Cognitive Reserve in Early Manifest Huntington Disease Patients: Leisure Time Is Associated with Lower Cognitive and Functional Impairment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical Measures
2.3. Cognitive Reserve
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Correlation between CR, Clinical and Cognitive Scores
3.3. Comparison between Groups with Impaired and Normal Cognitive Reserve Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Luca, A.; Morella, A.; Consoli, F.; Fanelli, S.; Thibert, J.R.; Statt, S.; Latham, G.J.; Squitieri, F. A Novel Triplet-Primed PCR Assay to Detect the Full Range of Trinucleotide CAG Repeats in the Huntingtin Gene (HTT). Int. J. Mol. Sci. 2021, 22, 1689. [Google Scholar] [CrossRef] [PubMed]
- Migliore, S.; Jankovic, J.; Squitieri, F. Genetic Counseling in Huntington’s Disease: Potential New Challenges on Horizon? Front. Neurol. 2019, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Trinkler, I.; Cleret de Langavant, L.; Bachoud-Lévi, A.C. Joint recognition-expression impairment of facial emotions in Huntington’s disease despite intact understanding of feelings. Cortex 2013, 49, 549–558. [Google Scholar] [CrossRef]
- Glikmann-Johnston, Y.; Mercieca, E.C.; Carmichael, A.M.; Alexander, B.; Harding, I.H.; Stout, J.C. Hippocampal and striatal volumes correlate with spatial memory impairment in Huntington’s disease. J. Neurosci. Res. 2021, 99, 2948–2963. [Google Scholar] [CrossRef]
- Migliore, S.; D’Aurizio, G.; Curcio, G.; Squitieri, F. Task-switching abilities in pre-manifest Huntington’s disease subjects. Parkinsonism Relat. Disord. 2019, 60, 111–117. [Google Scholar] [CrossRef]
- Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef] [PubMed]
- Barulli, D.; Stern, Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn. Sci. 2013, 17, 502–509. [Google Scholar] [CrossRef] [Green Version]
- McDowell, I.; Xi, G.; Lindsay, J.; Tierney, M. Mapping the connections between education and dementia. J. Clin. Exp. Neuropsychol. 2007, 29, 127–141. [Google Scholar] [CrossRef]
- Staff, R.T.; Murray, A.D.; Deary, I.J.; Whalley, L.J. What provides cerebral reserve? Brain 2004, 127, 1191–1199. [Google Scholar] [CrossRef]
- Solé-Padullés, C.; Bartrés-Faz, D.; Junqué, C.; Vendrell, P.; Rami, L.; Clemente, I.C.; Bosch, B.; Villar, A.; Bargalló, N.; Jurado, M.A.; et al. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2009, 30, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Hindle, J.V.; Martyr, A.; Clare, L. Cognitive reserve in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat. Disord. 2014, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sumowski, J.F.; Leavitt, V.M. Cognitive reserve in multiple sclerosis. Mult. Scler. J. 2013, 19, 1122–1127. [Google Scholar] [CrossRef]
