Assessment of Biventricular Myocardial Function with 2-Dimensional Strain and Conventional Echocardiographic Parameters: A Comparative Analysis in Healthy Infants and Patients with Severe and Critical Pulmonary Stenosis
Abstract
1. Introduction
2. Materials and Methods
3. Ethics
4. Statistical Analysis
5. Results
5.1. Description of Baseline Characteristics of PS Patients and Controls
5.2. Comparison of PS Cases with Control Group in Terms of Baseline Regional and Global Longitudinal Strain
5.3. Comparison of PS Cases with Control Group in Terms of Regional and Global Longitudinal Strains Measured after Interventional Procedure for the PS
5.4. Changes in Baseline and Post-Procedural Biventricular Regional Strains in PS Patients
5.5. Comparison of Longitudinal Strain and Conventional Ecocardiographic Parameters Measured before and after Interventionin in PS Patients
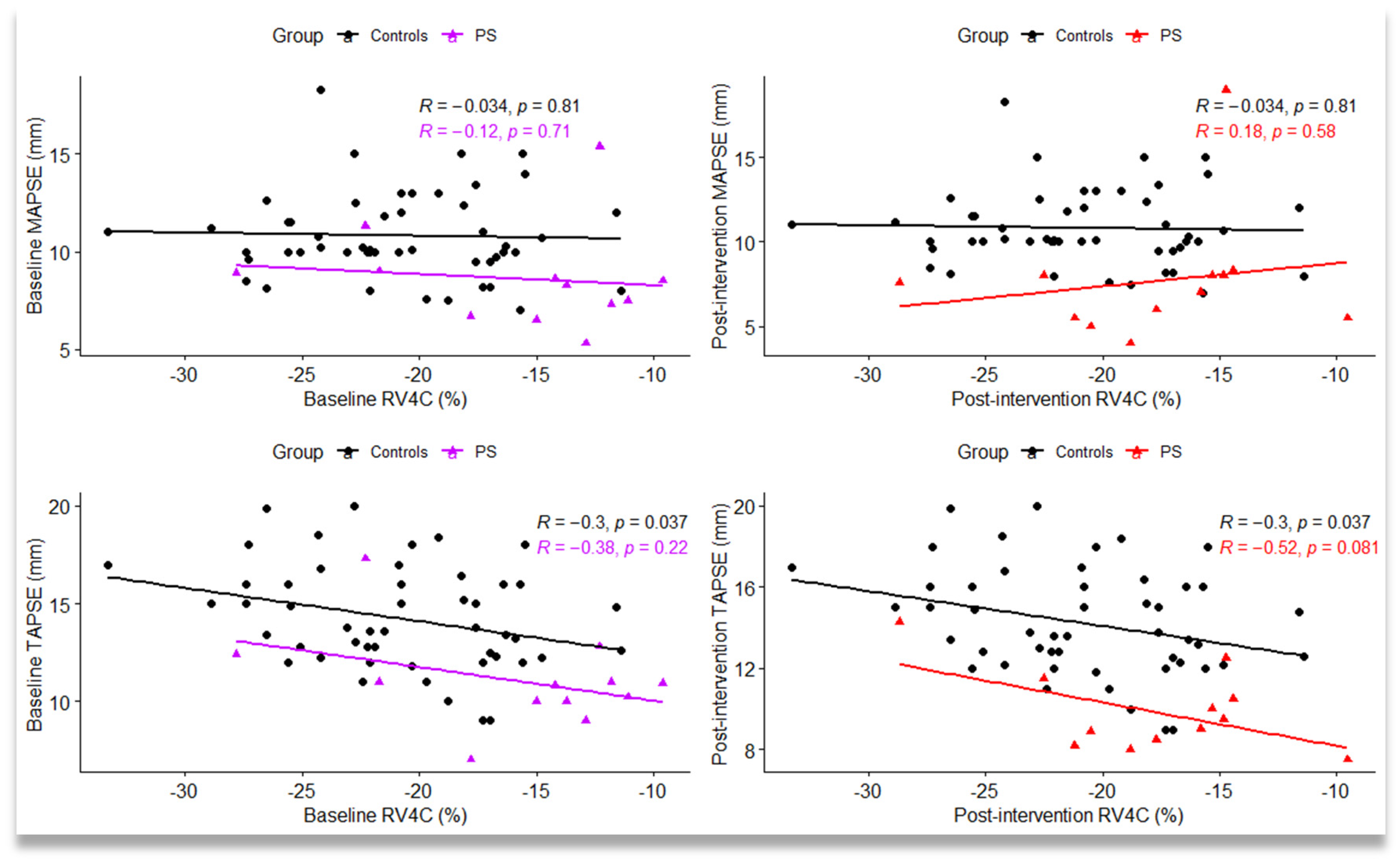
5.6. Correlations between Global Longitudinal Strain Variables and Conventional Ecocardiographic Parameters by Group Type
6. Discussion
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, L.F. Pulmonic Stenosis in Infants and Children: Clinical Manifestations and Diagnosis. Available online: www.uptodate.com (accessed on 14 October 2020).
