Real-Time Diabetic Retinopathy Severity Score Level versus Ultra-Widefield Leakage Index-Guided Management of Diabetic Retinopathy: Two-Year Outcomes from the Randomized PRIME Trial
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Re-Treatment Requirements in Year 2
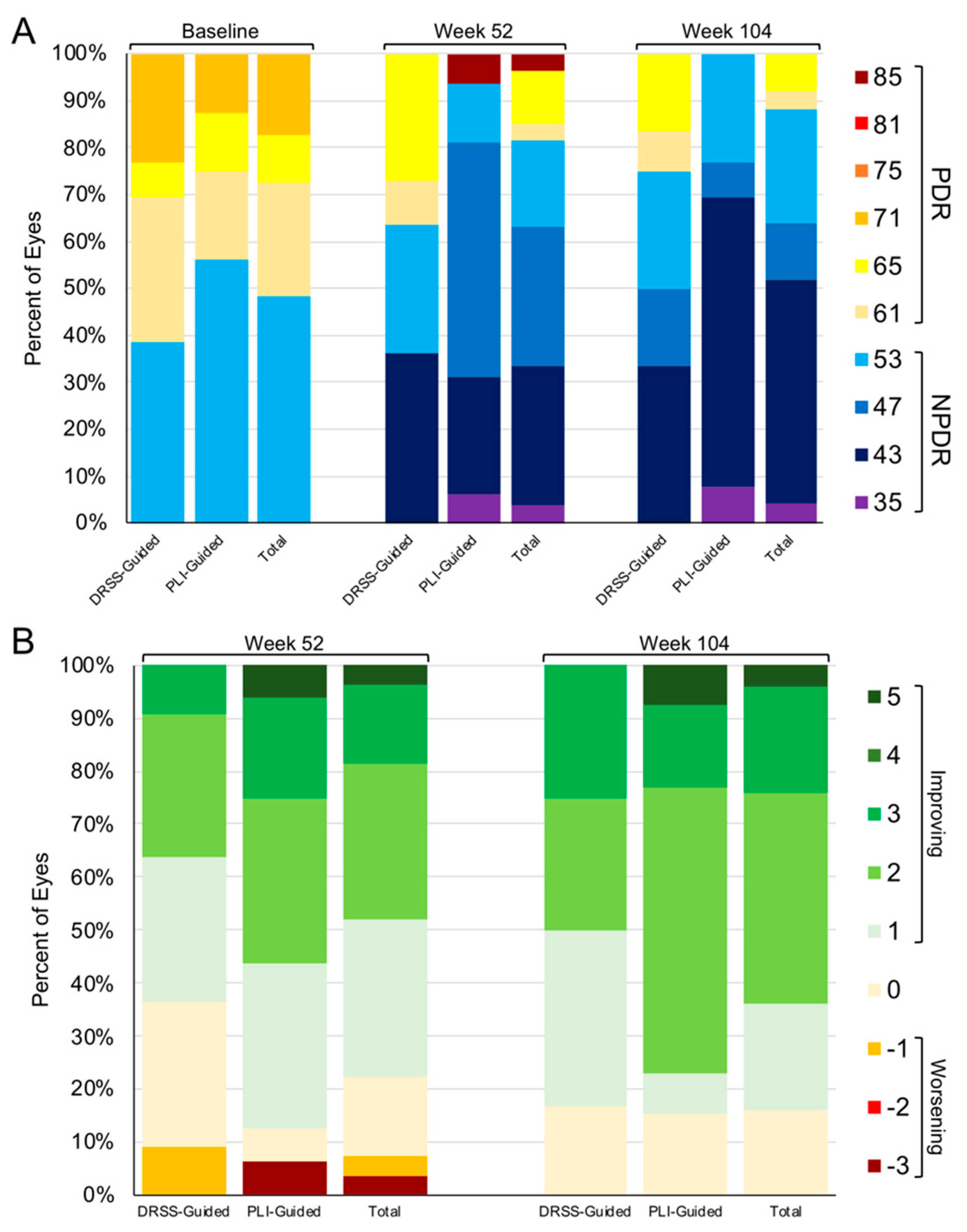
3.2. DRSS and PLI Outcomes through Year 2
3.3. Indications for PLI as a Precursor to DRSS Worsening
3.4. Visual and OCT-Based Anatomic Outcomes through Year 2
3.5. Adverse Events through Year 2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kempen, J.H.; O’Colmain, B.J.; Leske, M.C.; Haffner, S.M.; Klein, R.; Moss, S.E.; Taylor, H.R.; Hamman, R.F.; Eye Diseases Prevalence Research Group. The Prevalence of Diabetic Retinopathy among Adults in the United States. Arch. Ophthalmol. 2004, 122, 552–563. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.M.; Nguyen, Q.D.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Schlottmann, P.G.; Rundle, A.C.; Zhang, J.; Rubio, R.G.; et al. Long-Term Outcomes of Ranibizumab Therapy for Diabetic Macular Edema: The 36-Month Results from Two Phase III Trials: RISE and RIDE. Ophthalmology 2013, 120, 2013–2022. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.M.; Schmidt-Erfurth, U.; Do, D.V.; Holz, F.G.; Boyer, D.S.; Midena, E.; Heier, J.S.; Terasaki, H.; Kaiser, P.K.; Marcus, D.M.; et al. Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results from the VISTA and VIVID Studies. Ophthalmology 2015, 122, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Marcus, D.M.; Midena, E.; Korobelnik, J.-F.; Saroj, N.; Gibson, A.; Vitti, R.; Berliner, A.J.; Williams Liu, Z.; Zeitz, O.; et al. Intravitreal Aflibercept Injection in Eyes With Substantial Vision Loss After Laser Photocoagulation for Diabetic Macular Edema: Subanalysis of the VISTA and VIVID Randomized Clinical Trials. JAMA Ophthalmol. 2017, 135, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.F.; Wykoff, C.C.; Clark, W.L.; Bruce, B.B.; Boyer, D.S.; Brown, D.M.; TREX-DME Study Group. Randomized Trial of Treat and Extend Ranibizumab With and Without Navigated Laser Versus Monthly Dosing for Diabetic Macular Edema: TREX-DME 2-Year Outcomes. Am. J. Ophthalmol. 2019, 202, 91–99. [Google Scholar] [CrossRef]
- Writing Committee for the Diabetic Retinopathy Clinical Research Network; Gross, J.G.; Glassman, A.R.; Jampol, L.M.; Inusah, S.; Aiello, L.P.; Antoszyk, A.N.; Baker, C.W.; Berger, B.B.; Bressler, N.M.; et al. Panretinal Photocoagulation vs. Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA 2015, 314, 2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, J.G.; Glassman, A.R.; Liu, D.; Sun, J.K.; Antoszyk, A.N.; Baker, C.W.; Bressler, N.M.; Elman, M.J.; Ferris, F.L.; Gardner, T.W.; et al. