Precision Medicine into Clinical Practice: A Web-Based Tool Enables Real-Time Pharmacogenetic Assessment of Tailored Treatments in Psychiatric Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Drugs and CYP Variants
2.2. DNA Purification and Quantification
2.3. OpenArray™ Technology
2.4. Statistical Analysis
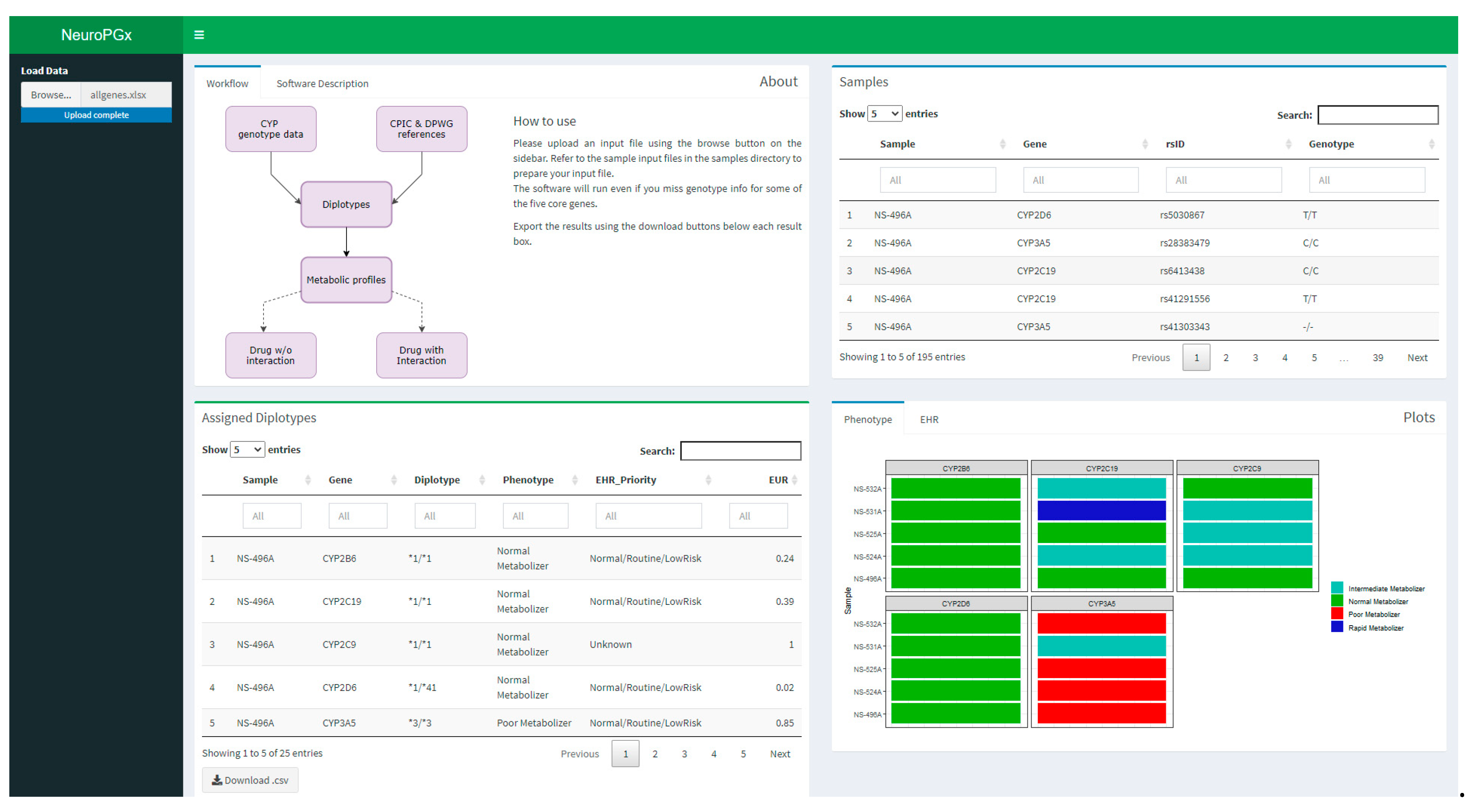
2.5. NeuroPGx Software Designing
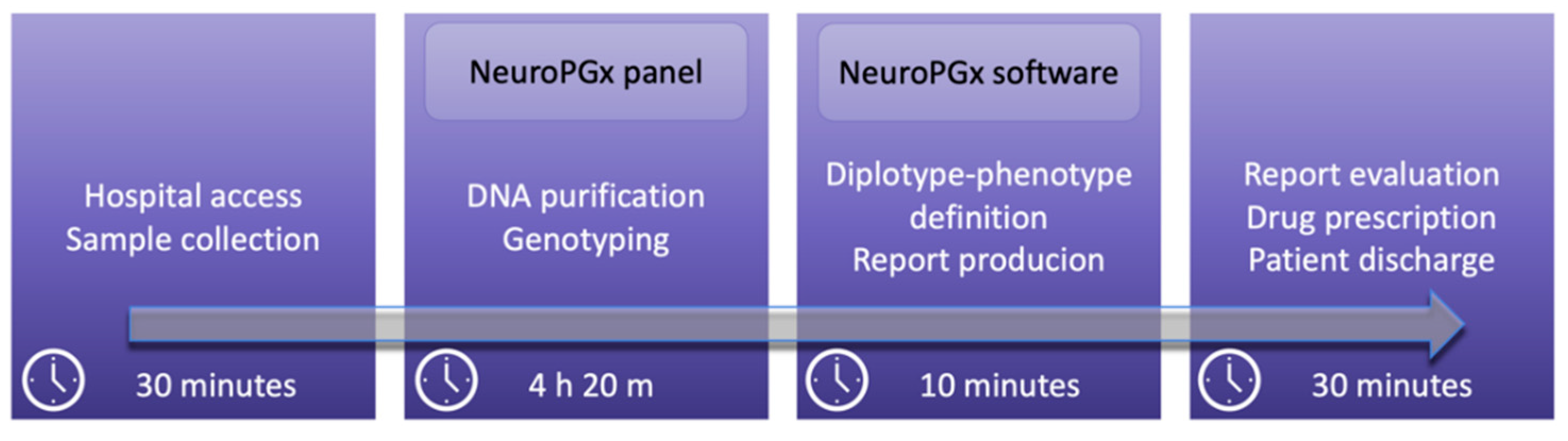
2.6. NeuroPGx Software Application
3. Results
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldberg, R.M.; Mabee, J.; Chan, L.; Wong, S. Drug-Drug and drug disease interactions in the ED: Analysis of a high-risk population. Am. J. Emerg. Med. 1996, 14, 447–450. [Google Scholar] [CrossRef]
- Storelli, F.; Samer, C.; Reny, J.L.; Desmeules, J.; Daali, Y. Complex Drug-Drug-Gene-Disease Interactions Involving Cytochromes P450: Systematic Review of Published Case Reports and Clinical Perspectives. Clin. Pharmacokinet. 2018, 57, 1267–1293. [Google Scholar] [CrossRef] [PubMed]
- Stocchi, L.; Cascella, R.; Zampatti, S.; Pirazzoli, A.; Novelli, G.; Giardina, E. The Pharmacogenomic HLA Biomarker Associated to Adverse Abacavir Reactions: Comparative Analysis of Different Genotyping Methods. Curr. Genom. 2012, 13, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Kheshti, R.; Aalipour, M.; Namazi, S. A comparison of five common drug-drug interaction software programs regarding accuracy and comprehensiveness. J. Res. Pharm. Pract. 2016, 5, 257–263. [Google Scholar] [PubMed]
- Clinical Pharmacogenetics Implementation Consortium (CPIC). Available online: https://cpicpgx.org/ (accessed on 20 June 2021).
- Dutch Pharmacogenetics Working Group (DPWG). Available online: https://www.pharmgkb.org/page/dpwg/ (accessed on 20 June 2021).
