schöffski, P.; Paridaens, R. Genetic variability in the multidrug resistance associated protein-1 (ABCC1/MRP1) predicts hematological toxicity in breast cancer patients receiving (neo-) adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide (FEC). Ann. Oncol. 2013, 24, 1513–1525. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, T.; Kang, P.; Qian, K.; Deng, B.; Zhou, J.; Wang, R.; Jiang, B.; Li, K.; Liu, F. Combined analysis of rearrangement of ALK, ROS1, somatic mutation of EGFR, KRAS, BRAF, PIK3CA, and mRNA expression of ERCC1, TYMS, RRM1, TUBB3, EGFR in patients with non-small cell lung cancer and their clinical significance. Cancer Chemother. Pharmacol. 2016, 77, 583–593. [Google Scholar] [CrossRef]
- Li, J.; Sun, P.; Chuang, T.; He, S.; Li, L.; Xue, G. Individualized chemotherapy guided by the expression of ERCC1, RRM1, TUBB3, TYMS and TOP2A genes versus classic chemotherapy in the treatment of breast cancer: A comparative effectiveness study. Oncol. Lett. 2020, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Cheng, D. Meta-analysis of ERCC1 protein expression and platinum chemosensitivity in non-small-cell lung cancer. Evid.-Based Complement. Altern. Med. 2020, 2020, 7376568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Dai, Y.; Zhu, L.; Wang, C.; Fei, X.; Pan, Q.; Chen, J.; Shi, X.; Yang, Y.; Tao, X. Poor response to platinum-based chemotherapy is associated with KRAS mutation and concomitant low expression of BRAC1 and TYMS in NSCLC. J. Int. Med. Res. 2016, 44, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhang, W.; Zhang, B.; Li, Y.; Tang, J.; Li, S.; Zhao, Y.; Zhao, Y.; Xia, H.; Yu, C. EGFR and K-RAS mutations and ERCC1, TUBB3, TYMS, RRM1 and EGFR mRNA expression in non-small cell lung cancer: Correlation with clinical response to gefitinib or chemotherapy. Mol. Clin. Oncol. 2015, 3, 1123–1128. [Google Scholar] [CrossRef] [PubMed]

| Complication | Number of Patients | p-Value | ||
|---|---|---|---|---|
| Control Group (n = 37) | Main Group (n = 48) | |||
| Anemia | 1–2 degrees | 8 (21.6) | 8 (16.7) | 1.00 |
| 3–4 degrees | 2 (5.4) | 2 (4.2) | ||
| Leukopenia | 1–2 degrees | 8 (21.6) | 8 (16.7) | 1.00 |
| 3–4 degrees | 2 (5.4) | 2 (4.2) | ||
| Thrombocytopenia | 1–2 degrees | 4 (10.8) | 7 (14.6) | 1.00 |
| 3–4 degrees | 1 (2.7) | 1 (2.1) | ||
| Hepatotoxicity | 7 (18.9) | 11 (22.9) | 1.00 | |
| Nephrotoxicity | 1 (2.7) | 2 (4.2) | 1.00 | |
| Nausea, vomiting | 6 (16.2) | 6 (12.5) | 1.00 | |
| Arthralgia/myalgia | 5 (13.5) | 12 (25.0) | 1.00 | |
| Clinical and Pathological Parameter | Number of Patients | p-Value | ||
|---|---|---|---|---|
| Control Group (n = 37) | Main Group (n = 48) | |||
| Gender | Male | 6 (16.2) | 6 (12.5) | 0.75 |
| Female | 31 (83.8) | 42 (87.5) | ||
| Age | Average | 57.2 ± 0.99 | 59.0 ± 1.02 | 0.12 |
| ≤50 years | 7 (18.9) | 7 (14.6) | 0.76 | |
| >50 years | 30 (81.1) | 41 (85.4) | ||
| Tumor size | T1 | 0 (0.0) | 4 (8.3) | 0.18 |
| T2 | 6 (16.2) | 11 (23.0) | ||
| T3 | 24 (64.9) | 28 (58.3) | ||
| T4 | 7 (18.9) | 5 (10.4) | ||
| Lymphogenous metastasis | N0 | 12 (32.4) | 13 (27.1) | 0.77 |
| N1 | 13 (35.1) | 19 (39.6) | ||
| N2 | 10 (27.0) | 15 (31.3) | ||
| N3 | 2 (5.5) | 1 (2.1) | ||
| TNM stage | IIB | 12 (32.4) | 13 (27.1) | 0.75 |
| IIIA | 21 (56.8) | 31 (64.6) | ||