- Bianchi, D.W.; Crombleholme, T.M.; D’Alton, M.E.; Malone, F.D. (Eds.) Pulmonary Stenosis and Atresia. In Fetology: Diagnosis and Management of the Fetal Patient, 2nd ed.; McGraw Hill: New York, NY, USA, 2010; Available online: https://obgyn.mhmedical.com/content.aspx?bookid=1306§ionid=75206254. (accessed on 15 November 2021).
- Kan, J.S.; White, R.I.; Mitchell, S.E.; Gardner, T.J. Percutaneous Balloon Valvuloplasty: A New Method for Treating Congenital Pulmonary-Valve Stenosis. N. Engl. J. Med. 1982, 307, 540–542. [Google Scholar] [CrossRef]
- Li, S.J.; Yu, H.K.; Wong, S.J.; Cheung, Y.F. Right and left ventricular mechanics and interaction late after balloon valvoplasty for pulmonary stenosis. Eur. Heart J.–Cardiovasc. Imaging 2014, 15, 1020–1028. [Google Scholar] [CrossRef][Green Version]
- Friedberg, M.K.; Wu, S.; Slorach, C. Left-Right Ventricular Interactions in Pediatric Aortic Stenosis: Right Ventricular Myocardial Strain before and after Aortic Valvuloplasty. J. Am. Soc. Echocardiogr. 2013, 26, 390–397. [Google Scholar] [CrossRef]
- Atsumi, A.; Seo, Y.; Ishizu, T.; Nakamura, A.; Enomoto, Y.; Harimura, Y.; Okazaki, T.; Abe, Y.; Aonuma, K. Right Ventricular Deformation Analyses Using a Three-Dimensional Speckle-Tracking Echocardiographic System Specialized for the Right Ventricle. J. Am. Soc. Echocardiogr. 2016, 29, 402–411.e2. [Google Scholar] [CrossRef] [PubMed]
- Ronai, C.; Ghelani, S.J.; Marshall, A.C.; Harrild, D.M.; Gauvreau, K.; Colan, S.D.; Brown, D.W. Characterization of Left Ventricular Dysfunction by Myocardial Strain in Critical Pulmonary Stenosis and Pulmonary Atresia After Neonatal Pulmonary Valve Balloon Dilation. Am. J. Cardiol. 2019, 123, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Ronai, C.; Rathod, R.H.; Marshall, A.C.; Oduor, R.; Gauvreau, K.; Colan, S.D.; Brown, D.W. Left Ventricular Dysfunction Following Neonatal Pulmonary Valve Balloon Dilation for Pulmonary Atresia or Critical Pulmonary Stenosis. Pediatr. Cardiol. 2015, 36, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Salte, I.M.; Østvik, A.; Smistad, E.; Melichova, D.; Nguyen, T.M.; Karlsen, S.; Brunvand, H.; Haugaa, K.H.; Edvardsen, T.; Lovstakken, L.; et al. Artificial Intelligence for Automatic Measurement of Left Ventricular Strain in Echocardiography. JACC Cardiovasc. Imaging 2021, 14, 1918–1928. [Google Scholar] [CrossRef]