- Bank, P.C.D.; Caudle, K.E.; Swen, J.J.; Gammal, R.S.; Whirl-Carrillo, M.; Klein, T.E.; Relling, M.V.; Guchelaar, H.J. Comparison of the Guidelines of the Clinical Pharmacogenetics Implementation Consortium and the Dutch Pharmacogenetics Working Group. Clin. Pharmacol. Ther. 2018, 103, 599–618. [Google Scholar] [CrossRef]
- Yoon, D.Y.; Lee, S.; Ban, M.S.; Jang, I.J.; Lee, S. Pharmacogenomic information from CPIC and DPWG guidelines and its application on drug labels. Transl. Clin. Pharmacol. 2020, 28, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Pharmacogene Variation Consortium. Available online: https://www.pharmvar.org/ (accessed on 20 June 2021).
- Lozupone, M.; Panza, F.; Stella, E.; La Montagna, M.; Bisceglia, P.; Miscio, G.; Galizia, I.; Daniele, A.; di Mauro, L.; Bellomo, A.; et al. Pharmacogenetics of neurological and psychiatric diseases at older age: Has the time come? Expert Opin. Drug Metab. Toxicol. 2017, 13, 259–277. [Google Scholar] [CrossRef]
- Saiz-Rodríguez, M.; Ochoa, D.; Belmonte, C.; Román, M.; Vieira de Lara, D.; Zubiaur, P.; Koller, D.; Mejía, G.; Abad-Santos, F. Polymorphisms in CYP1A2, CYP2C9 and ABCB1 affect agomelatine pharmacokinetics. J. Psychopharmacol. 2019, 33, 522–531. [Google Scholar] [CrossRef]
- Zhou, S.F.; Zhou, Z.W.; Yang, L.P.; Cai, J.P. Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr. Med. Chem. 2009, 16, 3480–3675. [Google Scholar] [CrossRef]
- Gashaw, I.; Kirchheiner, J.; Goldammer, M.; Bauer, S.; Seidemann, J.; Zoller, K.; Mrozikiewicz, P.M.; Roots, I.; Brockmöller, J. Cytochrome p450 3A4 messenger ribonucleic acid induction by rifampin in human peripheral blood mononuclear cells: Correlation with alprazolam pharmacokinetics. Clin. Pharmacol. Ther. 2003, 74, 448–457. [Google Scholar] [CrossRef]
- Williams, J.A.; Ring, B.J.; Cantrell, V.E.; Jones, D.R.; Eckstein, J.; Ruterbories, K.; Hamman, M.A.; Hall, S.D.; Wrighton, S.A. Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab. Dispos. 2002, 30, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Eap, C.B.; Gründer, G.; Baumann, P.; Ansermot, N.; Conca, A.; Corruble, E.; Crettol, S.; Dahl, M.L.; de Leon, J.; Greiner, C.; et al. Tools for optimising pharmacotherapy in psychiatry (therapeutic drug monitoring, molecular brain imaging and pharmacogenetic tests): Focus on antidepressants. World J. Biol. Psychiatry 2021, 12, 1–68. [Google Scholar] [CrossRef] [PubMed]
- Bauman, J.N.; Frederick, K.S.; Sawant, A.; Walsky, R.L.; Cox, L.M.; Obach, R.S.; Kalgutkar, A.S. Comparison of the bioactivation potential of the antidepressant and hepatotoxin nefazodone with aripiprazole, a structural analog and marketed drug. Drug Metab. Metab. Dispos. 2008, 36, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Winans, E. Aripiprazole. Am. J. Health Syst. Pharm. 2003, 60, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Li, G.F.; Markowitz, J.S. Atomoxetine: A Review of Its Pharmacokinetics and Pharmacogenomics Relative to Drug Disposition. J. Child. Adolesc. Psychopharmacol. 2016, 26, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.I.; Bae, J.W.; Lee, Y.J.; Lee, H.I.; Jang, C.G.; Lee, S.Y. Effects of CYP2C19 genetic polymorphisms on atomoxetine pharmacokinetics. J. Clin. Psychopharmacol. 2014, 34, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Foti, R.S.; Rock, D.A.; Wienkers, L.C.; Wahlstrom, J.L. Selection of alternative CYP3A4 probe substrates for clinical drug interaction studies using in vitro data and in vivo simulation. Drug Metab. Dispos. 2010, 38, 981–987. [Google Scholar] [CrossRef]
