Quantifying Left Atrial Size in the Context of Atrial Fibrillation Ablation: Which Echocardiographic Method Correlates to Outcome of Pulmonary Venous Isolation?
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Echocardiography
2.3. Ablation Procedure
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Procedural Findings
3.3. Follow-Up
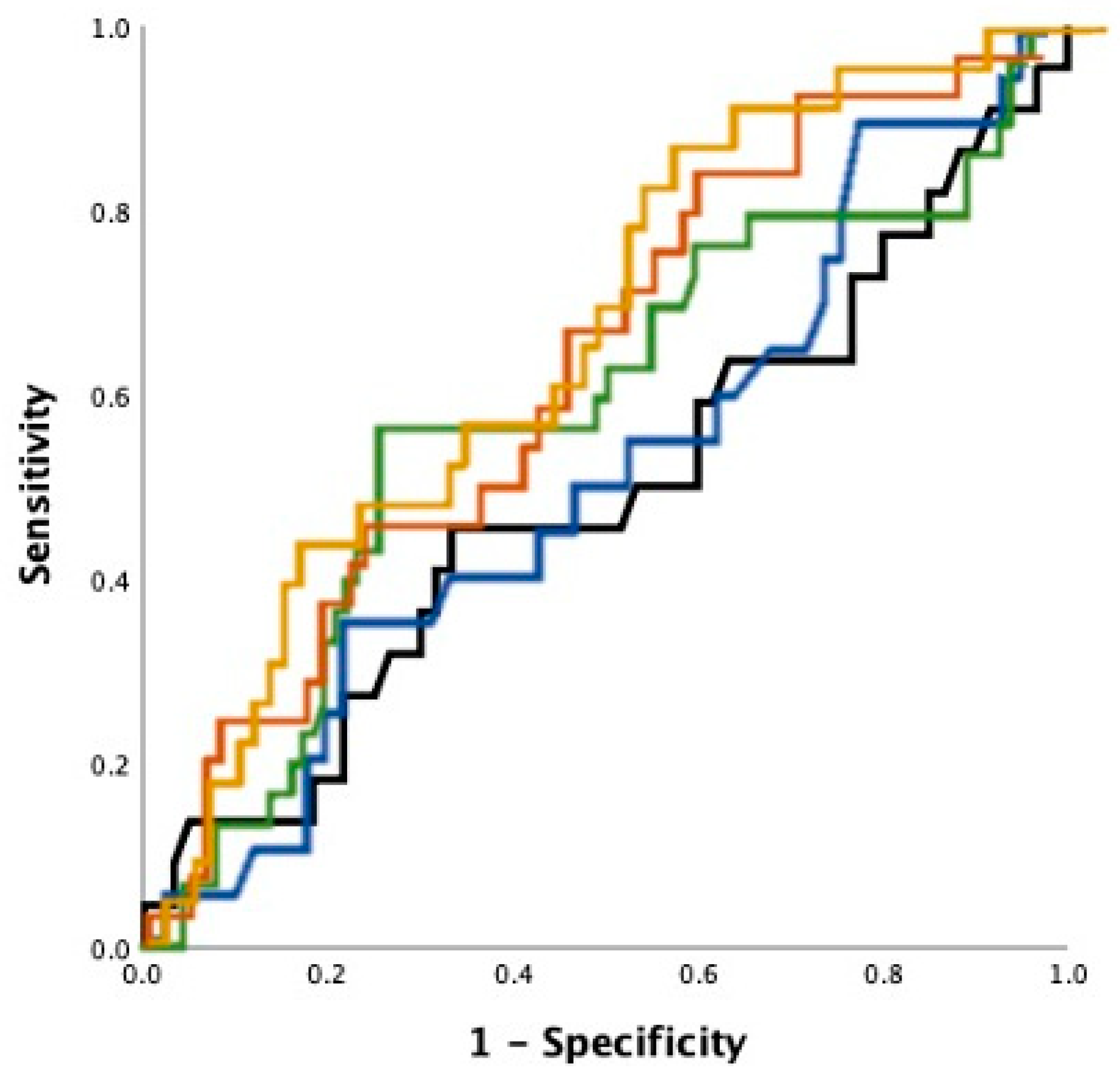
3.4. Echocardiography as Outcome Predictor
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, E.P.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Wyse, D.G.; Waldo, A.L.; DiMarco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; Kellen, J.C.; Greene, H.L.; Mickel, M.C.; Dalquist, J.E.; et al. A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Wang, Y.; Tang, K.; Li, X.; Peng, W.; Liang, C.; Xu, Y. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: A systematic review and meta-analysis of observational studies. Europace 2011, 14, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Leitz, P.; Mönnig, G.; Güner, F.; Dechering, D.G.; Wasmer, K.; Reinke, F.; Lange, P.S.; Eckardt, L.; Frommeyer, G. Comparing learning curves of two established “single-shot” devices for ablation of atrial fibrillation. J. Interv. Card. Electrophysiol. 2018, 53, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Knight, B.P.; Novak, G.P.; Sangrigoli, R.; Champagne, J.; Dubuc, M.; Adler, W.S.; Svinarich, J.T.; Essebag, V.; Hokanson, R.; Kueffer, F.; et al. Long-term outcomes after ablation for paroxysmal atrial fibrillation using the second-generation cryoballoon: Final results from STOP AF post-approval study. JACC Clin. Electrophysiol. 2019, 5, 306–314. [Google Scholar] [PubMed]
- Mor-Avi, V.; Yodwut, C.; Jenkins, C.; Kühl, H.; Nesser, H.-J.; Marwick, T.H.; Franke, A.; Weinert, L.; Niel, J.; Steringer-Mascherbauer, R.; et al. Real-time 3D echocardiographic quantification of left atrial volume: Multicenter study for validation with CMR. JACC Cardiovasc. Imaging 2012, 5, 769–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balk, E.M.; Garlitski, A.C.; Alsheikh-Ali, A.A.; Terasawa, T.; Chung, M.; Ip, S. Predictors of Atrial Fibrillation Recurrence After Radiofrequency Catheter Ablation: A Systematic Review. J. Cardiovasc. Electrophysiol. 