- Elkins, J.S.; Longstreth, W.T., Jr.; Manolio, T.A.; Newman, A.B.; Bhadelia, R.A.; Johnston, S.C. Education and the cognitive decline associated with MRI-defined brain infarct. Neurology 2006, 67, 435–440. [Google Scholar] [CrossRef]
- Kesler, S.R.; Adams, H.F.; Blasey, C.M.; Bigler, E.D. Premorbid intellectual functioning, education, and brain size in traumatic brain injury: An investigation of the cognitive reserve hypothesis. Appl. Neuropsychol. 2003, 10, 153–162. [Google Scholar] [CrossRef]
- Dufouil, C.; Alpérovitch, A.; Tzourio, C. Influence of education on the relationship between white matter lesions and cognition. Neurology 2003, 60, 831–836. [Google Scholar] [CrossRef]
- Andrews, S.C.; Domínguez, J.F.; Mercieca, E.C.; Georgiou-Karistianis, N.; Stout, J.C. Cognitive interventions to enhance neural compensation in Huntington’s disease. Neurodegener. Dis. Manag. 2015, 5, 155–164. [Google Scholar] [CrossRef]
- van Dellen, A.; Blakemore, C.; Deacon, R.; York, D.; Hannan, A.J. Delaying the onset of Huntington’s in mice. Nature 2000, 404, 721–722. [Google Scholar] [CrossRef]
- Curtin, P.C.; Farrar, A.M.; Oakeshott, S.; Sutphen, J.; Berger, J.; Mazzella, M.; Cox, K.; He, D.; Alosio, W.; Park, L.C.; et al. Cognitive Training at a Young Age Attenuates Deficits in the zQ175 Mouse Model of HD. Front. Behav. Neurosci. 2016, 9, 361. [Google Scholar] [CrossRef] [Green Version]
- Pang, T.; Stam, N.C.; Nithianantharajah, J.; Howard, M.L.; Hannan, A.J. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington’s disease transgenic mice. Neuroscience 2006, 141, 569–584. [Google Scholar] [CrossRef]
- Bonner-Jackson, A.; Long, J.D.; Westervelt, H.; Tremont, G.; Aylward, E.; Paulsen, J.S.; PREDICT-HD Investigators and Coordinators of the Huntington Study Group (2013). Cognitive reserve and brain reserve in prodromal Huntington’s disease. J. Int. Neuropsychol. Soc. 2013, 19, 739–750. [Google Scholar] [CrossRef]
- Garcia-Gorro, C.; Garau-Rolandi, M.; Escrichs, A.; Rodriguez-Dechicha, N.; Vaquer, I.; Subira, S.; Calopa, M.; Martinez-Horta, S.; Perez-Perez, J.; Kulisevsky, J.; et al. An active cognitive lifestyle as a potential neuroprotective factor in Huntington’s disease. Neuropsychologia 2019, 122, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Soloveva, M.V.; Jamadar, S.D.; Poudel, G.; Georgiou-Karistianis, N. A critical review of brain and cognitive reserve in Huntington’s disease. Neurosci. Biobehav. Rev. 2018, 88, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Cain, K.K.; Flanigan, J.L.; Dalrymple, W.A.; Patrie, J.; Harrison, M.B.; Barrett, M.J. The Effect of Education on Symptom Onset and Severity of Huntington’s Disease. Mov. Disord. Clin. Pract. 