- Djordjevic, N.; Milovanovic, D.D.; Radovanovic, M.; Radosavljevic, I.; Obradovic, S.; Jakovljevic, M.; Milovanovic, D.; Milovanovic, J.R.; Jankovic, S. CYP1A2 genotype affects carbamazepine pharmacokinetics in children with epilepsy. Eur. J. Clin. Pharmacol. 2016, 72, 439–445. [Google Scholar] [CrossRef]
- Iannaccone, T.; Sellitto, C.; Manzo, V.; Colucci, F.; Giudice, V.; Stefanelli, B.; Iuliano, A.; Corrivetti, G.; Filippelli, A. Pharmacogenetics of Carbamazepine and Valproate: Focus on Polymorphisms of Drug Metabolizing Enzymes and Transporters. Pharmaceuticals 2021, 14, 204. [Google Scholar] [CrossRef]
- Yoshimura, R.; Yanagihara, N.; Terao, T.; Minami, K.; Toyohira, Y.; Ueno, S.; Uezono, Y.; Abe, K.; Izumi, F. An active metabolite of carbamazepine, carbamazepine-10,11-epoxide, inhibits ion channel-mediated catecholamine secretion in cultured bovine adrenal medullary cells. Psychopharmacology 1998, 135, 368–373. [Google Scholar] [CrossRef]
- Murray, M. Role of CYP pharmacogenetics and drug-drug interactions in the efficacy and safety of atypical and other antipsychotic agents. J. Pharm. Pharmacol. 2006, 58, 871–885. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, X.; Li, M.; Huang, H.; Minica, C.; Yi, Z.; Wang, G.; Shen, L.; Xing, Q.; Shi, Y.; et al. Association studies of genomic variants with treatment response to risperidone, clozapine, quetiapine and chlorpromazine in the Chinese Han population. Pharm. J. 2016, 16, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Wójcikowski, J.; Boksa, J.; Daniel, W.A. Main contribution of the cytochrome P450 isoenzyme 1A2 (CYP1A2) to N-demethylation and 5-sulfoxidation of the phenothiazine neuroleptic chlorpromazine in human liver--A comparison with other phenothiazines. Biochem Pharmacol. 2010, 80, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Bezchlibnyk-Butler, K.; Aleksic, I.; Kennedy, S.H. Citalopram--a review of pharmacological and clinical effects. J. Psychiatry Neurosci. 2000, 25, 241–254. [Google Scholar] [PubMed]
- Tolbert, D.; Larsen, F. A Comprehensive Overview of the Clinical Pharmacokinetics of Clobazam. J. Clin. Pharmacol. 2019, 59, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Tóth, K.; Csukly, G.; Sirok, D.; Belic, A.; Kiss, Á.; Háfra, E.; Déri, M.; Menus, Á.; Bitter, I.; Monostory, K. Optimization of Clonazepam Therapy Adjusted to Patient’s CYP3A Status and NAT2 Genotype. Int. J. Neuropsychopharmacol. 2016, 19, pyw083. [Google Scholar] [CrossRef][Green Version]
- Pereira, N.L.; Rihal, C.S.; So, D.Y.F.; Rosenberg, Y.; Lennon, R.J.; Mathew, V.; Goodman, S.G.; Weinshilboum, R.M.; Wang, L.; Baudhuin, L.M.; et al. Clopidogrel Pharmacogenetics. Circ. Cardiovasc. Interv. 2019, 12, e007811. [Google Scholar] [CrossRef]
- Dean, L.; Kane, M. Clozapine Therapy and CYP Genotype. 2016 [updated 2021 May 26]. In Medical Genetics Summaries [Internet]; Pratt, V.M., Scott, S.A., Pirmohamed, M., Esquivel, B., Kane, M.S., Kattman, B.L., Malheiro, A.J., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Furman, K.D.; Grimm, D.R.; Mueller, T.; Holley-Shanks, R.R.; Bertz, R.J.; Williams, L.A.; Spear, B.B.; Katz, D.A. Impact of CYP2D6 intermediate metabolizer alleles on single-dose desipramine pharmacokinetics. Pharmacogenetics 2004, 14, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.K.; Sangkuhl, K.; Swen, J.J.; Ellingrod, V.L.; Müller, D.J.; Shimoda, K.; Bishop, J.R.; Kharasch, E.D.; Skaar, T.C.; Gaedigk, A.; et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin. Pharmacol. Ther. 2017, 102, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kirchheiner, J.; Meineke, I.; Müller, G.; Roots, I.; Brockmöller, J. Contributions of CYP2D6, CYP2C9 and CYP2C19 to the biotransformation of E- and Z-doxepin in healthy volunteers. Pharmacogenetics 2002, 12, 571–580. [Google Scholar] [CrossRef]