| IIIB | 4 (10.8) | 4 (8.3) | ||
| Clinical and anatomical form | Peripheral | 18 (48.6) | 21 (43.8) | 0.66 |
| Central | 19 (51.4) | 27 (56.3) | ||
| Histological type of the tumor | Squamous cell carcinoma | 23 (62.2) | 35 (72.9) | 0.35 |
| Adenocarcinoma | 14 (37.8) | 13 (27.1) | ||
| Effect of NAC | Full regression | 1 (2.7) | 1 (2.1) | 0.10 |
| Partial regression | 7 (18.9) | 20 (41.7) | ||
| Stabilization | 29 (78.4) | 26 (54.1) | ||
| Progression | 0 (0.0) | 1 (2.1) | ||
| Nature of surgery | Pneumonectomy | 19 (51.4) | 10 (20.8) | 0.005 |
| Lobectomy | 18 (48.6) | 38 (79.2) | ||
| ACT scheme | Vinorelbine/carboplatin | 37 (100.0) | 17 (35.4) | - |
| Vinorelbine/cisplatin | - | 2 (4.2) | ||
| Gemcitabine/carboplatin | - | 4 (8.3) | ||
| Gemcitabine/cisplatin | - | 16 (33.3) | ||
| Paclitaxel/carboplatin | - | 4 (8.3) | ||
| Paclitaxel/cisplatin | - | 1 (2.1) | ||
| Doxorubicin/carboplatin | - | 3 (6.3) | ||
| Irinotecan/carboplatin | - | 1 (2.1) | ||
| Hematogenous metastasis | Yes | 17 (45.9) | 15 (31.3) | 0.18 |
| No | 20 (54.1) | 33 (68.8) | ||
| Gene | Amplicon (bp) | Sequence |
|---|---|---|
| GAPDH | 124 bp | F 5′-gccagccgagccacatc-3′ |
| R 5′-ggcaacaatatccactttaccaga-3′ | ||
| Probe 5′-cgcccaatacgaccaaatccg-3′ | ||
| ACTB | 73 bp | F 5′-gagaagatgacccagatcatgtt-3′ |
| R 5′-atagcacagcctggatagcaa-3′ | ||
| Probe 5′-agaccttcaacaccccagccat-3′ | ||
| RRM1 | 94 bp | F 5′-actaagcaccctgactatgctatcc-3′ |
| R 5′-cttccatcacatcactgaacacttt-3′ | ||
| Probe 5′-cagccaggatcgctgtctctaacttgca-3′ | ||
| ERCC1 | 121 bp | F 5′-ggcgacgtaattcccgacta-3′ |
| R 5′-agttcttccccaggctctgc-3′ | ||
| Probe 5′-accacaacctgcacccagactacatcca-3′ | ||
| BRCA1 | 107 bp | F 5′-acagctgtgtggtgcttctgtg-3′ |
| R 5′-cattgtcctctgtccaggcatc-3′ | ||
| Probe 5′-catcattcacccttggcacaggtgt-3′ | ||
| TOP1 | 97 bp | F 5′-ggcgagtgaatctaaggataatgaa-3′ |
| R 5′-tggatatcttaaagggtacagcgaa-3′ | ||
| Probe 5′-accattttcccatcatcctttgttctgagc-3′ | ||
| TOP2α | 75 bp | F 5′-agtcgctttcagggttcttgag-3′ |
| R 5′-tttcatttacaggctgcaatgg-3′ | ||
| Probe 5′-cccttcacgaccgtcaccatgga-3′ | ||
| TUBΒ3 | 71 bp | F 5′-gggccaagttctgggaagtc-3′ |
| R 5′-cgagtcgcccacgtagttg-3′ | ||
| Probe 5′-atgagcatggcatcgaccccagc-3′ | ||
| TYMS | 91 bp | F 5′-tctggaagggtgttttgga-3′ |
| R 5′-tcccagattttcactccctt-3′ | ||
| Probe 5′-tctttagcatttgtggatcccttga-3′ | ||
| GSTP1 | 84 bp | F 5′-ctggtggacatggtgaatgac-3′ |
| R 5′-cttgcccgcctcatagttg-3′ | ||
| Probe 5′-aggacctccgctgcaaatacatctc-3′ |
| No. of Cycles | |||||
|---|---|---|---|---|---|
| Chemotherapy Regimens | 1 | 2 | 3 | 4 | n |
| Personalized chemotherapy | |||||
| Vinorelbine (25 mg/m2)/carboplatin (AUC 6) | 1 | 2 | 10 | 4 | 17 |
| Vinorelbine (25 mg/m2)/cisplatin (75 mg/m2) | 2 | 2 | |||
| Gemcitabine (1250 mg/m2)/carboplatin (AUC 6) | 3 | 1 | 4 | ||
| Gemcitabine (1250 mg/m2)/cisplatin (AUC 6) | 3 | 2 | 10 | 1 | 16 |
| Paclitaxel (175 mg/m2)/carboplatin (AUC 6) | 2 | 1 | 1 | 4 | |
| Paclitaxel (175 mg/m2)/cisplatin (75 mg/m2) | 1 | 1 | |||
| Doxorubicin (50 mg/m2)/carboplatin (AUC 6) | 3 | 3 | |||
| Irinotecan (75 mg/m2)/carboplatin (AUC 6) | 1 | 1 | |||
| Classic chemotherapy | |||||
| Vinorelbine (25 mg/m2)/carboplatin (AUC 6) | 3 | 17 | 15 | 2 | 37 |
| Factor | MFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Gender | ||||
| Male | 1.00 | 1.00 | ||