2010, 21, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Aurigemma, G.P.; Gottdiener, J.S.; Arnold, A.M.; Chinali, M.; Hill, J.C.; Dalane Kitzman, D. Left atrial volume and geometry in healthy aging: The Cardiovascular Health Study. Circ. Cardiovasc. Imaging 2009, 2, 282–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zacà, V.; Galderisi, M.; Mondillo, S.; Focardi, M.; Ballo, P.; Guerrini, F. Left atrial enlargement as a predictor of recurrences in lone paroxysmal atrial fibrillation. Can. J. Cardiol. 2007, 23, 869–872. [Google Scholar] [CrossRef] [Green Version]
- Suarez, G.S.; Lampert, S.; Ravid, S.; Lown, B. Changes in left atrial size in patients with lone atrial fibrillation. Clin. Cardiol. 1991, 14, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, C.; Olivo, G.; Comeglio, M.; Bertini, G.; Gensini, G.F.; Galanti, G. Left atrial size changes in patients with paroxysmal lone atrial fibrillation. An echocardiographic follow-up. Angiology 1996, 47, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Straube, F.; Wegscheider, K.; Kuniss, M.; Andresen, D.; Wu, L.-Q.; Tebbenjohanns, J.; Noelker, G.; Tilz, R.R.; Chun, J.K.R.; et al. Outcomes of cryoballoon or radiofrequency ablation in symptomatic paroxysmal or persistent atrial fibrillation. EP Eur. 2019, 21, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Njoku, A.; Kannabhiran, M.; Arora, R.; Reddy, P.; Gopinathannair, R.; Lakkireddy, D.; Dominic, P. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: A meta-analysis. EP Eur. 2018, 20, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Forleo, G.B.; Tondo, C.; De Luca, L.; Russo, A.D.; Casella, M.; De Sanctis, V.; Clementi, F.; Fagundes, R.L.; Leo, R.; Romeo, F.; et al. Gender-related differences in catheter ablation of atrial fibrillation. Europace 2007, 9, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Mohanty, P.; Di Biase, L.; Sanchez, J.E.; Shaheen, M.H.; Burkhardt, J.D.; Bassouni, M.; Cummings, J.; Wang, Y.; Lewis, W.R.; et al. Outcomes and complications of catheter ablation for atrial fibrillation in females. Heart Rhythm 2010, 7, 167–172. [Google Scholar] [CrossRef] [PubMed]

| Complete Collective | Patients Free from AF at 12 Month FU (n = 93) | Patients with AF Recurrence at 12 Month FU (n = 38) | p-Values | |
|---|---|---|---|---|
| Age (Years) | 61 ± 12 | 60 ± 12 | 62 ± 9 | 0.5 |
| Persistent AF (%) | 40% | 30% | 45% | 0.38 |
| CHA2DS2-Vasc Score | 1.6 ± 1.3 | 1.6 ± 1.4 | 1.8 ± 1.2 | 0.29 |