- Knadler, M.P.; Lobo, E.; Chappell, J.; Bergstrom, R. Duloxetine: Clinical pharmacokinetics and drug inter-actions. Clin. Pharmacokinet. 2011, 50, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Rao, N. The clinical pharmacokinetics of escitalopram. Clin. Pharmacokinet. 2007, 46, 281–290. [Google Scholar] [CrossRef]
- Margolis, J.M.; O’Donnell, J.P.; Mankowski, D.C.; Ekins, S.; Obach, R.S. (R)-, (S)-, and racemic fluoxetine N-demethylation by human cytochrome P450 enzymes. Drug Metab. Dispos. 2000, 28, 1187–1191. [Google Scholar] [PubMed]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Müller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; LLerena, A.; et al. Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Prommer, E. Role of haloperidol in palliative medicine: An update. Am. J. Hosp. Palliat. Care. 2012, 29, 295–301. [Google Scholar] [CrossRef]
- Anttila, S.A.; Leinonen, E.V. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001, 7, 249–264. [Google Scholar] [CrossRef]
- Callaghan, J.T.; Bergstrom, R.F.; Ptak, L.R.; Beasley, C.M. Olanzapine. Pharmacokinetic and pharmacody-namic profile. Clin. Pharmacokinet. 1999, 37, 177–1793. [Google Scholar] [CrossRef]
- Bang, L.; Goa, K. Oxcarbazepine: A review of its use in children with epilepsy. Paediatr. Drugs. 2003, 5, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Waade, R.B.; Solhaug, V.; Høiseth, G. Impact of CYP2D6 on serum concentrations of flupentixol, haloperidol, perphenazine and zuclopenthixol. Br. J. Clin. Pharmacol. 2021, 87, 2228–2235. [Google Scholar] [CrossRef]
- Fohner, A.E.; Rettie, A.E.; Thai, K.K.; Ranatunga, D.K.; Lawson, B.L.; Liu, V.X.; Schaefer, C.A. Associations of CYP2C9 and CYP2C19 Pharmacogenetic Variation with Phenytoin-Induced Cutaneous Adverse Drug Reactions. Clin. Transl. Sci. 2020, 13, 1004–1009. [Google Scholar] [CrossRef]
- Chapron, B.D.; Dinh, J.C.; Toren, P.C.; Gaedigk, A.; Leeder, J.S. The Respective Roles of CYP3A4 and CYP2D6 in the Metabolism of Pimozide to Established and Novel Metabolites. Drug Metab. Dispos. 2020, 48, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- DeVane, C.L.; Nemeroff, C.B. Clinical pharmacokinetics of quetiapine: An atypical antipsychotic. Clin. Pharmacokinet. 2001, 40, 509–522. [Google Scholar] [CrossRef]
- Cui, Y.; Yan, H.; Su, Y.; Wang, L.; Lu, T.; Zhang, D.; Yue, W. CYP2D6 Genotype-Based Dose Recommendations for Risperidone in Asian People. Front. Pharmacol. 2020, 11, 936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Brown, S.J.; Shan, Y.; Lee, A.M.; Allen, J.D.; Eum, S.; de Leon, J.; Bishop, J.R. CYP2D6 Genetic Polymorphisms and Risperidone Pharmacokinetics: A Systematic Review and Meta-analysis. Pharmacotherapy 2020, 40, 632–647. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Rodríguez, M.; Belmonte, C.; Román, M.; Ochoa, D.; Koller, D.; Talegón, M.; Ovejero-Benito, M.C.; López-Rodríguez, R.; Cabaleiro, T.; Abad-Santos, F. Effect of Polymorphisms on the Pharmacokinetics, Pharmacodynamics and Safety of Sertraline in Healthy Volunteers. Basic Clin. Pharmacol. Toxicol. 2018, 122, 501–511. [Google Scholar] [CrossRef]