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wykoff, C.C.; Nittala, M.G.; Zhou, B.; Fan, W.; Velaga, S.B.; Lampen, S.I.R.; Rusakevich, A.M.; Ehlers, J.P.; Babiuch, A.; Brown, D.M.; et al. Intravitreal Aflibercept for Retinal Nonperfusion in Proliferative Diabetic Retinopathy: Outcomes from the Randomized RECOVERY Trial. Ophthalmol. Retina 2019, 3, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Sivaprasad, S.; Prevost, A.T.; Vasconcelos, J.C.; Riddell, A.; Murphy, C.; Kelly, J.; Bainbridge, J.; Tudor-Edwards, R.; Hopkins, D.; Hykin, P.; et al. Clinical Efficacy of Intravitreal Aflibercept versus Panretinal Photocoagulation for Best Corrected Visual Acuity in Patients with Proliferative Diabetic Retinopathy at 52 Weeks (CLARITY): A Multicentre, Single-Blinded, Randomised, Controlled, Phase 2b, Non-Inferiority Trial. Lancet 2017, 389, 2193–2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, D.M.; Wykoff, C.C.; Boyer, D.; Heier, J.S.; Clark, W.L.; Emanuelli, A.; Higgins, P.M.; Singer, M.; Weinreich, D.M.; Yancopoulos, G.D.; et al. Evaluation of Intravitreal Aflibercept for the Treatment of Severe Nonproliferative Diabetic Retinopathy: Results From the PANORAMA Randomized Clinical Trial. JAMA Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Maturi, R.K.; Glassman, A.R.; Josic, K.; Antoszyk, A.N.; Blodi, B.A.; Jampol, L.M.; Marcus, D.M.; Martin, D.F.; Melia, M.; Salehi-Had, H.; et al. Effect of Intravitreous Anti–Vascular Endothelial Growth Factor vs Sham Treatment for Prevention of Vision-Threatening Complications of Diabetic Retinopathy: The Protocol W Randomized Clinical Trial. JAMA Ophthalmol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.C.; Brown, D.M.; Payne, J.F.; Wykoff, C.C. Relationship Between Visual Acuity and Retinal Thickness During Anti-Vascular Endothelial Growth Factor Therapy for Retinal Diseases. Am. J. Ophthalmol. 2017, 180, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Diabetic Retinopathy Clinical Research Network; Browning, D.J.; Glassman, A.R.; Aiello, L.P.; Beck, R.W.; Brown, D.M.; Fong, D.S.; Bressler, N.M.; Danis, R.P.; Kinyoun, J.L.; et al. Relationship between Optical Coherence Tomography-Measured Central Retinal Thickness and Visual Acuity in Diabetic Macular Edema. Ophthalmology 2007, 114, 525–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.J.; Ehlers, J.P.; Sevgi, D.D.; Hach, J.; O’Connell, M.; Reese, J.L.; Srivastava, S.K.; Wykoff, C.C. Real-Time Photographic- and Fluorescein Angiographic-Guided Management of Diabetic Retinopathy: Randomized PRIME Trial Outcomes. Am. J. Ophthalmol. 2021, 226, 126–136. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group Fundus Photographic Risk Factors for Progression of Diabetic Retinopathy. Ophthalmology 1991, 98, 823–833. [CrossRef]
- Sun, J.K.; Wang, P.-W.; Taylor, S.; Haskova, Z. Durability of Diabetic Retinopathy Improvement with As-Needed Ranibizumab: Open-Label Extension of RIDE and RISE Studies. Ophthalmology 2019, 126, 712–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wykoff, C.C.; Ou, W.C.; Khurana, R.N.; Brown, D.M.; Lloyd Clark, W.; Boyer, D.S.; ENDURANCE Study Group. Long-Term Outcomes with as-Needed Aflibercept in Diabetic Macular Oedema: 2-Year Outcomes of the ENDURANCE Extension Study. Br. J. Ophthalmol. 2018, 102, 631–636. [Google Scholar] [CrossRef]
