Correlation between Peri-Implant Marginal Bone Loss Progression and Peri-Implant Sulcular Fluid Levels of Metalloproteinase-8
Abstract
:1. Introduction
- (1)
- to determine a cutoff point for discriminating between low and high bone loss-type (BLT) implants, considering a threshold of 2 mm at 24 months [6];
- (2)
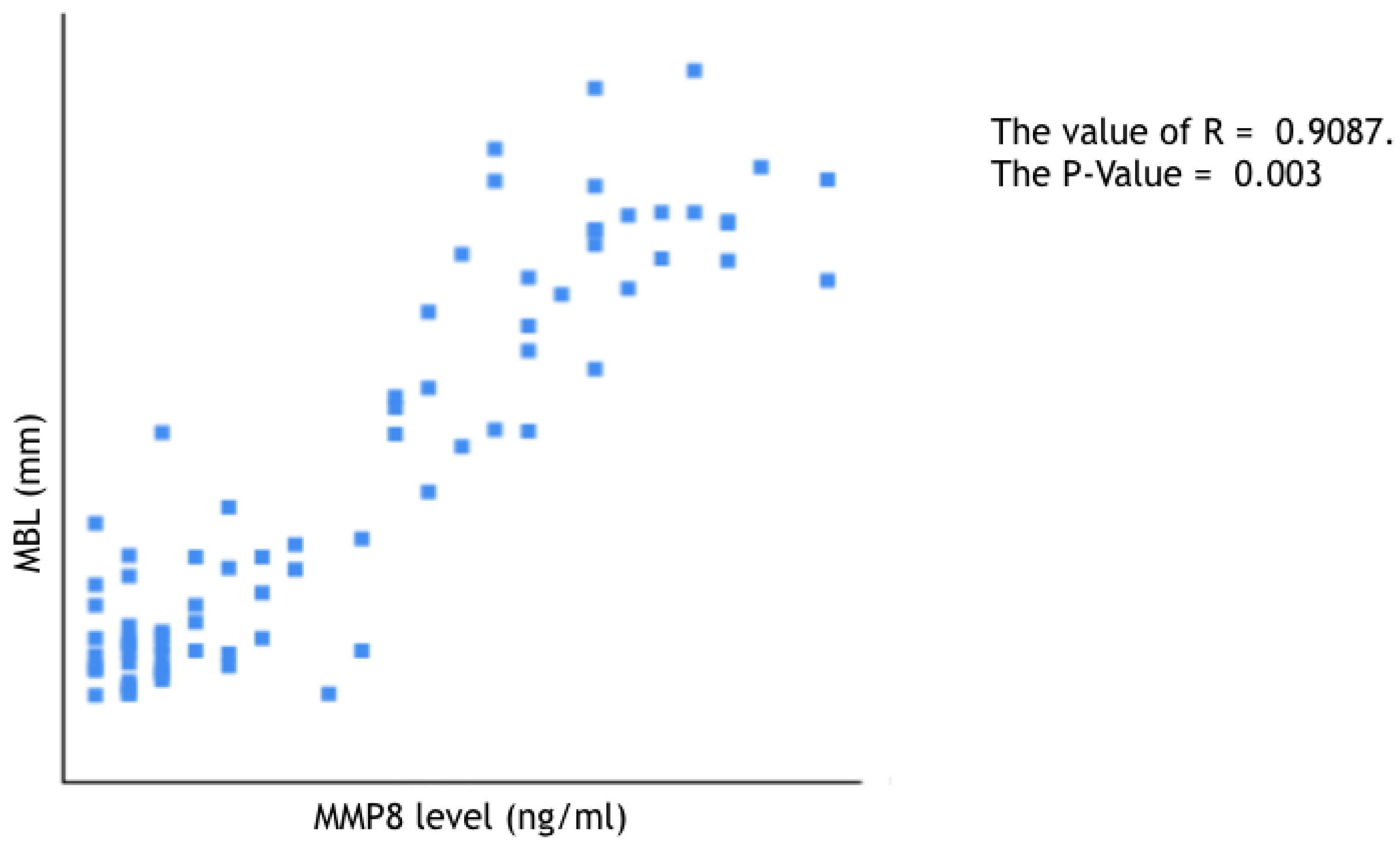
- to evaluate a possible correlation between peri-implant marginal bone loss progression and peri-implant sulcular fluid levels of aMMP-8; and
- (3)
- to examine the patient and clinical variables that might influence MBL progression.
2. Materials and Methods
- -
- age > 18 years,
- -
- presence of at least one edentulous site in posterior areas,
- -
- physical status of I or II according to the American Society of Anesthesiologists (ASA) classification system, absence of systemic diseases or conditions known to alter bone metabolism,
- -
- presence of stable periodontal condition (patient had to present at the first visit an inter-proximal attachment loss < 3 mm and/or radiographic bone loss < 30% of root length in <30% of sites) [37].
- -
- radiographic implant length (IL): distance (in mm) between the implant coronal margin and the implant apex, as assessed at the midportion of the implant; and
- -
- residual bone height at the mesial (MI) and distal (DI) aspects of the implant: distance (in mm) between the line linking the coronal implant margin, and the first contact of the crestal bone on both mesial and distal sides of the implant.
