Systolic Arterial Pressure Control Using an Automated Closed-Loop System for Vasopressor Infusion during Intermediate-to-High-Risk Surgery: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Patients
2.3. Clinical Care
2.4. CLV Controller
2.5. Primary Objective
2.6. Secondary Objectives
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
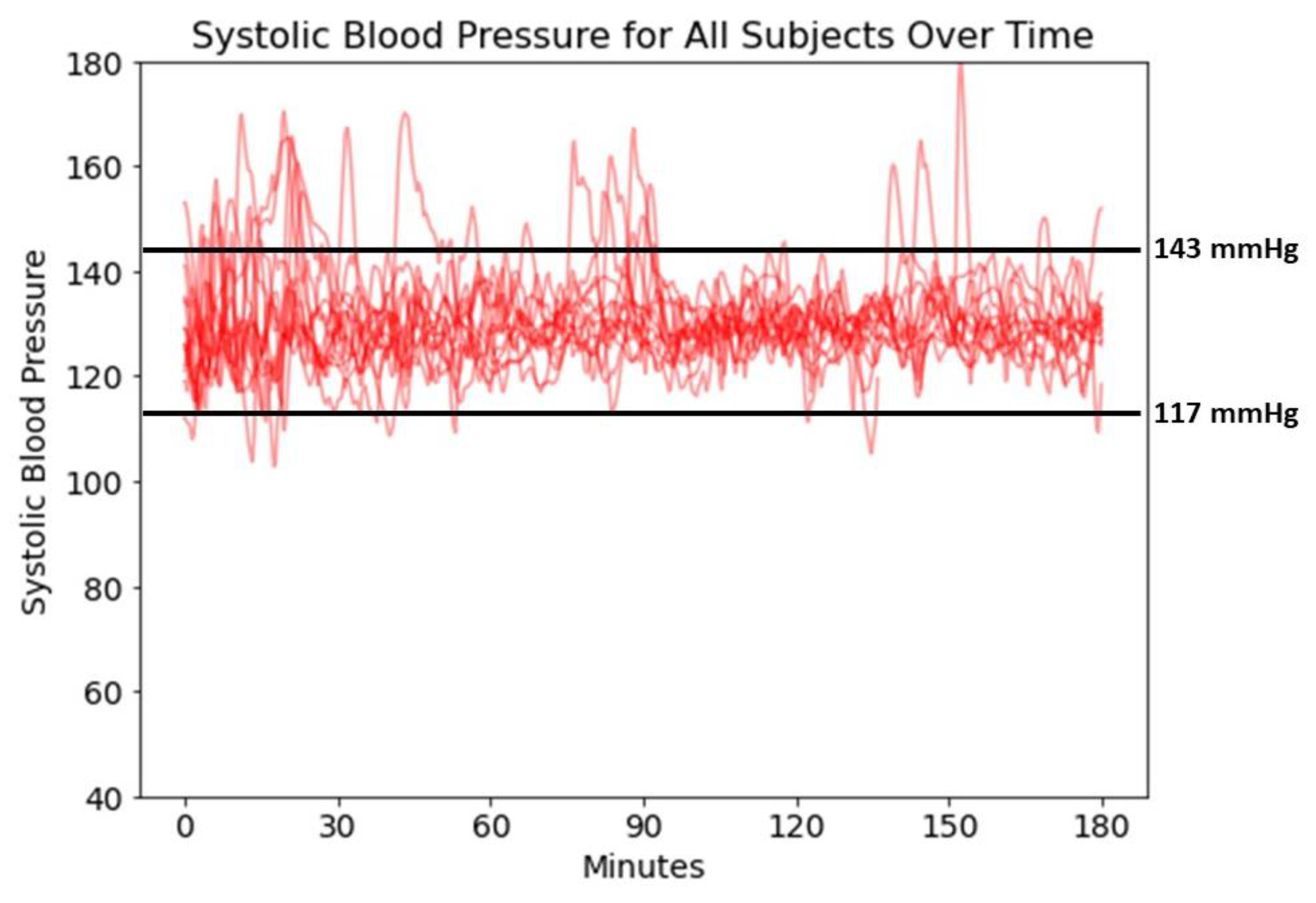
3.2. CLV Control Characteristics
3.3. Study Objectives
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christensen, A.L.; Jacobs, E.; Maheshwari, K.; Xing, F.; Zhao, X.; Simon, S.E.; Domino, K.B.; Posner, K.L.; Stewart, A.F.; Sanford, J.A.; et al. Development and Evaluation of a Risk-Adjusted Measure of Intraoperative Hypotension in Patients Having Nonemergent, Noncardiac Surgery. Anesth. Analg. 2021, 133, 445–454. [Google Scholar] [CrossRef]
- Shah, N.J.; Mentz, G.; Kheterpal, S. The incidence of intraoperative hypotension in moderate to high risk patients undergoing non-cardiac surgery: A retrospective multicenter observational analysis. J. Clin. Anesth. 2020, 66, 109961. [Google Scholar] [CrossRef]
- Sessler, D.I.; Bloomstone, J.A.; Aronson, S.; Berry, C.; Gan, T.J.; Kellum, J.A.; Plumb, J.; Mythen, M.G.; Grocott, M.P.W.; Edwards, M.R.; et al. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br. J. Anaesth. 2019, 122, 563–574. [Google Scholar] [CrossRef]
- Gregory, A.; Stapelfeldt, W.H.; Khanna, A.K.; Smischney, N.J.; Boero, I.J.; Chen, Q.; Stevens, M.; Shaw, A.D. Intraoperative Hypotension Is Associated With Adverse Clinical Outcomes After Noncardiac Surgery. Anesth. Analg. 2021, 132, 1654–1665. [Google Scholar] [CrossRef]
- Joosten, A.; Lucidi, V.; Ickx, B.; Van Obbergh, L.; Germanova, D.; Berna, A.; Alexander, B.; Desebbe, O.; Carrier, F.M.; Cherqui, D.; et al. Intraoperative hypotension during liver transplant surgery is associated with postoperative acute kidney injury: A historical cohort study. BMC Anesth. 2021, 21, 12. [Google Scholar] [CrossRef]
- Rinehart, J.; Ma, M.; Calderon, M.D.; Bardaji, A.; Hafiane, R.; Van der Linden, P.; Joosten, A. Blood pressure variability in surgical and intensive care patients: Is there a potential for closed-loop vasopressor administration? Anaesth. Crit. Care Pain Med. 2019, 38, 69–71. [Google Scholar] [CrossRef]
- Sessler, D.I.; Turan, A.; Stapelfeldt, W.H.; Mascha, E.J.; Yang, D.; Farag, E.; Cywinski, J.; Vlah, C.; Kopyeva, T.; Keebler, A.L.; et al. Triple-low Alerts Do Not Reduce Mortality: A Real-time Randomized Trial. Anesthesiology 2019, 130, 72–82. [Google Scholar] [CrossRef]
- Joosten, A.; Alexander, B.; Duranteau, J.; Taccone, F.S.; Creteur, J.; Vincent, J.L.; Cannesson, M.; Rinehart, J. Feasibility of closed-loop titration of norepinephrine infusion in patients undergoing moderate- and high-risk surgery. Br. J. Anaesth. 2019, 123, 430–438. [Google Scholar] [CrossRef]