- Geyer, H.; Caracciolo, G.; Abe, H.; Wilansky, S.; Carerj, S.; Gentile, F.; Nesser, H.J.; Khandheria, B.; Narula, J.; Sengupta, P.P. Assessment of myocardial mechanics using speckle tracking echocardiography: Fundamentals and clinical applications. J. Am. Soc. Echocardiogr. 2010, 23, 351–369. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Daraban, A.M.; Ünlü, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J. Am. Soc. Echocardiogr. 2015, 28, 1171–1181.e2. [Google Scholar] [CrossRef]
- Cvijic, M.; Voigt, J.U. Application of strain echocardiography in valvular heart diseases. Anatol. J. Cardiol. 2020, 23, 244–253. [Google Scholar] [CrossRef]
- Cohen, J.; Binka, E.; Woldu, K.; Levasseur, S.; Glickstein, J.; Freud, L.R.; Chelliah, A.; Chiu, J.S.; Shah, A. Myocardial strain abnormalities in fetuses with pulmonary atresia and intact ventricular septum. Ultrasound Obstet. Gynecol. 2019, 53, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, C.; Rougon, N.; Cluzel, P. Assessment of myocardial function: A review of quantification methods and results using tagged MRI. J. Cardiovasc. Magn. Reson. 2005, 7, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Pena, J.L.; da Silva, M.G.; Alves, J.M., Jr.; Salemi, V.M.; Mady, C.; Baltabaeva, A.; Sutherland, G.R. Sequential changes of longitudinal and radial myocardial deformation indices in the healthy neonate heart. J. Am. Soc. Echocardiogr. 2010, 23, 294–300. [Google Scholar] [CrossRef]
- Eyskens, B.; Brown, S.C.; Claus, P.; Dymarkowski, S.; Gewillig, M.; Bogaert, J.; Mertens, L. The influence of pulmonary regurgitation on regional right ventricular function in children after surgical repair of tetralogy of Fallot. Eur. J. Echocardiogr. 2010, 11, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.T.; Sanchez Mejia, A.A.; Machefsky, A.; Fowler, S.; Holland, M.R.; Singh, G.K. Normal ranges of right ventricular systolic and diastolic strain measures in children: A systematic review and meta-analysis. J. Am. Soc. Echocardiogr. 2014, 27, 549–560.e3. [Google Scholar] [CrossRef] [PubMed]
- Jashari, H.; Rydberg, A.; Ibrahimi, P.; Bajraktari, G.; Kryeziu, L.; Jashari, F.; Henein, M.Y. Normal ranges of left ventricular strain in children: A meta-analysis. Cardiovasc. Ultrasound 2015, 13, 37. [Google Scholar] [CrossRef]
- Schubert, U.; Müller, M.; Norman, M.; Abdul-Khaliq, H. Transition from fetal to neonatal life: Changes in cardiac function assessed by speckle-tracking echocardiography. Early Hum. Dev. 2013, 89, 803–808. [Google Scholar] [CrossRef]
- Levy, P.T.; Machefsky, A.; Sanchez, A.A.; Patel, M.D.; Rogal, S.; Fowler, S.; Yaeger, L.; Hardi, A.; Holland, M.R.; Hamvas, A.; et al. Reference Ranges of Left Ventricular Strain Measures by Two-Dimensional Speckle-Tracking Echocardiography in Children: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2016, 29, 209–225.e6. [Google Scholar] [CrossRef]