Statistical Analysis
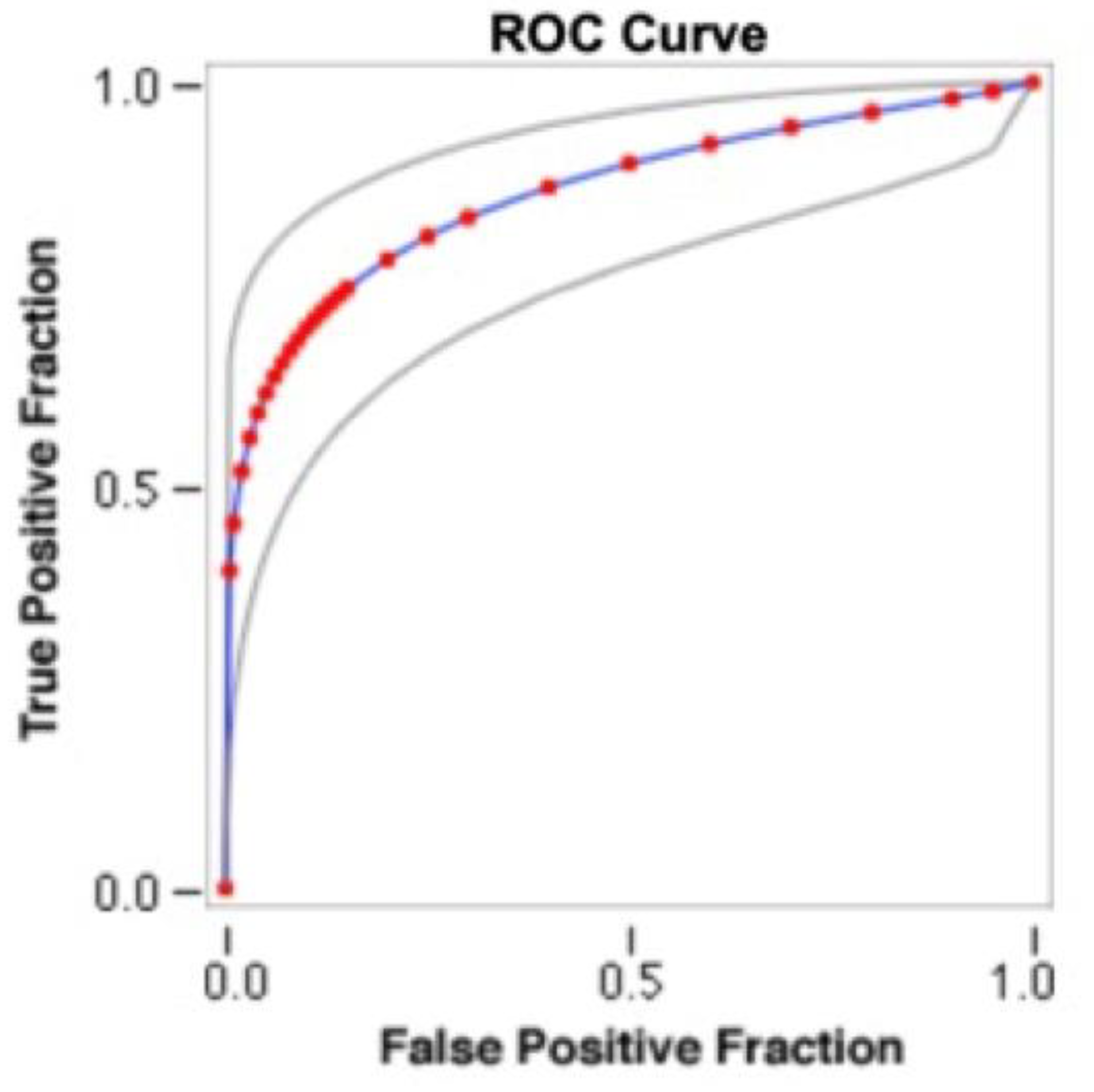
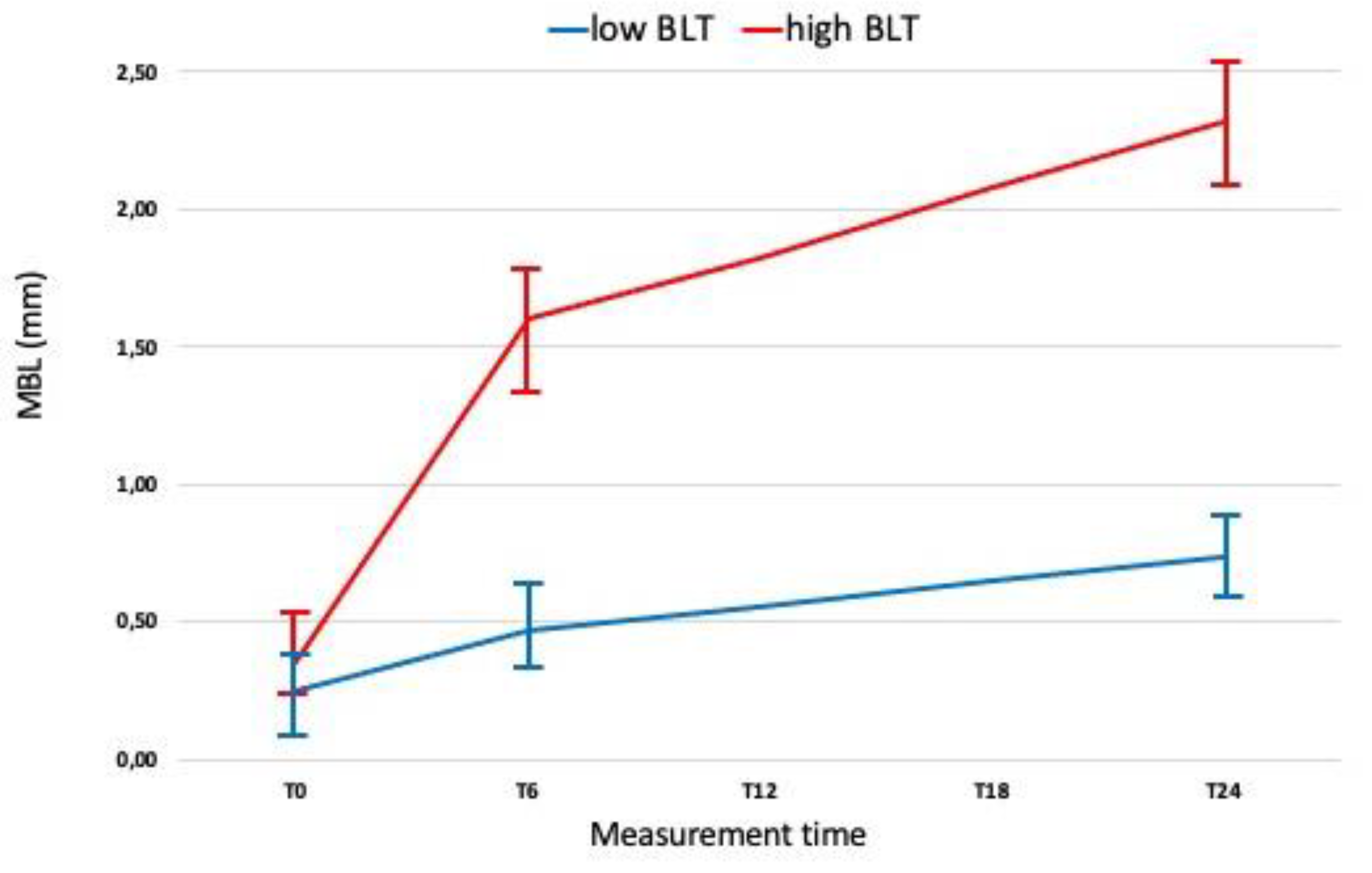