- Rinehart, J.; Joosten, A.; Ma, M.; Calderon, M.D.; Cannesson, M. Closed-loop vasopressor control: In-silico study of robustness against pharmacodynamic variability. J. Clin. Monit. Comput. 2019, 33, 795–802. [Google Scholar] [CrossRef]
- Rinehart, J.; Ma, M.; Calderon, M.D.; Cannesson, M. Feasibility of automated titration of vasopressor infusions using a novel closed-loop controller. J. Clin. Monit. Comput. 2018, 32, 5–11. [Google Scholar] [CrossRef]
- Desebbe, O.; Rinehart, J.; Van der Linden, P.; Cannesson, M.; Delannoy, B.; Vigneron, M.; Curtil, A.; Hautin, E.; Vincent, J.L.; Duranteau, J.; et al. Control of Postoperative Hypotension Using a Closed-Loop System for Norepinephrine Infusion in Patients After Cardiac Surgery: A Randomized Trial. Anesth. Analg. 2022, 134, 964–973. [Google Scholar] [CrossRef]
- Joosten, A.; Rinehart, J.; Van der Linden, P.; Alexander, B.; Penna, C.; De Montblanc, J.; Cannesson, M.; Vincent, J.L.; Vicaut, E.; Duranteau, J. Computer-assisted Individualized Hemodynamic Management Reduces Intraoperative Hypotension in Intermediate- and High-risk Surgery: A Randomized Controlled Trial. Anesthesiology 2021, 135, 258–272. [Google Scholar] [CrossRef]
- Joosten, A.; Chirnoaga, D.; Van der Linden, P.; Barvais, L.; Alexander, B.; Duranteau, J.; Vincent, J.L.; Cannesson, M.; Rinehart, J. Automated closed-loop versus manually controlled norepinephrine infusion in patients undergoing intermediate- to high-risk abdominal surgery: A randomised controlled trial. Br. J. Anaesth. 2021, 126, 210–218. [Google Scholar] [CrossRef]
- Futier, E.; Lefrant, J.Y.; Guinot, P.G.; Godet, T.; Lorne, E.; Cuvillon, P.; Bertran, S.; Leone, M.; Pastene, B.; Piriou, V.; et al. Effect of Individualized vs Standard Blood Pressure Management Strategies on Postoperative Organ Dysfunction Among High-Risk Patients Undergoing Major Surgery: A Randomized Clinical Trial. JAMA 2017, 318, 1346–1357. [Google Scholar] [CrossRef]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287-91SA. [Google Scholar] [CrossRef]
- Davoud, S.; Gao, W.; Riveros-Perez, E. Adaptive optimal target controlled infusion algorithm to prevent hypotension associated with labor epidural: An adaptive dynamic programming approach. ISA Trans 2020, 100, 74–81. [Google Scholar] [CrossRef]
- Soltesz, K.; Sjöberg, T.; Jansson, T.; Johansson, R.; Robertsson, A.; Paskevicius, A.; Liao, Q.; Qin, G.; Steen, S. Closed-loop regulation of arterial pressure after acute brain death. J. Clin. Monit. Comput. 2018, 32, 429–437. [Google Scholar] [CrossRef]