- Beyar, R.; Dong, S.J.; Smith, E.R.; Belenkie, I.; Tyberg, J.V. Ventricular interaction and septal deformation: A model compared with experimental data. Am. J. Physiol. 1993, 265 Pt 2, H2044–H2056. [Google Scholar] [CrossRef]
- Kemal, H.S.; Kayıkçıoğlu, M.; Nalbantgil, S.; Can, L.H.; Moğulkoç, N.; Kültürsay, H. Assessment of right ventricular function in patients with pulmonary arterial hypertension-congenital heart disease and repaired and unrepaired defects: Correlation among speckle tracking, conventional echocardiography, and clinical parameters. Anatol. J. Cardiol. 2020, 23, 277–287. [Google Scholar] [CrossRef]
- Kempny, A.; Diller, G.P.; Orwat, S.; Kaleschke, G.; Kerckhoff, G.; Bunck, A.C.; Maintz, D.; Baumgartner, H. Right ventricular-left ventricular interaction in adults with Tetralogy of Fallot: A combined cardiac magnetic resonance and echocardiographic speckle tracking study. Int. J. Cardiol. 2012, 154, 259–264. [Google Scholar] [CrossRef] [PubMed]
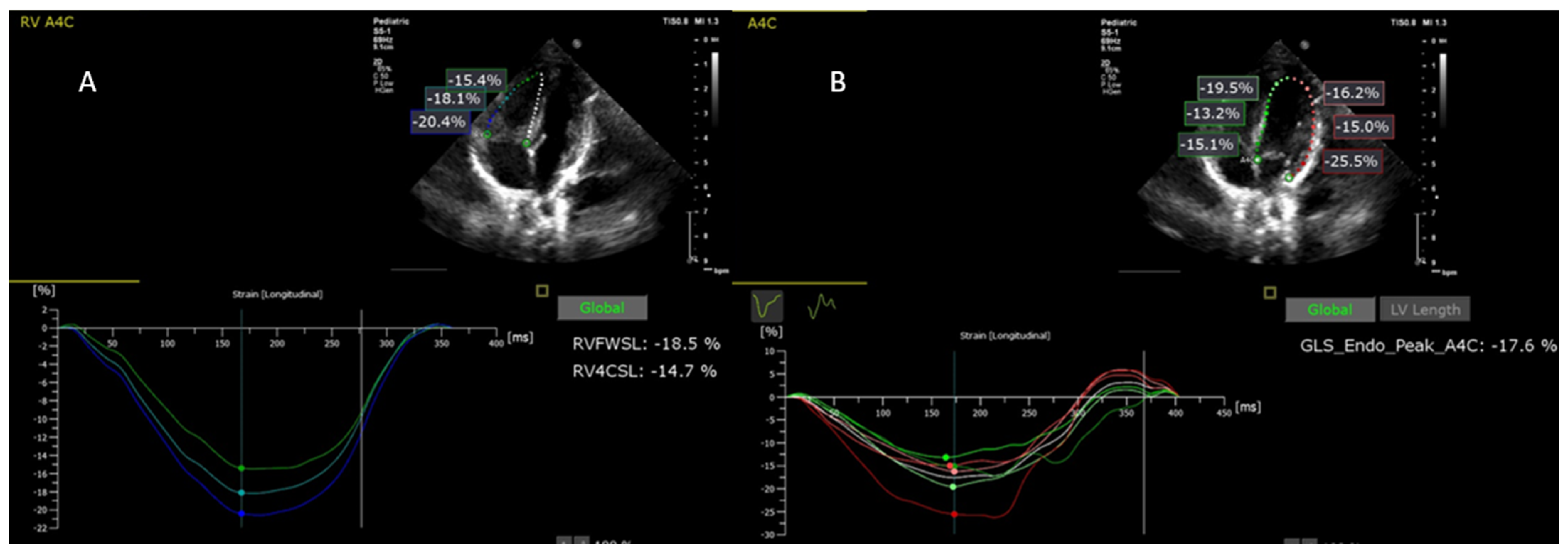

| Variables | Description |
|---|---|
| RV Basal | The basal segment of the right ventricle lateral wall |
| RV Medial | The medial segment of the right ventricle lateral wall |
| RV Apical | The apical segment of the right ventricle lateral wall |
| RV free wall | Longitudinal strain of the right ventricle free wall |
| RV4C | Global longitudinal strain of the right ventricle including interventricular septum |
| LV Basal | The basal segment of the left ventricle lateral wall |
| LV Medial | The medial segment of the left ventricle lateral wall |
| LV Apical | The apical segment of the left ventricle lateral wall |
| Inter V Basal | The basal segment of the inter-ventricular septum |
| Inter V Medial | The medial segment of the inter-ventricular septum |
| Inter V Apical | The apical segment of the inter-ventricular septum |
| LV pGLS | Peak global longitudinal strain of the left ventricle |
| GP max | Anterograde peak pressure gradient measured transpulmonary |
| EF M mode | Ejection Fraction |
| TAPSE | Tricuspid annular plane systolic excursion |
| MAPSE | Mitral annular plane systolic excursion |
| Control Group (n1 = 50) | PS Group (n2 = 12) | p-Value | |
|---|---|---|---|
| Gestational age (weeks) (1) | 38.24 ± 0.77 | 38.92 ± 0.79 | 0.0086 * |
| Age (weeks) (2) | 13 (8, 16) | 4.5 (2, 14.75) | 0.027 * |
| Gender (male) (3) | 22 (44) | 3 (25) | 0.330 |
| Body surface area (m2) (1) | 0.27 ± 0.03 | 0.26 ± 0.06 | 0.766 |