- Berecz, R.; de la Rubia, A.; Dorado, P.; Fernández-Salguero, P.; Dahl, M.L.; LLerena, A. Thioridazine steady-state plasma concentrations are influenced by tobacco smoking and CYP2D6, but not by the CYP2C9 genotype. Eur. J. Clin. Pharmacol. 2003, 59, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.P. Does trazodone have a role in palliating symptoms? Support. Care Cancer. 2007, 15, 221–224. [Google Scholar] [CrossRef]
- Kirchheiner, J.; Müller, G.; Meineke, I.; Wernecke, K.D.; Roots, I.; Brockmöller, J. Effects of polymorphisms in CYP2D6, CYP2C9, and CYP2C19 on trimipramine pharmacokinetics. J. Clin. Psychopharmacol. 2003, 23, 459–466. [Google Scholar] [CrossRef]
- Zhu, M.M.; Li, H.L.; Shi, L.H.; Chen, X.P.; Luo, J.; Zhang, Z.L. The pharmacogenomics of valproic acid. J. Hum. Genet. 2017, 62, 1009–1014. [Google Scholar] [CrossRef]
- McAlpine, D.E.; Biernacka, J.M.; Mrazek, D.A.; O’Kane, D.J.; Stevens, S.R.; Langman, L.J.; Courson, V.L.; Bhagia, J.; Moyer, T.P. Effect of cytochrome P450 enzyme polymorphisms on pharmacokinetics of venlafaxine. Ther Drug Monit. 2011, 33, 14–20. [Google Scholar] [CrossRef]
- Chen, G.; Højer, A.M.; Areberg, J.; Nomikos, G. Vortioxetine: Clinical Pharmacokinetics and Drug Interactions. Clin. Pharmacokinet. 2018, 57, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.; Kamel, A.; Cui, D.; Whalen, R.D.; Miceli, J.J.; Tweedie, D. Identification of the major human liver cytochrome P450 isoform(s) responsible for the formation of the primary metabolites of ziprasidone and prediction of possible drug interactions. Br. J. Clin. Pharmacol. 2000, 49 (Suppl. 1), 35S–42S. [Google Scholar] [CrossRef]
- Beedham, C.; Miceli, J.J.; Obach, R.S. Ziprasidone metabolism, aldehyde oxidase, and clinical implications. J. Clin. Psychopharmacol. 2003, 23, 229–232. [Google Scholar] [CrossRef]
- Pichard, L.; Gillet, G.; Bonfils, C.; Domergue, J.; Thénot, J.P.; Maurel, P. Oxidative metabolism of zolpidem by human liver cytochrome P450S. Drug Metab. Dispos. 1995, 23, 1253–1262. [Google Scholar] [PubMed]
- Okada, Y.; Seo, T.; Ishitsu, T.; Wanibuchi, A.; Hashimoto, N.; Higa, Y.; Nakagawa, K. Population estimation regarding the effects of cytochrome P450 2C19 and 3A5 polymorphisms on zonisamide clearance. Ther. Drug. Monit. 2008, 30, 540–543. [Google Scholar] [CrossRef]
- Saruwatari, J.; Ishitsu, T.; Nakagawa, K. Update on the Genetic Polymorphisms of Drug-Metabolizing Enzymes in Antiepileptic Drug Therapy. Pharmaceuticals 2010, 3, 2709–2732. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Westin, A.A.; Castberg, I.; Lewis, G.; Lennard, M.S.; Taylor, S.; Spigset, O. Characterisation of zuclopenthixol metabolism by in vitro and therapeutic drug monitoring studies. Acta Psychiatr. Scand. 2010, 122, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Broccanello, C.; Gerace, L.; Stevanato, P. QuantStudio™ 12K Flex OpenArray® System as a Tool for High-Throughput Genotyping and Gene Expression Analysis. In Quantitative Real-Time PCR. Methods in Molecular Biology; Humana: New York, NY, USA, 2020; Volume 2065, pp. 199–208. [Google Scholar]
- Chang, W.; Cheng, J.; Allaire, J.; Xie, Y.; McPherson, J. Shiny: Web application framework for R. R Package Version 2017, 1, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 20 June 2021).