- Ngan Kee, W.D.; Tam, Y.H.; Khaw, K.S.; Ng, F.F.; Lee, S.W. Closed-Loop Feedback Computer-Controlled Phenylephrine for Maintenance of Blood Pressure During Spinal Anesthesia for Cesarean Delivery: A Randomized Trial Comparing Automated Boluses Versus Infusion. Anesth. Analg. 2017, 125, 117–123. [Google Scholar] [CrossRef]
- Ngan Kee, W.D.; Khaw, K.S.; Tam, Y.H.; Ng, F.F.; Lee, S.W. Performance of a closed-loop feedback computer-controlled infusion system for maintaining blood pressure during spinal anaesthesia for caesarean section: A randomized controlled comparison of norepinephrine versus phenylephrine. J. Clin. Monit. Comput. 2017, 31, 617–623. [Google Scholar] [CrossRef]
- Marques, N.R.; Whitehead, W.E.; Kallu, U.R.; Kinsky, M.P.; Funston, J.S.; Wassar, T.; Khan, M.N.; Milosch, M.; Jupiter, D.; Grigoriadis, K.; et al. Physician-Directed Versus Computerized Closed-Loop Control of Blood Pressure Using Phenylephrine in a Swine Model. Anesth. Analg. 2017, 125, 110–116. [Google Scholar] [CrossRef]
- Libert, N.; Chenegros, G.; Harrois, A.; Baudry, N.; Decante, B.; Cordurie, G.; Benosman, R.; Mercier, O.; Vicaut, E.; Duranteau, J. Performance of closed-loop resuscitation in a pig model of haemorrhagic shock with fluid alone or in combination with norepinephrine, a pilot study. J. Clin. Monit. Comput. 2021, 35, 835–847. [Google Scholar] [CrossRef]
- Libert, N.; Chenegros, G.; Harrois, A.; Baudry, N.; Cordurie, G.; Benosman, R.; Vicaut, E.; Duranteau, J. Performance of closed-loop resuscitation of haemorrhagic shock with fluid alone or in combination with norepinephrine: An experimental study. Ann. Intensive Care 2018, 8, 89. [Google Scholar] [CrossRef]
| Variables | |
|---|---|
| Age (year) | 67 (61–72) |
| Male gender (%) | 9 [75] |
| Weight (kg) | 75 (65–84) |
| Height (cm) | 172 (165–178) |
| American Society of Anesthesiologists status III | 12 (100) |
| Preoperative systolic blood pressure (mmHg) | 130 (128–136) |
| Preoperative plasma creatinine level (mmol/L) | 0.98 (0.80–1.07) |
| Postoperative plasma creatinine level (mmol/L) * | 0.87 (0.72–0.97) |
| |
| Aspirin ẞ blocker Angiotensin-converting-enzyme inhibitor Statin Calcium channel blocker
Arterial hypertension Hypercholesterolemia Diabetes | 3 [25] 1 [8.3] 3 [25] 4 [33] 3 [25] 10 [83] 4 [33] 4 [33] |
| |
| Pancreatectomy | 3 [25] |
| Liver surgery | 3 [25] |
| Cystectomy | 2 [17] |
| Other (nephrectomy, colorectal surgery, gastrectomy) | 4 [33] |
| Laparoscopic surgery | 4 [33] |
| Anesthesia duration (min) | 394 (351–431) |
| Surgery duration (min) | 303 (250–345) |
| |
| Stroke volume index (mL/m2) Stroke volume variation (%) | 37.3 (35.4–49.8) 7.7 (5.5–9.7) |
| Total cristalloids (mL) Total colloids (mL) | 2500 (2000–3063) 375 (0–625) |
| Estimated blood loss (mL) Urine output (mL) Net fluid balance (mL) | 550 (238–881) 450 (338–592) 2247 (1075–2579) |
| Length of stay in hospital (days) | 9 (7–11) |