| Female | 0.78 (0.43–1.42) | 0.43 | 0.95 (0.45–2.00) | 0.90 |
| Age | ||||
| >50 | 1.00 | 1.00 | ||
| ≤50 | 1.13 (0.66–1.90) | 0.64 | 0.59 (0.35–1.00) | 0.05 |
| Tumor size | ||||
| T1–2 | 1.00 | 1.00 | ||
| T3–4 | 1.07 (0.48–2.38) | 0.86 | 5.95 (0.78–44.93) | 0.08 |
| Lymphogenous metastasis | ||||
| N0 | 1.00 | 1.00 | ||
| N1 | 0.60 (0.22–1.62) | 0.32 | 0.92 (0.24–3.44) | 0.90 |
| N2 | 2.55 (1.23–5.28) | 0.01 | 2.00 (0.74–5.41) | 0.16 |
| N3 | 2.69 (0.63–11.39) | 0.17 | 2.53 (0.33–19.29) | 0.37 |
| TNM stage | ||||
| IIB | 1.00 | 1.00 | ||
| IIIA | 1.50 (0.62–3.60) | 0.35 | 1.40 (0.37–5.17) | 0.61 |
| IIIB | 3.32 (1.33–8.30) | 0.01 | 6.84 (2.26–20.73) | 0.001 |
| Clinical and anatomical form | ||||
| Central | 1.00 | 1.00 | ||
| Peripheral | 0.99 (0.49–1.98) | 0.97 | 0.62 (0.23–1.69) | 0.35 |
| Histological type of the tumor | ||||
| Squamous cell carcinoma | 1.00 | 1.00 | ||
| Adenocarcinoma | 0.65 (0.29–1.46) | 0.30 | 0.66 (0.21–2.05) | 0.48 |
| Effect of NAC | ||||
| Full/partial regression | 1.00 | 1.00 | ||
| Stabilization/progression | 1.87 (0.83–4.19) | 0.12 | 47.25 (0.84–2630.40) | 0.06 |
| Nature of surgery | ||||
| Lobectomy | 1.00 | 1.00 | ||
| Pneumonectomy | 1.45 (0.71–2.94) | 0.30 | 7.43 (2.41–22.85) | <0.000 |
| Chemotherapy strategy | ||||
| Personalized chemotherapy regimen | 1.00 | 1.00 | ||
| Classic chemotherapy regimen | 1.95 (0.96–3.98) | 0.06 | 14.82 (3.33–65.86) | <0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsyganov, M.M.; Rodionov, E.O.; Ibragimova, M.K.; Miller, S.V.; Cheremisina, O.V.; Frolova, I.G.; Tuzikov, S.A.; Litviakov, N.V. Personalized Prescription of Chemotherapy Based on Assessment of mRNA Expression of BRCA1, RRM1, ERCC1, TOP1, TOP2α, TUBβ3, TYMS, and GSTP1 Genes in Tumors Compared to Standard Chemotherapy in the Treatment of Non-Small-Cell Lung Cancer. J. Pers. Med. 2022, 12, 1647. https://doi.org/10.3390/jpm12101647
Tsyganov MM, Rodionov EO, Ibragimova MK, Miller SV, Cheremisina OV, Frolova IG, Tuzikov SA, Litviakov NV. Personalized Prescription of Chemotherapy Based on Assessment of mRNA Expression of BRCA1, RRM1, ERCC1, TOP1, TOP2α, TUBβ3, TYMS, and GSTP1 Genes in Tumors Compared to Standard Chemotherapy in the Treatment of Non-Small-Cell Lung Cancer. Journal of Personalized Medicine. 2022; 12(10):1647. https://doi.org/10.3390/jpm12101647
Chicago/Turabian StyleTsyganov, Matvey M., Evgeny O. Rodionov, Marina K. Ibragimova, Sergey V. Miller, Olga V. Cheremisina, Irina G. Frolova, Sergey A. Tuzikov, and Nikolai V. Litviakov. 2022. "Personalized Prescription of Chemotherapy Based on Assessment of mRNA Expression of BRCA1, RRM1, ERCC1, TOP1, TOP2α, TUBβ3, TYMS, and GSTP1 Genes in Tumors Compared to Standard Chemotherapy in the Treatment of Non-Small-Cell Lung Cancer" Journal of Personalized Medicine 12, no. 10: 1647. https://doi.org/10.3390/jpm12101647
APA StyleTsyganov, M. M., Rodionov, E. O., Ibragimova, M. K., Miller, S. V., Cheremisina, O. V., Frolova, I. G., Tuzikov, S. A., & Litviakov, N. V. (2022). Personalized Prescription of Chemotherapy Based on Assessment of mRNA Expression of BRCA1, RRM1, ERCC1, TOP1, TOP2α, TUBβ3, TYMS, and GSTP1 Genes in Tumors Compared to Standard Chemotherapy in the Treatment of Non-Small-Cell Lung Cancer. Journal of Personalized Medicine, 12(10), 1647. https://doi.org/10.3390/jpm12101647