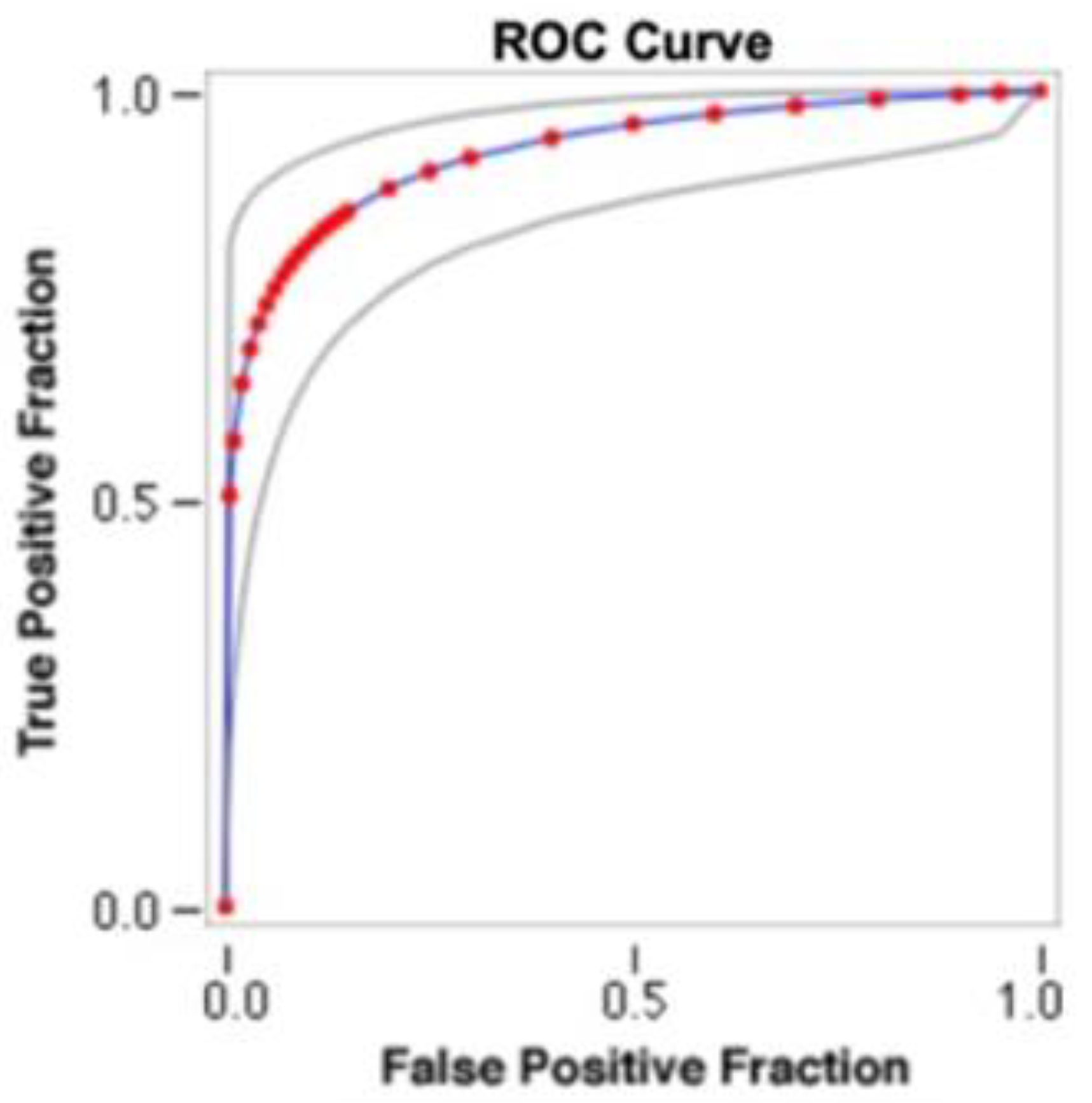
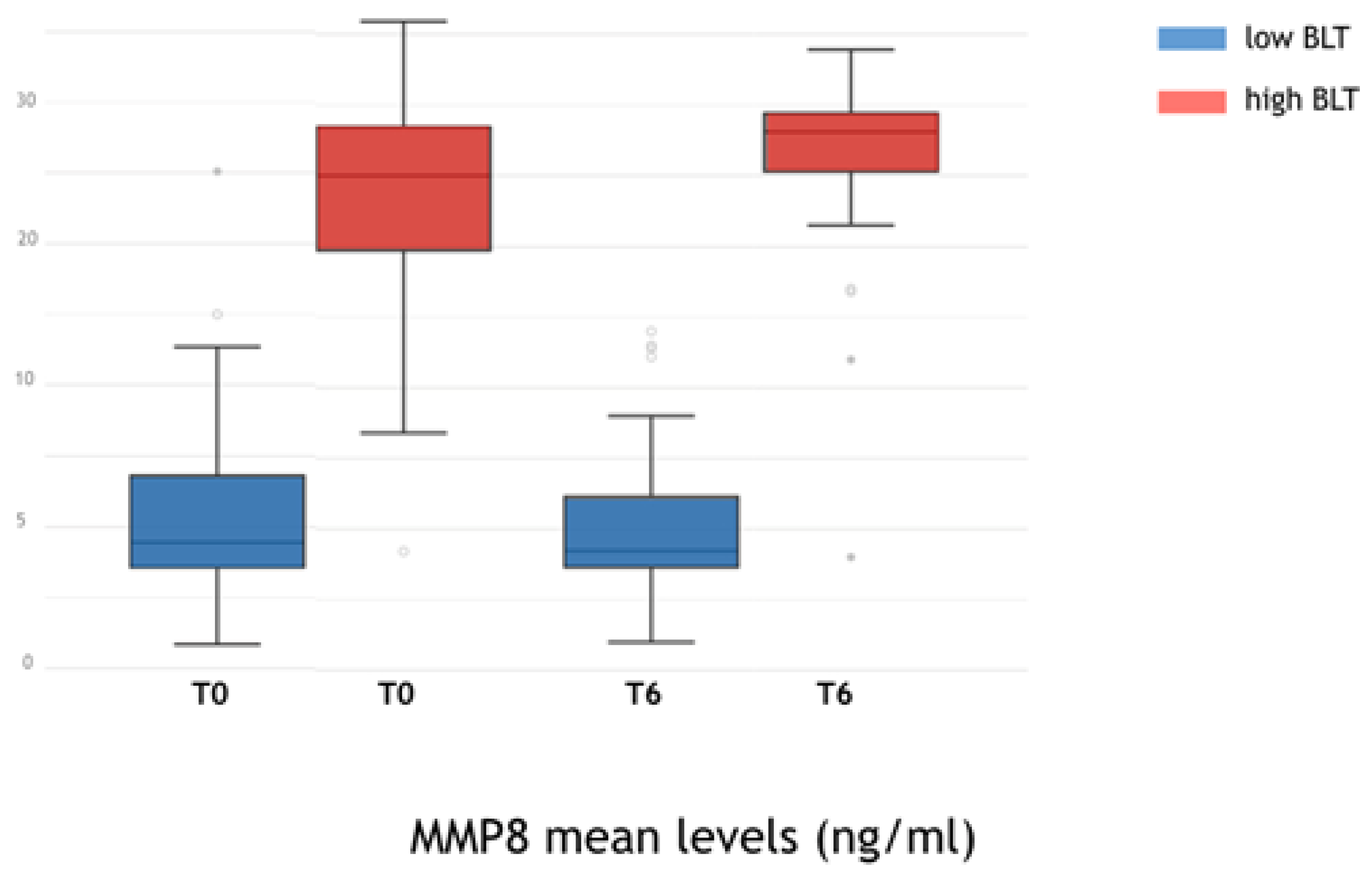
3. Results
4. Discussion
5. Conclusions
- -
- Implants with increased MBL rates at 6 months after loading are likely to achieve additional MBL values.