- Muether, P.S.; Hermann, M.M.; Viebahn, U.; Kirchhof, B.; Fauser, S. Vascular Endothelial Growth Factor in Patients with Exudative Age-Related Macular Degeneration Treated with Ranibizumab. Ophthalmology 2012, 119, 2082–2086. [Google Scholar] [CrossRef]
- Muether, P.S.; Hermann, M.M.; Dröge, K.; Kirchhof, B.; Fauser, S. Long-Term Stability of Vascular Endothelial Growth Factor Suppression Time under Ranibizumab Treatment in Age-Related Macular Degeneration. Am. J. Ophthalmol. 2013, 156, 989–993.e2. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Jiang, A.C.; Boss, J.D.; Hu, M.; Figueiredo, N.; Babiuch, A.; Talcott, K.; Sharma, S.; Hach, J.; Le, T.; et al. Quantitative Ultra-Widefield Angiography and Diabetic Retinopathy Severity: An Assessment of Panretinal Leakage Index, Ischemic Index and Microaneurysm Count. Ophthalmology 2019, 126, 1527–1532. [Google Scholar] [CrossRef]
- Lechner, J.; O’Leary, O.E.; Stitt, A.W. The Pathology Associated with Diabetic Retinopathy. Vis. Res. 2017, 139, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Ip, M.S.; Zhang, J.; Ehrlich, J.S. The Clinical Importance of Changes in Diabetic Retinopathy Severity Score. Ophthalmology 2017, 124, 596–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campochiaro, P.A.; Marcus, D.M.; Awh, C.C.; Regillo, C.; Adamis, A.P.; Bantseev, V.; Chiang, Y.; Ehrlich, J.S.; Erickson, S.; Hanley, W.D.; et al. The Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration: Results from the Randomized Phase 2 Ladder Clinical Trial. Ophthalmology 2019, 126, 1141–1154. [Google Scholar] [CrossRef] [Green Version]
- A Trial to Evaluate the Efficacy, Durability, and Safety of KSI-301 Compared to Aflibercept in Participants with Diabetic Macular Edema (DME) (GLEAM). Available online: https://clinicaltrials.gov/ct2/show/NCT04611152 (accessed on 30 June 2021).
- RGX-314 Gene Therapy Administered in the Suprachoroidal Space for Participants with Diabetic Retinopathy (DR) without Center Involved-Diabetic Macular Edema (CI-DME) (ALTITUDE). Available online: https://clinicaltrials.gov/ct2/show/NCT04567550 (accessed on 30 June 2021).
- ADVM-022 Intravitreal Gene Therapy for DME (INFINITY). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04418427 (accessed on 30 June 2021).
- Heier, J.S.; Singh, R.P.; Wykoff, C.C.; Csaky, K.G.; Lai, T.Y.Y.; Loewenstein, A.; Schlottmann, P.G.; Paris, L.P.; Westenskow, P.D.; Quezada-Ruiz, C. The Angiopoietin/Tie Pathway in Retinal Vascular Diseases: A Review. Retina 2021, 41, 1–19. [Google Scholar] [CrossRef]
- New Phase III Data Show Roche’s Faricimab Is the First Investigational Injectable Eye Medicine to Extend Time between Treatments up to Four Months in Two Leading Causes of Vision Loss, Potentially Reducing Treatment Burden for Patients. Available online: https://www.roche.com/media/releases/med-cor-2021-02-12.htm (accessed on 30 June 2021).