| SaO2 (%) (2) | 99 (99, 100) | 94.5 (85, 98.25) | <0.0001 * |
| Systolic blood pressure (mmHg) (2) | 75.5 (70.5, 85) | 83 (75.5, 93) | <0.041 * |
| Diastolic blood pressure (mmHg) (2) | 51.4 (46.25, 55) | 49.5 (39.5, 60) | 0.604 |
| Heart rate (bpm) (1) | 128.5 ± 9.20 | 133.50 ± 18.41 | 0.380 |
| Birth weight (g) (1) | 3285 ± 541.85 | 3300 ± 657.99 | 0.935 |
| Apgar score at 1-minute (2) | 10 (9, 10) | 9 (8, 10) | 0.025 * |
| Apgar score at 5-minute (2) | 10 (10, 10) | 9.5 (9, 10) | 0.0006 * |
| C-section (3) | 11 (22) | 5 (41.67) | 0.268 |
| Pathological pregnancy (3) | 4 (8) | 5 (41.67) | 0.010 * |
| Genetic syndrome (3) | NA | 4 (33.33) | NA |
| PS requiring prostaglandin infusion (3) | NA | 5 (41.67) | NA |
| Ecocardiografic parameters | |||
| EF | 71.70 ± 6.34 | 70.33 ± 10.07 | 0.661 |
| MAPSE | 10.82 ± 2.24 | 8.61 ± 2.62 | 0.004 * |
| TAPSE | 14.25 ± 2.65 | 11.03 ± 2.48 | 0.0003 * |
| Effects | Control Group | PS Group | Adjusted Difference between Groups (a) | Mixed Models Analysis, p-Values (c) | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Estimated Regression Parameter [95% CI] | p-Value (b) | PS Group (vs. Control) | Medial Location (Vs. Basal) | Apical Location (vs. Basal) | |
| Location of the left ventricle 2D strain measurements | 0.223 | <0.0001 * | <0.0001 * | ||||
| Basal | −28.83 ± 9.70 | −30.22 ± 11.87 | 3.12 (−4.22, 10.45) | 0.841 | |||
| Medial | −16.70 ± 4.21 | −11.37± 4.26 | −3.62 (−10.95, 3.72) | 0.715 | |||
| Apical | −14.95 ± 7.67 | −18.85 ± 8.04 | 5.62 (−1.72, 12.93) | 0.239 | |||
| Location of the right ventricle 2D strain measurements | 0.0324 * | <0.0001 * | <0.0001 * | ||||
| Basal | −28.68 ± 6.72 | −22.75 ± 8.69 | −4.82 (−11.27, 1.64) | 0.261 | |||
| Medial | −23.43 ± 6.19 | −18.86 ± 6.88 | −3.46 (−9.91, 2.99) | 0.628 | |||
| Apical | −18.98 ± 7.78 | −16.69 ± 5.99 | −1.18 (−7.63, 5.28) | 0.994 | |||
| Location of the inter-ventricular septum 2D strain measurements | 0.3589 | <0.0001 * | 0.0066 * | ||||
| Basal | −18.17 ± 5.36 | −15.10 ± 7.25 | −2.08 (−8.63, 4.46) | 0.941 | |||
| Medial | −22.96 ± 4.73 | −15.59 ± 7.02 | −6.39 (−12.93, 0.16) | 0.060 | |||
| Apical | −21.08 ± 8.19 | −24.92 ± 10.70 | 4.83 (−1.71, 11.38) | 0.275 | |||
| Effects | Control Group | PS Group | Adjusted Difference between Groups (a) | Mixed Models Analysis, p-Values (c) | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Estimated Regression Parameter [95% CI] | p-Value (b) | PS Group (vs. Control) | Medial Location (vs. Basal) | Apical Location (vs. Basal) | |
| Location of the left ventricle 2D strain measurements | 0.539 | <0.0001 * | <0.0001 * | ||||