- -
- The initial high level of aMMP8 can be considered as indicators of the subsequent progression of peri-implant bone loss.
- -
- MMP-8 could be used as biomarker for identifying implants and patients that could present an HBL.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A

References
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S286–S291. [Google Scholar] [CrossRef] [Green Version]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S278–S285. [Google Scholar] [CrossRef] [Green Version]
- No authors: Peri-implant mucositis and peri-implantitis: A current understanding of their diagnoses and clinical implications. J. Periodontol. 2013, 84, 436–443. [CrossRef]
- Albrektsson, T.; Chrcanovic, B.; Östman, P.O.; Sennerby, L. Initial and long-term crestal bone responses to modern dental implants. Periodontology 2017, 73, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Klinge, B. Peri-implant marginal bone loss: An academic controversy or a clinical challenge? Eur. J. Oral Implantol. 2012, 5, S13–S19. [Google Scholar] [PubMed]
- Galindo-Moreno, P.; León-Cano, A.; Ortega-Oller, I.; Monje, A.; O’Valle, F.; Catena, A. Marginal bone loss as success criterion in implant dentistry: Beyond 2 mm. Clin. Oral Implant. Res. 2015, 26, e28–e34. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Leung, M.C.T.; Ng, K.T.; Fan, M.H.M.; Fokas, G.; Mattheos, N. Peri-implant marginal bone loss rate pre- and post-loading: An exploratory analysis of associated factors. Clin. Oral Implant. Res. 2019, 30, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Lang, N.P. Diagnostic parameters for monitoring peri-implant conditions. Int. J. Oral Maxillofac. Implant. 2004, 19, 116–127. [Google Scholar] [PubMed]
- Dursun, E.; Tözüm, T.F. Peri-Implant Crevicular Fluid Analysis, Enzymes and Biomarkers: A Systemetic Review. J. Oral Maxillofac Res. 2016, 7, e9. [Google Scholar] [CrossRef]
- Hentenaar, D.F.; De Waal, Y.C.; Vissink, A.; Van Winkelhoff, A.J.; Meijer, H.J.; Liefers, S.C.; Kroese, F.G.; Raghoebar, G.M. Biomarker levels in peri-implant crevicular fluid of healthy implants, untreated and non-surgically treated implants with peri-implantitis. J. Clin Periodontol. 2021, 48, 590–601. [Google Scholar] [CrossRef]
- Kahari, V.M.; Saarialho-Kere, U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann. Med. 1999, 31, 34–45. [Google Scholar] [CrossRef]
- Saarialho-Kere, U.; Kerkelä, E.; Jeskanen, L.; Ranki, A.; Vaalamo, M.; Hasan, T.; Pierce, R.; Starcher, B.; Raudasoja, R.; Oikarinen, A. Accumulation of matrilysin (MMP-7) and macrophage metalloelastase (MMP-12) in actinic damage. J. Investig. Dermatol. 1999, 113, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.L.; Ouellette, A.J.; Satchell, D.P.; Ayabe, T.; López-Boado, Y.S.; Stratman, J.L.; Hultgren, S.J.; Matrisian, L.M.; Parks, W.C. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 1999, 286, 113–117. [Google Scholar] [CrossRef]
- Sorsa, T.; Uitto, V.J.; Suomalainen, K.; Vauhkonen, M.; Lindy, S. Comparison of interstitial collagenases from human gingiva, sulcular fluid and polymorphonuclear leukocytes. J. Periodontal Res. 1988, 23, 386–393. [Google Scholar] [CrossRef]
- Kiili, M.; Cox, S.W.; Chen, H.W.; Wahlgren, J.; Maisi, P.; Eley, B.M.; Salo, T.; Sorsa, T. Collagenase-2 (MMP-8) and collagenase-3 (MMP-13) in adult periodontitis: Molecular forms and levels in gingival crevicular fluid and immunolocalisation in gingival tissue. J. Clin. Periodontol. 2002, 29, 224–232. [Google Scholar] [CrossRef]
- Golub, L.M.; Sorsa, T.; Lee, H.-M.; Ciancio, S.; Sorbi, D.; Ramamurthy, N.S.; Gruber, B.; Salo, T.; Konttinen, Y.T. Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinases in human adult periodontitis gingiva. J. Clin. Periodontol. 1995, 22, 100–109. [Google Scholar] [CrossRef]
- Tervahartiala, T.; Pirilä, E.; Ceponis, A.; Maisi, P.; Salo, T.; Tuter, G.; Kallio, P.; Törnwall, J.; Srinivas, R.; Konttinen, Y.; et al. The in vivo Expression of the Collagenolytic Matrix Metalloproteinases (MMP-2, -8, -13, and -14) and Matrilysin (MMP-7) in Adult and Localized Juvenile Periodontitis. J. Dent. Res. 2000, 79, 1969–1977. [Google Scholar] [PubMed]