2021, 8, 555–562. [Google Scholar] [CrossRef] [PubMed]
- López-Sendón, J.L.; Royuela, A.; Trigo, P.; Orth, M.; Lange, H.; Reilmann, R.; Keylock, J.; Rickards, H.; Piacentini, S.; Squitieri, F.; et al. European HD Network. What is the impact of education on Huntington’s disease? Mov. Disord. 2011, 26, 1489–1495. [Google Scholar] [CrossRef]
- Borroni, B.; Premi, E.; Bozzali, M.; Padovani, A. Reserve mechanisms in neurodegenerative diseases: From bench to bedside and back again. Curr. Med. Chem. 2012, 19, 6112–6118. [Google Scholar] [CrossRef]
- Papoutsi, M.; Labuschagne, I.; Tabrizi, S.J.; Stout, J.C. The cognitive burden in Huntington’s disease: Pathology, phenotype, and mechanisms of compensation. Mov. Disord. 2014, 29, 673–683. [Google Scholar] [CrossRef]
- Landwehrmeyer, G.B.; Fitzer-Attas, C.J.; Giuliano, J.D.; Gonçalves, N.; Anderson, K.E.; Cardoso, F.; Ferreira, J.J.; Mestre, T.A.; Stout, J.C.; Sampaio, C. Data Analytics from Enroll-HD, a Global Clinical Research Platform for Huntington’s Disease. Mov. Disord. Clin. Pract. 2016, 4, 212–224. [Google Scholar] [CrossRef] [Green Version]
- Kremer, H.P.H.; Hungtington Study Group. Unified Huntington’s Disease Rating Scale: Reliability and consistency. Huntington Study Group. Mov. Disord. 1996, 11, 136–142. [Google Scholar] [CrossRef]
- Marder, K.; Zhao, H.; Myers, R.H.; Cudkowicz, M.; Kayson, E.; Kieburtz, K.; Orme, C.; Paulsen, J.; Penney, J.B., Jr.; Siemers, E.; et al. Rate of functional decline in Huntington’s disease. Huntington Study Group. Neurology 2000, 54, 452–458. [Google Scholar] [CrossRef]
- Schobel, S.A.; Palermo, G.; Auinger, P.; Long, J.D.; Ma, S.; Khwaja, O.S.; Trundell, D.; Cudkowicz, M.; Hersch, S.; Sampaio, C.; et al. TRACK-HD, COHORT, CARE-HD, and 2CARE Huntington Study Group Investigators. Motor, cognitive, and functional declines contribute to a single progressive factor in early HD. Neurology 2017, 89, 2495–2502. [Google Scholar] [CrossRef]
- Nucci, M.; Mapelli, D.; Mondini, S. Cognitive Reserve Index questionnaire (CRIq): A new instrument for measuring cognitive reserve. Aging Clin. Exp. Res. 2012, 24, 218–226. [Google Scholar] [CrossRef]