- Suresh, R.; Yu, H.; Thoveson, A.; Swisher, J.; Apolinario, M.; Zhou, B.; Shah, A.R.; Fish, R.H.; Wykoff, C.C. Loss to Follow-Up Among Patients with Proliferative Diabetic Retinopathy in Clinical Practice. Am. J. Ophthalmol. 2020, S0002939420301112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Mitchell, T.C.; Rusakevich, A.M.; Brown, D.M.; Wykoff, C.C. Noncompliance in Prospective Retina Clinical Trials: Analysis of Factors Predicting Loss to Follow-Up. Am. J. Ophthalmol. 2020, 210, 86–96. [Google Scholar] [CrossRef]





| Ocular Adverse Events | |||
| DRSS-Guided | PLI-Guided | Total | |
| Worsening of Cataracts | 1 | 1 | 2 |
| Worsening of PDR | 1 | 0 | 1 |
| Floaters | 1 | 0 | 1 |
| Flashes | 1 | 0 | 1 |
| Ocular Pain | 1 | 0 | 1 |
| Glaucoma | 0 | 1 | 1 |
| Cotton Wool Spots | 0 | 1 | 1 |
| Total # of AEs | 5 | 3 | 8 |
| Total # of Patients | 4 | 3 | 7 |
| Serious Systemic Adverse Events | |||
| DRSS-Guided | PLI-Guided | Total | |
| Cerebrovascular Accident | 0 | 1 | 1 |
| Arthritic Hip Pain | 0 | 1 | 1 |
| Infection of Left Foot on Hallux | 0 | 1 | 1 |
| Bone Destruction of Right Foot | 1 | 0 | 1 |
| Pneumonia | 1 | 1 | 2 |
| COVID-19 | 0 | 1 | 1 |
| Acute Chronic Renal Failure | 0 | 1 | 1 |
| Asthma | 1 | 0 | 1 |
| Worsening Anemia | 1 | 0 | 1 |
| Transient Ischemic Attack | 1 | 0 | 1 |
| Stage 2 Kidney Failure | 1 | 0 | 1 |
| Fatal Cardiovascular Disease/Diabetes | 0 | 1 | 1 |
| Fatal Cardiac Arrest | 0 | 1 | 1 |
| Total # of SAEs | 6 | 8 | 14 |
| Total # of Patients | 4 | 6 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.J.; Ehlers, J.P.; Sevgi, D.D.; O’Connell, M.; Reese, J.L.; Srivastava, S.K.; Wykoff, C.C. Real-Time Diabetic Retinopathy Severity Score Level versus Ultra-Widefield Leakage Index-Guided Management of Diabetic Retinopathy: Two-Year Outcomes from the Randomized PRIME Trial. J. Pers. Med. 2021, 11, 885. https://doi.org/10.3390/jpm11090885
Yu HJ, Ehlers JP, Sevgi DD, O’Connell M, Reese JL, Srivastava SK, Wykoff CC. Real-Time Diabetic Retinopathy Severity Score Level versus Ultra-Widefield Leakage Index-Guided Management of Diabetic Retinopathy: Two-Year Outcomes from the Randomized PRIME Trial. Journal of Personalized Medicine. 2021; 11(9):885. https://doi.org/10.3390/jpm11090885
Chicago/Turabian StyleYu, Hannah J., Justis P. Ehlers, Duriye Damla Sevgi, Margaret O’Connell, Jamie L. Reese, Sunil K. Srivastava, and Charles C. Wykoff. 2021. "Real-Time Diabetic Retinopathy Severity Score Level versus Ultra-Widefield Leakage Index-Guided Management of Diabetic Retinopathy: Two-Year Outcomes from the Randomized PRIME Trial" Journal of Personalized Medicine 11, no. 9: 885. https://doi.org/10.3390/jpm11090885
APA StyleYu, H. J., Ehlers, J. P., Sevgi, D. D., O’Connell, M., Reese, J. L., Srivastava, S. K., & Wykoff, C. C. (2021). Real-Time Diabetic Retinopathy Severity Score Level versus Ultra-Widefield Leakage Index-Guided Management of Diabetic Retinopathy: Two-Year Outcomes from the Randomized PRIME Trial. Journal of Personalized Medicine, 11(9), 885. https://doi.org/10.3390/jpm11090885