| GENEs | HGVS | Alleles 1 | Major Nucleotide Variation | RS ID | AssayID | Nucleotide Change | Effect on Protein |
|---|---|---|---|---|---|---|---|
| CYP2B6 | NC_000019; NG_007929.1; NM_000767; NP_000758.1 | *22, *34, *35, *36 | −82T > C | rs34223104 | C__27830964_10 | g.40991224T > C | Upstream Transcript Variant |
| *16, *18 | 983T > C | rs28399499 | C__60732328_20 | c.983T > C | p.Ile328Thr | ||
| *5, *7 | 25505C > T | rs3211371 | C__30634242_40 | c.1459C > T | p.Arg487Cys | ||
| CYP2C19 | NC_000010; NG_055436; NM_000769; NP_000760 | *17 | −806C > T | rs12248560 | C____469857_10 | −806C > T | Upstream Transcript Variant |
| *4A/B | 1A > G | rs28399504 | C__30634136_10 | c.1A > G | p.Met1Leu | ||
| *8 | 12711T > C | rs41291556 | C__30634130_30 | c.358T > C | p.Trp120Arg | ||
| *6 | 12748G > A | rs72552267 | C__27531918_10 | c.395G > A | p.Arg132Gln | ||
| *9 | 12784G > A | rs17884712 | C__25745302_30 | c.431G > A | p.Arg144His | ||
| *3 | 17948G > A | rs4986893 | C__27861809_10 | c.636G > A | p.Trp212Ter | ||
| *10 | 19153C > T | rs6413438 | C__30634128_10 | 19153C > T | p.Pro227Leu | ||
| *2 | 19154G > A | rs4244285 | C__25986767_70 | c.681G > A/19154G > A | p.Pro227 = | ||
| *7 | 19294T > A | rs72558186 | C__30634127_10 | g.94781999T > A | Splice Donor Variant | ||
| *5 | 90033C > T | rs56337013 | C__27861810_10 | c.1297C > T | p.Arg433Trp | ||
| CYP2C9 | NC_000010; NG_008385; NM_000771; NP_000762 | *2, *35, *61 | 3608C > T | rs1799853 | C__25625805_10 | c.430C > T | p.Arg144Cys |
| *6 | 10601delA | rs9332131; hcv32287221 | C__32287221_20 | c.818delA | p.Lys273ArgfsTer34 | ||
| *11 | 42542C > T | rs28371685 | C__30634132_70 | c.1003C > T | p.Arg335Trp | ||
| *3, *18 | 42614A > C | rs1057910 | C__27104892_10 | c.1075A > G | p.Ile359Val | ||
| *4 | 42615T > C | rs56165452 | C__30634131_20 | c.1076T > C | p.Ile359Thr | ||
| *5 | 42619C > G | rs28371686 | C__27859817_40 | c.1080C > G | p.Asp360Glu | ||
| CYP2D6 | NC_000022.11; NG_008376; NM_001025161; NP_000097 | *10, *36, *37, *47, *49, *52, *54, *57, *64, *65, *69, *72, *87, *94, *95, *99, *100, *101, *114, *132 | 100C > T | rs1065852 | C__11484460_40 | c.100C > T | p.Pro34Ser |
| *12 | 124G > A | rs5030862 | C__27531552_A0 | c.124G > A | P.Gly42Arg | ||
| *17, *40, *58, *64, *82 | 1022C > T/A | rs28371706 | C___2222771_A0 | c.320C > T; c.320C > A | p.Thr107Ile; p.Thr107Asn | ||