- Tjaderhane, L.; Larjava, H.; Sorsa, T.; Uitto, V.J.; Larmas, M.; Salo, T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J. Dent. Res. 1998, 77, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Sulkala, M.; Larmas, M.; Sorsa, T.; Salo, T.; Tjaderhane, L. The localization of matrix metalloproteinase-20 (MMP-20, enamelysin) in mature human teeth. J. Dent. Res. 2002, 81, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Prikk, K.; Maisi, P.; Pirila, E.; Sepper, R.; Salo, T.; Wahlgren, J.; Sorsa, T. In vivo collagenase-2 (MMP-8) expression by human bronchial epithelial cells and monocytes/macrophages in bronchiectasis. J. Pathol. 2001, 194, 232–238. [Google Scholar] [CrossRef]
- Elliott, S.; Cawston, T. The clinical potential of matrix metalloproteinase inhibitors in the rheumatic disorders. Drugs Aging 2001, 18, 87–99. [Google Scholar] [CrossRef]
- Chang, C.; Werb, Z. The many faces of metalloproteases: Cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001, 11, S37–S43. [Google Scholar] [CrossRef]
- Salo, T.; Makela, M.; Kylmaniemi, M.; Autio-Harmainen, H.; Larjava, H. Expression of matrix metalloproteinase-2 and -9 during early human wound healing. Lab. Investig. 1994, 70, 176–182. [Google Scholar] [PubMed]
- Tsubota, M.; Sasano, Y.; Takahashi, I.; Kagayama, M.; Shimauchi, H. Expression of MMP-8 and MMP-13 mRNAs in rat periodontium during tooth eruption. J. Dent. Res. 2002, 81, 673–678. [Google Scholar] [CrossRef]
- Chin, J.R.; Werb, Z. Matrix metalloproteinases regulate morphogenesis, migration and remodeling of epithelium, tongue skeletal muscle and cartilage in the mandibular arch. Development 1997, 124, 1519–1530. [Google Scholar] [CrossRef]
- Kivelä-Rajamäki, M.J.; Teronen, O.P.; Maisi, P.; Husa, V.; Tervahartiala, T.I.; Pirilä, E.M.; Salo, T.A.; Mellanen, L.; Sorsa, T.A. Laminin-5 gamma2-chain and collagenase-2 (MMP-8) in human peri-implant sulcular fluid. Clin. Oral Implant. Res. 2003, 14, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Kitti, U.; Teronen, O.; Sorsa, T.; Husa, V.; Laine, P.; Rönkä, H.; Salo, T.; Lindqvist, C.; Konttinen, Y. Collagenases in different categories of peri-implant vertical bone loss. J. Dent. Res. 2000, 79, 1870–1873. [Google Scholar] [CrossRef]
- Nomura, T.; Ishii, A.; Shimizu, H.; Taguchi, N.; Yoshie, H.; Kusakari, H.; Hara, K. Tissue inhibitor of metalloproteinases-1, matrix metalloproteinases-1 and -8, and collagenase activity levels in peri-implant crevicular fluid after implantation. Clin. Oral Implant. Res. 2000, 11, 430–440. [Google Scholar] [CrossRef]
- Basegmez, C.; Yalcin, S.; Yalcin, F.; Ersanli, S.; Mijiritsky, E. Evaluation of periimplant crevicular fluid prostaglandin E2 and matrix metalloproteinase-8 levels from health to periimplant disease status: A prospective study. Implant. Dent. 2012, 21, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, C.A.; Eick, S.; Brönnimann, C.; Buser, D.; Brägger, U.; Salvi, G.E. Host-derived biomarkers at teeth and implants in partially edentulous patients. A 10-year retrospective study. Clin. Oral Implant. Res. 2016, 27, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Alassiri, S.; Parnanen, P.; Rathnayake, N.; Johannsen, G.; Heikkinen, A.M.; Lazzara, R.; van der Schoor, P.; van der Schoor, J.G.; Tervahartiala, T.; Gieselmann, D.; et al. The Ability of Quantitative, Specific, and Sensitive Point-of-Care/Chair-Side Oral Fluid Immunotests for aMMP-8 to Detect Periodontal and Peri-Implant Diseases. Dis. Markers 2018, 2018, 1306396. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, I.T.; Heikkinen, A.M.; Nwhator, S.O.; Umeizudike, K.A.; Tervahartiala, T.; Sorsa, T. On the diagnostic discrimination ability of mouthrinse and salivary aMMP-8 point-of-care testing regarding periodontal health and disease. Diagn. Microbiol. Infect. Dis. 2019, 95, 114871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorsa, T.; Gieselmann, D.; Arweiler, N.B.; Hernández, M. A quantitative point-of-care test for periodontal and dental peri-implant diseases. Nat. Rev. Dis. Prim. 2017, 3, 17069. [Google Scholar] [CrossRef] [PubMed]