| Diabetes (%) | 8% | 10% | 3% | 0.27 |
| Arterial Hypertension (%) | 51% | 52% | 58% | 0.59 |
| Prior Stroke (%) | 9% | 8% | 3% | 0.34 |
| BMI (kg/m2) | 26 ± 3 | 27 ± 3 | 25 ± 2 | 0.1 |
| EHRA Class | 2.5 ± 0.5 | 2.5 ± 0.5 | 2.7 ± 0.4 | 0.01 |
| Female gender | n = 48 | n = 26 | n = 22 | 0.01 |
| Univariate OR | Univariate p | Multivariate OR | Multivariate p | C Statistic | |
|---|---|---|---|---|---|
| PLAX | 1.05/10 cm | 0.91 | 1.7/10 cm | 0.2 | 0.51 |
| Area 2CH | 1.4/10 cm2 | 0.28 | 1.9/10 cm2 | 0.05 | 0.59 |
| Area 4CH | 1.3/10 cm2 | 0.48 | 1.9/10 cm2 | 0.07 | 0.57 |
| LAV | 1.1/10ml | 0.19 | 1.3/10ml | 0.02 | 0.61 |
| LAVI | 1.4/10 mL/m2 | 0.03 | 1.58/10 mL/m2 | 0.02 | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitz, P.; Stebel, L.M.; Andresen, C.; Ellermann, C.; Güner, F.; Reinke, F.; Kochhäuser, S.; Frommeyer, G.; Köbe, J.; Wasmer, K.; et al. Quantifying Left Atrial Size in the Context of Atrial Fibrillation Ablation: Which Echocardiographic Method Correlates to Outcome of Pulmonary Venous Isolation? J. Pers. Med. 2021, 11, 913. https://doi.org/10.3390/jpm11090913
Leitz P, Stebel LM, Andresen C, Ellermann C, Güner F, Reinke F, Kochhäuser S, Frommeyer G, Köbe J, Wasmer K, et al. Quantifying Left Atrial Size in the Context of Atrial Fibrillation Ablation: Which Echocardiographic Method Correlates to Outcome of Pulmonary Venous Isolation? Journal of Personalized Medicine. 2021; 11(9):913. https://doi.org/10.3390/jpm11090913
Chicago/Turabian StyleLeitz, Patrick, Lena Marie Stebel, Christian Andresen, Christian Ellermann, Fatih Güner, Florian Reinke, Simon Kochhäuser, Gerrit Frommeyer, Julia Köbe, Kristina Wasmer, and et al. 2021. "Quantifying Left Atrial Size in the Context of Atrial Fibrillation Ablation: Which Echocardiographic Method Correlates to Outcome of Pulmonary Venous Isolation?" Journal of Personalized Medicine 11, no. 9: 913. https://doi.org/10.3390/jpm11090913
APA StyleLeitz, P., Stebel, L. M., Andresen, C., Ellermann, C., Güner, F., Reinke, F., Kochhäuser, S., Frommeyer, G., Köbe, J., Wasmer, K., Lange, P. S., Orwat, S., Eckardt, L., & Dechering, D. G. (2021). Quantifying Left Atrial Size in the Context of Atrial Fibrillation Ablation: Which Echocardiographic Method Correlates to Outcome of Pulmonary Venous Isolation? Journal of Personalized Medicine, 11(9), 913. https://doi.org/10.3390/jpm11090913