| Basal | −28.83 ± 9.70 | −25.33± 15.51 | −1.65 (−9.36, 6.07) | 0.989 | |||
| Medial | −16.70 ± 4.21 | −14.37± 5.88 | −0.49 (−8.20, 7.23) | 1.000 | |||
| Apical | −14.95 ± 7.67 | −17.52 ± 7.56 | 4.42 (−3.29, 12.14) | 0.565 | |||
| Location of the right ventricle 2D strain measurements | 0.0141 * | <0.0001 * | <0.0001 * | ||||
| Basal | −28.68 ± 6.72 | −22.89 ± 6.58 | −5.25 (−11.35, 0.86) | 0.133 | |||
| Medial | −23.43 ± 6.19 | −22.42 ± 6.79 | −0.47 (−6.57, 5.63) | 0.999 | |||
| Apical | −18.98 ± 7.78 | −21.22 ± 6.71 | 2.04 (−4.07, 8.14) | 0.924 | |||
| Location of the inter-ventricular septum 2D strain measurements | 0.111 | <0.0001 * | 0.0082 * | ||||
| Basal | −18.17 ± 5.36 | −13.58 ± 5.07 | −3.43 (−9.60, 2.75) | 0.598 | |||
| Medial | −22.96 ± 4.73 | −15.59 ± 6.16 | −6.20 (−12.38, −0.03) | 0.048 * | |||
| Apical | −21.08 ± 8.19 | −20.79 ± 10.13 | 0.88 (−5.29, 7.06) | 0.998 | |||
| Effects | Baseline (T0) | Post-Intervention (T1) | p-Values for Main Effects | |||
|---|---|---|---|---|---|---|
| Estimated Marginal Means [95% CI] | Estimated Marginal Means [95% CI] | p-Value | Time T1 vs. T0 | Medial Location (vs. Basal) | Apical Location (vs. Basal) | |
| Location of the left ventricle 2D strain measurements | 0.206 | <0.0001 * | 0.004 * | |||
| Basal | −30.2 (−35.6, −24.82] | −25.3 (−30.7, −19.93) | 0.795 | |||
| Medial | −11.4 (−16.8, −5.96] | −14.4 (−19.8, −8.96) | 0.969 | |||
| Apical | −18.9 (−24.3, −13.45) | −17.5 (−22.9, −12.12) | 0.999 | |||
| Location of the right ventricle 2D strain measurements | 0.946 | 0.065 | 0.005 * | |||
| Basal | −22.8 (−26.4, −19.1) | −22.9 (−26.5, −19.2) | 1.000 | |||
| Medial | −18.9 (−22.5, −15.2) | −22.4 (−26.1, −18.8) | 0.525 | |||
| Apical | −16.7 (−20.3, −13.00) | −21.2 (−24.9, −17.6) | 0.259 | |||
| Location of the inter-ventricular septum 2D strain measurements | 0.591 | 0.862 | 0.010 * | |||
| Basal | −15.1 (−19.6, −10.6) | −13.6 (−18.1, −9.1) | 0.994 | |||
| Medial | −15.6 (−20.1, −11.08) | −15.6 (−20.1, −11.08) | 1.000 | |||
| Apical | −24.9 (−29.4, −20.4) | −20.8 (−25.3, −16.28) | 0.688 | |||
| Effects | Baseline (T0) | Post-Intervention (T1) | p-Value |
|---|---|---|---|
| Estimated Marginal Means (95% CI) | Estimated Marginal Means (95% CI) | ||
| LVpGLS (%) | −19.2 (−21.8, −16.5) | −17.4 (−20.0, −14.8) | 0.337 |
| RVfree (%) | −19.9 (−23.4, −16.3) | −22.7 (−26.2, −19.1) | 0.252 |
| RV4C (%) | −15.8 (−18.4, −13.3) | −17.8 (−20.3, −15.3) | 0.195 |
| EF (%) | 70.3 (64.8, 75.8) | 62.2 (56.7, 67.8) | 0.039 * |
| PG | 108.0 (90.8, 125.2) | 37.2 (20.0, 54.5) | <0.0001 * |