- Golub, L.M.; Räisänen, I.T.; Sorsa, T.; Preshaw, P.M. An Unexplored Pharmacologic/Diagnostic Strategy for Peri-Implantitis: A Protocol Proposal. Diagnostics 2020, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Lähteenmäki, H.; Umeizudike, K.A.; Heikkinen, A.M.; Räisänen, I.T.; Rathnayake, N.; Johannsen, G.; Tervahartiala, T.; Nwhator, S.O.; Sorsa, T. aMMP-8 Point-of-Care/Chairside Oral Fluid Technology as a Rapid, Non-Invasive Tool for Periodontitis and Peri-Implantitis Screening in a Medical Care Setting. Diagnostics 2020, 10, 562. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S1–S8. [Google Scholar] [CrossRef]
- Rathnayake, N.; Gieselmann, D.R.; Heikkinen, A.M.; Tervahartiala, T.; Sorsa, T. Salivary Diagnostics-Point-of-Care diagnostics of MMP-8 in dentistry and medicine. Diagnostics 2017, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Misch, C.E.; Perel, M.L.; Wang, H.L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A.; et al. Implant success, survival, and failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant. Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Berglundh, T.; Persson, L.; Klinge, B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J. Clin. Periodontol. 2002, 29 (Suppl. 3), 197–212. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42, S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Koldsland, O.C.; Scheie, A.A.; Aass, A.M. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J. Periodontol. 2010, 81, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Y.; Li, J.; Wang, H.L.; Yu, H. Influence of Laser-Microtextured Surface Collar on Marginal Bone Loss and Peri-Implant Soft Tissue Response: A Systematic Review and Meta-Analysis. J. Periodontol. 2017, 88, 651–662. [Google Scholar] [CrossRef]
- Arakawa, H.; Uehara, J.; Hara, E.S.; Sonoyama, W.; Kimura, A.; Kanyama, M.; Matsuka, Y.; Kuboki, T. Matrix metalloproteinase-8 is the major potential collagenase in active peri-implantitis. J. Prosthod. Res. 2012, 56, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Al-Majid, A.; Alassiri, S.; Rathnayake, N.; Tervahartiala, T.; Gieselmann, D.R.; Sorsa, T. Matrix Metalloproteinase-8 as an Inflammatory and Prevention Biomarker in Periodontal and Peri-Implant Diseases. Int. J. Dent. 2018, 2018, 7891323. [Google Scholar] [CrossRef]
- Thierbach, R.; Maier, K.; Sorsa, T.; Mäntylä, P. Peri-implant sulcus fluid (PISF) matrix metalloproteinase (MMP)-8 levels in peri-implantitis. J. Clin. Diagn. Res. 2016, 10, ZC34–ZC38. [Google Scholar] [CrossRef]
- Salvi, G.E.; Aglietta, M.; Eick, S.; Sculean, A.; Lang, N.P.; Ramseier, C.A. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin. Oral Implant. Res. 2012, 23, 182–190. [Google Scholar] [CrossRef]
- Qian, J.; Wennerberg, A.; Albrektsson, T. Reasons for marginal bone loss around oral implants. Clin. Implant Dent. Relat. Res. 2012, 14, 792–807. [Google Scholar] [CrossRef]
- Albrektsson, T.; Canullo, L.; Cochran, D.; De Bruyn, H. “Peri-Implantitis”: A Complication of a Foreign Body or a Man-Made “Disease”. Facts and Fiction. Clin. Implant. Dent. Relat. Res. 2016, 18, 840–849. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin. Implant. Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef]
- Derks, J.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Larsson, M.; Berglundh, T. Effectiveness of implant therapy analyzed in a Swedish population: Early and late implant loss. J. Dent. Res. 2015, 94, 44S–51S. [Google Scholar] [CrossRef] [Green Version]
- Becker, S.T.; Beck-Broichsitter, B.E.; Graetz, C.; Dörfer, C.E.; Wiltfang, J.; Häsler, R. Peri-implantitis versus periodontitis: Functional differences indicated by transcriptome profiling. Clin. Implant Dent. Relat. Res. 2014, 16, 401–411. [Google Scholar] [CrossRef]
- Xu, L.; Yu, Z.; Lee, H.M.; Wolff, M.S.; Golub, L.M.; Sorsa, T.; Kuula, H. Characteristics of collagenase-2 from gingival crevicular fluid and peri-implant sulcular fluid in periodontitis and peri-implantitis patients: Pilot study. Acta Odontol. Scand. 2008, 66, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Dahlin, C.; Jemt, T.; Sennerby, L.; Turri, A.; Wennerberg, A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin. Implant Dent. Relat. Res. 2014, 16, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Holliday, L.S.; Welgus, H.G.; Fliszar, C.J.; Veith, G.M.; Jeffrey, J.J.; Gluck, S.L. Initiation of osteoclast bone resorption by interstitial collagenase. J. Biol. Chem. 1997, 272, 22053–22058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Collin-Osdoby, P.; Osdoby, P. SDF-1 increases recruitment of osteoclast precursors by upregulation of matrix metalloproteinase activity. Connect. Tis. Res. 2003, 44 (Suppl. 1), 79–84. [Google Scholar] [CrossRef]