- Martínez-Horta, S.; Moreu, A.; Perez-Perez, J.; Sampedro, F.; Horta-Barba, A.; Pagonabarraga, J.; Gomez-Anson, B.; Lozano-Martinez, G.A.; Lopez-Mora, D.A.; Camacho, V.; et al. The impact of bilingualism on brain structure and function in Huntington’s disease. Parkinsonism Relat. Disord. 2019, 60, 92–97. [Google Scholar] [CrossRef]
- Nithianantharajah, J.; Hannan, A.J. Mechanisms mediating brain and cognitive reserve: Experience-dependent neuroprotection and functional compensation in animal models of neurodegenerative diseases. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 331–339. [Google Scholar] [CrossRef]
- Cruickshank, T.; Bartlett, D.; Govus, A.; Hannan, A.; Teo, W.P.; Mason, S.; Lo, J.; Ziman, M. The relationship between lifestyle and serum neurofilament light protein in Huntington’s disease. Brain Behav. 2020, 10, e01578. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.P.; Kang, J.M.; Kim, W.H.; Maeng, S.; Cho, S.E.; Na, K.S.; Oh, S.H.; Park, J.W.; Cho, S.J.; et al. Cognitive reserve and the effects of virtual reality-based cognitive training on elderly individuals with mild cognitive impairment and normal cognition. Psychogeriatrics 2021, 21, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kang, J.M.; Kim, D.J.; Woo, S.K.; Lee, J.Y.; Cho, S.J. Cognitive Reserve, Leisure Activity, and Neuropsychological Profile in the Early Stage of Cognitive Decline. Front. Aging Neurosci. 2020, 12, 590607. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.M.; Stern, Y. Cognitive reserve in aging. Curr. Alzheimer Res. 2011, 8, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Bennett, D.A.; Wilson, R.S.; Schneider, J.A.; Evans, D.A.; Mendes de Leon, C.F.; Arnold, S.E.; Barnes, L.L.; Bienias, J.L. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 2003, 60, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Sumowski, J.F.; Rocca, M.A.; Leavitt, V.M.; Riccitelli, G.; Comi, G.; DeLuca, J.; Filippi, M. Brain reserve and cognitive reserve in multiple sclerosis: What you’ve got and how you use it. Neurology 2013, 80, 2186–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neubauer, A.C.; Grabner, R.H.; Fink, A.; Neuper, C. Intelligence and neural efficiency: Further evidence of the influence of task content and sex on the brain-IQ relationship. Brain Res. Cogn. Brain Res. 2005, 25, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Berchtold, N.C. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002, 25, 295–301. [Google Scholar] [CrossRef]
| Early Manifest HD Cohort | |
|---|---|
| Sample Number | 75 |
| Male-Female | 47-28 |