| TAPSE | 11.03 (9.81, 12.3) | 9.87 (8.64, 11.1) | 0.015 * |
| MAPSE | 8.61 (6.34, 10.9) | 7.66 (5.39, 9.93) | 0.106 |
| ∆LVpGLS (%) | ∆Rvfree (%) | ∆RV4C (%) | ∆EF (%) | ΔTAPSE (%) | ∆MAPSE (%) | |
|---|---|---|---|---|---|---|
| ∆LVpGLS (%) | −15.04 (−29.37, 23.88) | −0.13 (0.683) | −0.03 (0.939) | 0.31 (0.343) | 0.19 (0.558) | 0.58 (0.052) |
| ∆RVfree wall (%) | 21.91 (−3.62, 38.39) | 0.91 (<0.0001) | −0.08 (0.800) | −0.41 (0.193) | −0.03 (0.921) | |
| ∆RV4C (%) | 16.58 (1.04, 37.61) | 0.05 (0.886) | −0.35 (0.266) | −0.16 (0.619) | ||
| ∆EF (%) | −8.09 (−15.87, −2.55) | 0.41 (0.185) | −0.16 (0.619) | |||
| ∆TAPSE (%) | −8.15 (−17.36, −4.89) | 0.47 (0.128) | ||||
| ∆MAPSE (%) | −9.84 (−33.05, −6.56) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gozar, L.; Iancu, M.; Gozar, H.; Sglimbea, A.; Cerghit Paler, A.; Gabor-Miklosi, D.; Toganel, R.; Făgărășan, A.; Iurian, D.R.; Toma, D. Assessment of Biventricular Myocardial Function with 2-Dimensional Strain and Conventional Echocardiographic Parameters: A Comparative Analysis in Healthy Infants and Patients with Severe and Critical Pulmonary Stenosis. J. Pers. Med. 2022, 12, 57. https://doi.org/10.3390/jpm12010057
Gozar L, Iancu M, Gozar H, Sglimbea A, Cerghit Paler A, Gabor-Miklosi D, Toganel R, Făgărășan A, Iurian DR, Toma D. Assessment of Biventricular Myocardial Function with 2-Dimensional Strain and Conventional Echocardiographic Parameters: A Comparative Analysis in Healthy Infants and Patients with Severe and Critical Pulmonary Stenosis. Journal of Personalized Medicine. 2022; 12(1):57. https://doi.org/10.3390/jpm12010057
Chicago/Turabian StyleGozar, Liliana, Mihaela Iancu, Horea Gozar, Anca Sglimbea, Andreea Cerghit Paler, Dorottya Gabor-Miklosi, Rodica Toganel, Amalia Făgărășan, Diana Ramona Iurian, and Daniela Toma. 2022. "Assessment of Biventricular Myocardial Function with 2-Dimensional Strain and Conventional Echocardiographic Parameters: A Comparative Analysis in Healthy Infants and Patients with Severe and Critical Pulmonary Stenosis" Journal of Personalized Medicine 12, no. 1: 57. https://doi.org/10.3390/jpm12010057
APA StyleGozar, L., Iancu, M., Gozar, H., Sglimbea, A., Cerghit Paler, A., Gabor-Miklosi, D., Toganel, R., Făgărășan, A., Iurian, D. R., & Toma, D. (2022). Assessment of Biventricular Myocardial Function with 2-Dimensional Strain and Conventional Echocardiographic Parameters: A Comparative Analysis in Healthy Infants and Patients with Severe and Critical Pulmonary Stenosis. Journal of Personalized Medicine, 12(1), 57. https://doi.org/10.3390/jpm12010057