- Imai, S.; Konttinen, Y.T.; Jumppanen, M.; Lindy, O.; Ceponis, A.; Kemppinen, P.; Sorsa, T.; Santavirta, S.; Xu, J.W.; Lopéz-Otín, C. High levels of expression of collagenase-3 (MMP-13) in pathological conditions associated with a foreign-body reaction. J. Bone Jt. Surg. Br. 1998, 80, 701–710. [Google Scholar] [CrossRef]
- Albrektsson, T.; Buser, D.; Chen, S.T.; Cochran, D.; Debruyn, H.; Jemt, T.; Koka, S.; Nevins, M.; Sennerby, L.; Simion, M.; et al. Statements from the Estepona Consensus Meeting on Peri-implantitis, February 2–4, 2012. Clin. Implant. Dent. Relat. Res. 2012, 14, 781–782. [Google Scholar] [CrossRef]


| Factors | Level | n | % |
|---|---|---|---|
| Location | Right mandible | 16 | 20 |
| Left mandible | 22 | 27.5 | |
| Right mandible | 24 | 30 | |
| Right maxilla | 18 | 22.5 | |
| Collar surface | LM | 41 | 51.2 |
| M | 39 | 48.8 | |
| Gender | Female | 52 | 65 |
| Male | 38 | 35 | |
| Alcohol | No | 72 | 90 |
| Yes | 8 | 10 | |
| Smoking | No | 60 | 75 |
| Yes | 20 | 25 | |
| Plaque Index | 0 | 3 | 3.7 |
| 1 | 48 | 60 | |
| 2 | 24 | 30 | |
| 3 | 5 | 6.3 | |
| PD | No | 11 | 13.7 |
| Yes | 69 | 86.3 | |
| BoP | No | 68 | 85 |
| At least 1 site | 12 | 15 |
| Mean (Median) | Standard Error of the Mean | Range | ||
|---|---|---|---|---|
| Age | 54.6 (53) | 0.42 | 29 | 74 |
| Smoking | 14.6 (11.5) | 0.47 | 0 | 30 |
| Implant diameter | 3.95 (4) | 0.02 | 3.8 | 4.6 |
| Implant length | 10.6 | 0.06 | 9.5 | 12 |
| MBL (mm) at T0 | 0.29 (0.21) | 0.018 | 0.2 | 0.6 |
| MBL (mm) at T6 | 0.95 (0.53) | 0.075 | 0.2 | 2.4 |
| MBL (mm) at T24 | 1.4 (1.22) | 0.10 | 0.2 | 3.2 |
| T0 | T6 | T24 | ||
|---|---|---|---|---|
| BLT | Low High | 0.041 (0.058 (0.03) 0.058 (0.03) [0.02] | 0.097 (0.04) [0.02] 0.192 (0.07) [0.12] | 0.014 (0.02) [0.01] 0.042 (0.02) [0.02] |
| Collar surface | LM M | 0.066 (0.01) [0] 0.112 (0.04) [0.08] | 0.082 (0.03) [0.05] 0.253 (0.18) [0.14] | 0.044 (0.02) [0.02] 0.063 (0.03) [0.03] |
| PD | No Yes | 0.031 (0.00) [0.00] 0.052 (0.00) [0.00] | 0.058 (0.00) [0.02] 0.074 (0.01) [0.01] | 0.037 (0.03) [0.02] 0.055 (0.019 [0.02] |
| BoP | No Yes * | 0.011 (0.00) [0.00] 0.064 (0.02) [0.01] | 0.097 (0.04) [0.02] 0.134 (0.03) [0.02] | 0.073 (0.029 [0.03] 0.65 (0.03) [0.03] |
| Smoking * | 0.094 (0.06) 0.00] | 0.134 (0.03) [0.02] | 0.65 (0.03) [0.03] | |
| Gender | Female Male | 0.038 (0.00) [0.00] 0.039 (0.00) [0.001] | 0.065 (0.00) [0.01] 0.061 (0.01) [0.01] | 0.029 (001) [0.01] 0.031 (0.00) [0.01] |
| Age * | 0.066 (0.03) | 0.021 (0.03) | 0.54 (0.03) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarnieri, R.; Zanza, A.; D’Angelo, M.; Di Nardo, D.; Del Giudice, A.; Mazzoni, A.; Reda, R.; Testarelli, L. Correlation between Peri-Implant Marginal Bone Loss Progression and Peri-Implant Sulcular Fluid Levels of Metalloproteinase-8. J. Pers. Med. 2022, 12, 58. https://doi.org/10.3390/jpm12010058
Guarnieri R, Zanza A, D’Angelo M, Di Nardo D, Del Giudice A, Mazzoni A, Reda R, Testarelli L. Correlation between Peri-Implant Marginal Bone Loss Progression and Peri-Implant Sulcular Fluid Levels of Metalloproteinase-8. Journal of Personalized Medicine. 2022; 12(1):58. https://doi.org/10.3390/jpm12010058
Chicago/Turabian StyleGuarnieri, Renzo, Alessio Zanza, Maurilio D’Angelo, Dario Di Nardo, Andrea Del Giudice, Alessandro Mazzoni, Rodolfo Reda, and Luca Testarelli. 2022. "Correlation between Peri-Implant Marginal Bone Loss Progression and Peri-Implant Sulcular Fluid Levels of Metalloproteinase-8" Journal of Personalized Medicine 12, no. 1: 58. https://doi.org/10.3390/jpm12010058
APA StyleGuarnieri, R., Zanza, A., D’Angelo, M., Di Nardo, D., Del Giudice, A., Mazzoni, A., Reda, R., & Testarelli, L. (2022). Correlation between Peri-Implant Marginal Bone Loss Progression and Peri-Implant Sulcular Fluid Levels of Metalloproteinase-8. Journal of Personalized Medicine, 12(1), 58. https://doi.org/10.3390/jpm12010058