| Age at baseline (range) | 47.2 ± 12.5 (27–78) |
| Education level in years (range) | 11.65 ± 4.6 (8–18) |
| Age at motor onset (range) | 47.5 ± 12.3 (22–70) |
| CAG repeat number (range) | 43.7 ± 2.3 (40–49) |
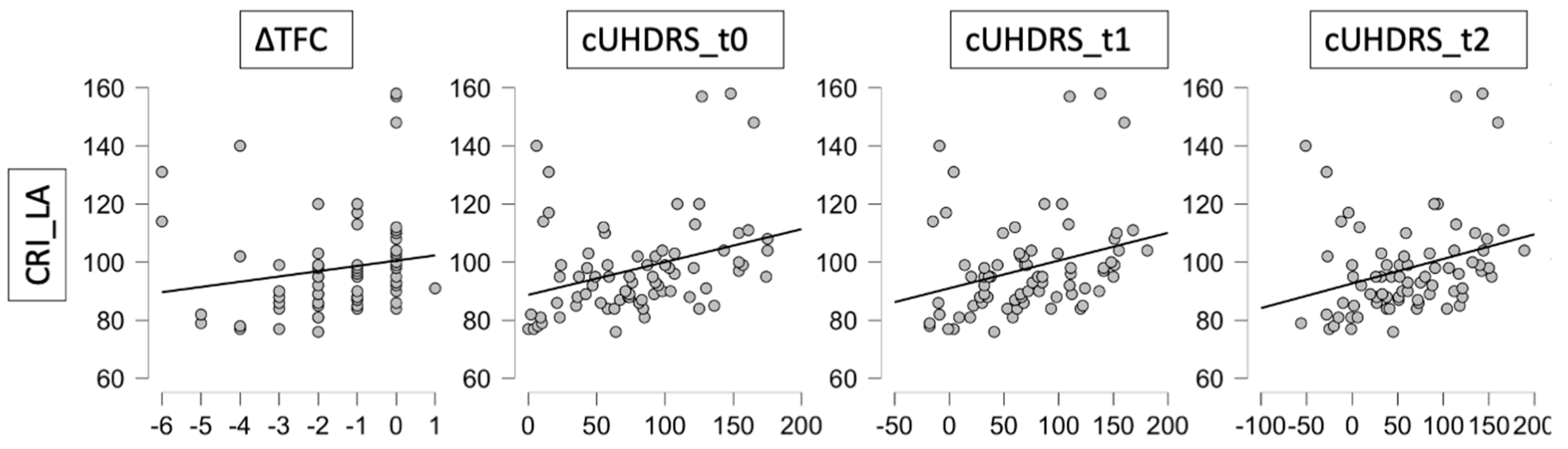
| Age of Onset | ∆TFC | ∆TMS | cUHDRS_t0 | cUHDRS_t1 | cUHDRS_t2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | |
| CRIq_Edu | 0.156 | 0.181 | 0.036 | 0.758 | −0.104 | 0.375 | 0.049 | 0.690 | 0.029 | 0.805 | 0.018 | 0.878 |
| CRIq_WA | 0.026 | 0.824 | 0.058 | 0.619 | −0.141 | 0.228 | 0.058 | 0.624 | 0.078 | 0.508 | 0.065 | 0.580 |
| CRIq_LA | 0.095 | 0.418 | 0.242 | 0.037 | −0.150 | 0.200 | 0.349 | 0.002 | 0.332 | 0.004 | 0.344 | 0.003 |
| CRIq_Tot | 0.170 | 0.146 | 0.117 | 0.316 | −0.117 | 0.319 | 0.191 | 0.100 | 0.179 | 0.124 | 0.149 | 0.203 |
| (a) | ||||||||||
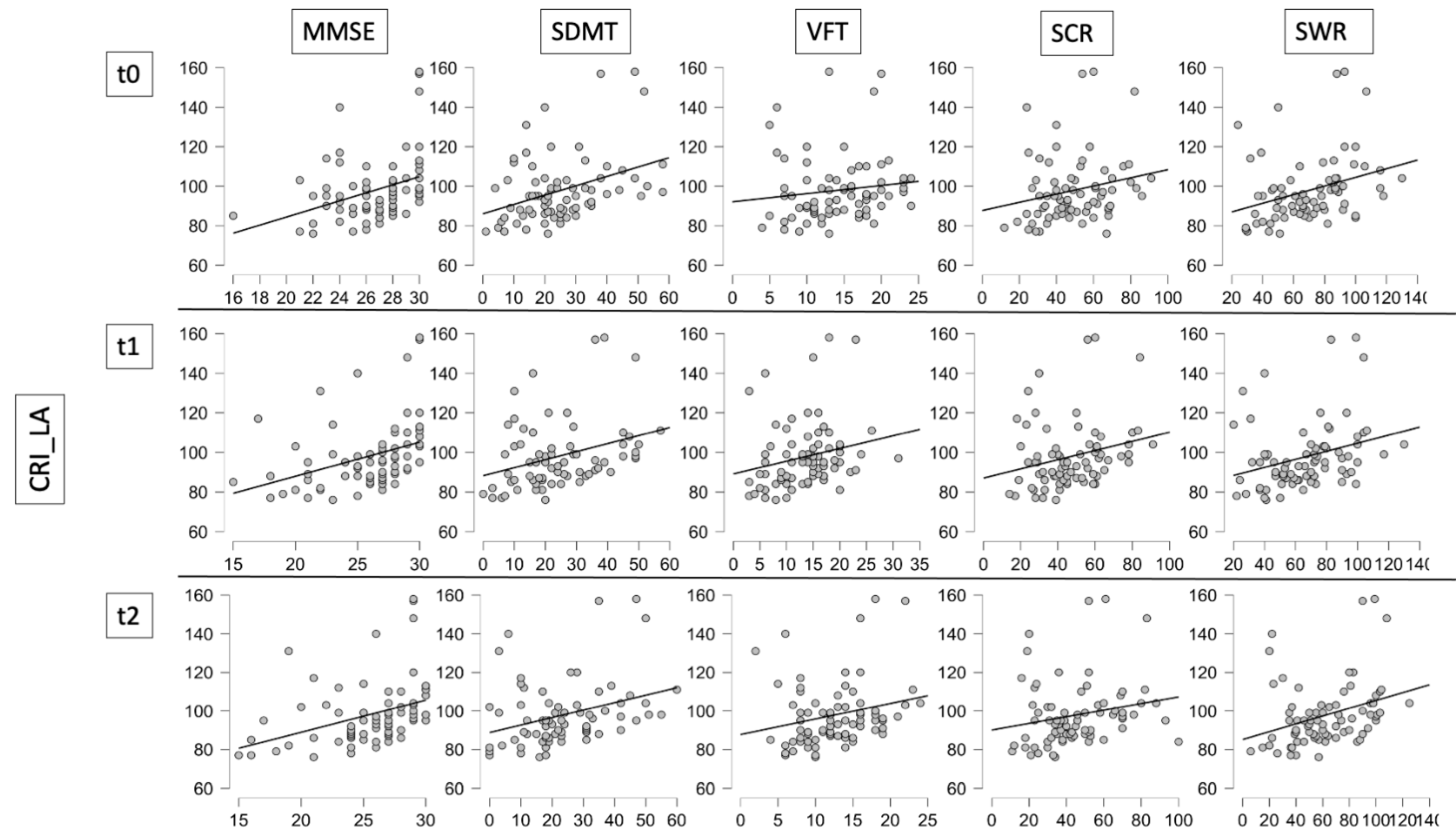
| MMSE | SDMT | VFT | SCR | SWR | ||||||
| r | p | r | p | r | p | r | p | r | p | |
| CRIq_Edu | 0.206 | 0.082 | 0.026 | 0.825 | 0.080 | 0.494 | 0.019 | 0.870 | 0.117 | 0.319 |
| CRIq_WA | 0.146 | 0.220 | 0.137 | 0.242 | −0.160 | 0.171 | −0.016 | 0.892 | 0.079 | 0.500 |
| CRIq_LA | 0.340 | 0.003 | 0.392 | 0.001 | 0.240 | 0.036 | 0.280 | 0.015 | 0.348 | 0.002 |
| CRIq_Tot | 0.294 | 0.012 | 0.239 | 0.039 | 0.025 | 0.831 | 0.100 | 0.393 | 0.220 | 0.058 |
| (b) | ||||||||||
| MMSE | SDMT | VFT | SCR | SWR | ||||||
| r | p | r | p | r | p | r | p | r | p | |
| CRIq_Edu | 0.205 | 0.083 | −0.005 | 0.970 | 0.106 | 0.365 | 0.071 | 0.547 | 0.075 | 0.522 |
| CRIq_WA | 0.192 | 0.098 | 0.114 | 0.337 | 0.027 | 0.820 | 0.064 | 0.588 | 0.082 | 0.485 |
| CRIq_LA | 0.396 | <0.001 | 0.359 | 0.002 | 0.265 | 0.022 | 0.294 | 0.010 | 0.330 | 0.004 |
| CRIq_Tot | 0.337 | 0.003 | 0.192 | 0.103 | 0.156 | 0.183 | 0.171 | 0.142 | 0.200 | 0.085 |
| (c) | ||||||||||
| MMSE | SDMT | VFT | SCR | SWR | ||||||
| r | p | r | p | r | p | r | p | r | p | |
| CRIq_Edu | 0.193 | 0.100 | −0.021 | 0.860 | 0.082 | 0.486 | −0.001 | 0.991 | 0.046 | 0.694 |
| CRIq_WA | 0.205 | 0.080 | 0.087 | 0.456 | 0.069 | 0.554 | 0.037 | 0.750 | 0.079 | 0.499 |
| CRIq_LA | 0.404 | <0.001 | 0.354 | 0.002 | 0.252 | 0.029 | 0.253 | 0.028 | 0.374 | 0.001 |
| CRIq_Tot | 0.326 | 0.005 | 0.178 | 0.128 | 0.167 | 0.153 | 0.113 | 0.334 | 0.200 | 0.085 |
| CRI_LA Impaired Group (n = 24) Mean ± SE | CRI_LA Normal Group (n = 51) Mean ± SE | p | ||
|---|---|---|---|---|
| Clinical variables | ∆_TFC | −2.08 ± 0.29 | −1.2 ± 0.21 | 0.017 |
| ∆_TMS | 9.79 ± 1.94 | 8.25 ± 1.24 | 0.496 | |
| cUHDRS_t0 | 54.34 ± 8.23 | 92.28 ± 6.69 | 0.001 | |