| Type of Surgery | Mean Percentage of Case Time with | CLV Rate Changes Per Minute | Total Dose of VP (mcg) | Mean Rate of VP (mcg/min) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Case | SAP < 117 mmHg | SAP 117–143 mmHg | SAP > 143 mmHg | MAP < 65 mmHg | CLV Giving VP | ||||
| 1 | Cystectomy | 3.6 | 89.1 | 7.3 | 0 | 9 | 4.7 | 1616 | 6.8 |
| 2 | Liver surgery | 0.6 | 95.6 | 3.8 | 0.1 | 94.5 | 4.9 | 1533 | 9.6 |
| 3 | Gastrectomy | 1.8 | 96.2 | 2 | 0 | 98.4 | 4.8 | 2325 | 5.2 |
| 4 | Liver surgery | 1.8 | 93.8 | 4.4 | 0 | 96.0 | 5.0 | 1240 | 11.7 |
| 5 | Cystectomy | 0.8 | 97.9 | 1.2 | 0 | 99.8 | 4.5 | 2604 | 6.5 |
| 6 | nephrectomy | 4.1 | 84.4 | 11.5 | 0.5 | 88.6 | 4.3 | 3099 | 2.4 |
| 7 | Pancreatectomy | 3.2 | 86.4 | 10.4 | 0 | 86.1 | 4.8 | 487 | 3.7 |
| 8 | Liver surgery | 3.7 | 90.4 | 5.9 | 0 | 94.6 | 4.5 | 926 | 4.5 |
| 9 | Pancreatectomy | 0.9 | 90.9 | 8.2 | 0 | 90.1 | 5.0 | 1074 | 15.3 |
| 10 | Pancreatectomy | 1.7 | 97.7 | 0.6 | 0 | 99.6 | 4.9 | 7678 | 6.1 |
| 11 | Colorectal surgery | 4.2 | 90.9 | 4.9 | 0 | 97.6 | 4.5 | 1387 | 1.9 |
| 12 | Nephrectomy | 0 | 96.4 | 3.6 | 0 | 89.5 | 4.7 | 396 | 6.8 |
| Median 25th percentile 75th percentile | 1.8 | 92.4 | 4.7 | 0 | 95.3 | 4.8 | 1460 | 6.3 | |
| 0.9 | 90.1 | 3.2 | 0 | 86.1 | 4.5 | 1037 | 4.3 | ||
| 3.6 | 96.3 | 7.5 | 0 | 98.6 | 4.9 | 2395 | 7.5 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinehart, J.; Desebbe, O.; Berna, A.; Lam, I.; Coeckelenbergh, S.; Cannesson, M.; Joosten, A. Systolic Arterial Pressure Control Using an Automated Closed-Loop System for Vasopressor Infusion during Intermediate-to-High-Risk Surgery: A Feasibility Study. J. Pers. Med. 2022, 12, 1554. https://doi.org/10.3390/jpm12101554
Rinehart J, Desebbe O, Berna A, Lam I, Coeckelenbergh S, Cannesson M, Joosten A. Systolic Arterial Pressure Control Using an Automated Closed-Loop System for Vasopressor Infusion during Intermediate-to-High-Risk Surgery: A Feasibility Study. Journal of Personalized Medicine. 2022; 12(10):1554. https://doi.org/10.3390/jpm12101554
Chicago/Turabian StyleRinehart, Joseph, Olivier Desebbe, Antoine Berna, Isaac Lam, Sean Coeckelenbergh, Maxime Cannesson, and Alexandre Joosten. 2022. "Systolic Arterial Pressure Control Using an Automated Closed-Loop System for Vasopressor Infusion during Intermediate-to-High-Risk Surgery: A Feasibility Study" Journal of Personalized Medicine 12, no. 10: 1554. https://doi.org/10.3390/jpm12101554
APA StyleRinehart, J., Desebbe, O., Berna, A., Lam, I., Coeckelenbergh, S., Cannesson, M., & Joosten, A. (2022). Systolic Arterial Pressure Control Using an Automated Closed-Loop System for Vasopressor Infusion during Intermediate-to-High-Risk Surgery: A Feasibility Study. Journal of Personalized Medicine, 12(10), 1554. https://doi.org/10.3390/jpm12101554