| cUHDRS_t1 | 41.09 ± 8.41 | 85.48 ± 7.14 | <0.001 | |
| cUHDRS_t2 | 30.09 ± 9.64 | 75.50 ± 8.13 | <0.001 | |
| Cognitive variables - Baseline | MMSE | 25.34 ± 0.60 | 26.98 ± 0.37 | 0.019 |
| SDMT | 17.54 ± 1.70 | 28.58 ± 1.92 | 0.001 | |
| VFT | 11.87 ± 0.84 | 14.94 ± 0.75 | 0.016 | |
| SCR | 41.41 ± 3.19 | 52.88 ± 2.42 | 0.007 | |
| SWR | 58.75 ± 4.39 | 75.02 ± 3.35 | 0.006 | |
| Cognitive variables - 1 year follow-up | MMSE | 23.62 ± 0.74 | 26.78 ± 0.41 | <0.001 |
| SDMT | 16.04 ± 1.84 | 28.06 ± 1.91 | <0.001 | |
| VFT | 10.7 ± 1 | 14.94 ± 0.8 | 0.003 | |
| SCR | 39.25 ± 2.71 | 50.17 ± 2.63 | 0.013 | |
| SWR | 54.16 ± 3.92 | 72.6 ± 3.49 | 0.002 | |
| Cognitive variables - 2 years follow-up | MMSE | 23.08 ± 0.8 | 26.56 ± 0.41 | <0.001 |
| SDMT | 15.5 ± 1.81 | 27.12 ± 2.02 | 0.001 | |
| VFT | 10.29 ± 0.74 | 13.58 ± 0.69 | 0.005 | |
| SCR | 36.75 ± 3.81 | 49.17 ± 2.78 | 0.012 | |
| SWR | 48.42 ± 4.58 | 69.16± 3.73 | 0.002 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliore, S.; D’Aurizio, G.; Scaricamazza, E.; Maffi, S.; Ceccarelli, C.; Ristori, G.; Romano, S.; Castaldo, A.; Fichera, M.; Curcio, G.; et al. Cognitive Reserve in Early Manifest Huntington Disease Patients: Leisure Time Is Associated with Lower Cognitive and Functional Impairment. J. Pers. Med. 2022, 12, 36. https://doi.org/10.3390/jpm12010036
Migliore S, D’Aurizio G, Scaricamazza E, Maffi S, Ceccarelli C, Ristori G, Romano S, Castaldo A, Fichera M, Curcio G, et al. Cognitive Reserve in Early Manifest Huntington Disease Patients: Leisure Time Is Associated with Lower Cognitive and Functional Impairment. Journal of Personalized Medicine. 2022; 12(1):36. https://doi.org/10.3390/jpm12010036
Chicago/Turabian StyleMigliore, Simone, Giulia D’Aurizio, Eugenia Scaricamazza, Sabrina Maffi, Consuelo Ceccarelli, Giovanni Ristori, Silvia Romano, Anna Castaldo, Mario Fichera, Giuseppe Curcio, and et al. 2022. "Cognitive Reserve in Early Manifest Huntington Disease Patients: Leisure Time Is Associated with Lower Cognitive and Functional Impairment" Journal of Personalized Medicine 12, no. 1: 36. https://doi.org/10.3390/jpm12010036
APA StyleMigliore, S., D’Aurizio, G., Scaricamazza, E., Maffi, S., Ceccarelli, C., Ristori, G., Romano, S., Castaldo, A., Fichera, M., Curcio, G., & Squitieri, F. (2022). Cognitive Reserve in Early Manifest Huntington Disease Patients: Leisure Time Is Associated with Lower Cognitive and Functional Impairment. Journal of Personalized Medicine, 12(1), 36. https://doi.org/10.3390/jpm12010036