| *6 | 1708delT | rs5030655 | C__32407243_20 | c.454delT | p.Trp152GlyfsTer2 | ||
| *8, *14, *114 | 1759G > A/T | rs5030865 | C_30634117D_M0/C_30634117C_K0 | c.505G > T;c.505G > C; c.505G > A | p.Gly169Ter; p.Gly169Arg; p.Gly169Arg | ||
| *4 | 1847G > A | rs3892097 | C__27102431_D0 | c.506-1G > A | Upstream Transcript Variant | ||
| *3 | 2550delA | rs35742686 | C__32407232_50 | c.775delA | p.Arg259GlyfsTer2 | ||
| *7 | 2936A > C | rs5030867 | C__32388575_A0 | c.971A > C | p.His324Pro | ||
| *32, *41, *69, *91, *119, *123, *132, *138 | 2989G > A | rs28371725 | C__34816116_20 | c.985+39G > A | Intron Variant | ||
| *29, *70, *109 | 3184G > A | rs59421388 | C__34816113_20 | c.1012G > A | p.Val338Met | ||
| *2, *8, *10, *11, *12, *14, *17, *19, *20, *21, *28, *29, *30, *31, *32, *35, *36, *37, *39, *40, *41, *42, *45, *46, *47, *49, *51, *52, *54, *55, *56, *57, *58, *59, *64, *65, *69, *70, *72, *73, *83, *84, *85, *87, *88, *94, *95, *98, *99, *100, *101, *102, *103, *104, *105, *111, *114, *117, *121, *123, *125, *126, *128, *129, *132, *133, *135, *136, *138 | 4181G > C | rs1135840 | C__27102414_10 | c.1457C > G | p.Thr486Ser | ||
| *2, *8, *11, *12, *14, *17, *19, *20, *21, *28, *29, *30, *31, *32, *34, *35, *40, *41, *42, *45, *46, *51, *55, *58, *59, *65, *69, *73, *84, *85, *91, *98, *102, *103, *104, *105, *111, *114, *117, *121, *123, *125, *126, *128, *129, *133, *135, *136, *138 | 2851C > T | rs16947 | C__27102425_10 | c.886T > C | p.Cys296Arg | ||
| *9, *109, *115 | 2616delAAG | rs5030656; HCV32407229 | C__32407229_60 | c.841_843delAAG | p.Lys281del | ||
| CYP3A5 | NC_000007.14; NG_007938; NM_000777; NP_000768 | *8 | 3699C > T | rs55817950 | C__30633872_10 | c.82C > T | p.Arg28Cys |
| *3 | 6986A > G | rs776746 | C__26201809_30 | c.−253-1G > A | Downstream Transcript Variant | ||
| *3 | 6986A > G + H30Y (3705C > T) | rs776746; rs28383468 | C__30633871_50 | c.88C > T | p.His30Tyr | ||
| *6 | 14690G > A | rs10264272 | C__30203950_10 | c.624G > A | p.Lys208 = | ||
| *9 | 19386G > A | rs28383479 | C__30633863_10 | c.1009G > A | p.Ala337Thr | ||
| *7 | 27131_27132insT | rs41303343 | C__32287188_10 | c.1035dupT | p.Thr346TyrfsTer3 | ||
| *2 | 27289C > A | rs28365083 | C__30633862_10 | c.1193C > A | p.Thr398Asn |
| Drug | Genes 1 | References |
|---|---|---|
| Agomelatine | CYP1A2, CYP2C9 | [10,11] |
| Alprazolam | CYP2C19, CYP2C9, CYP3A4, CYP3A5, CYP3A7 | [10,12,13,14] |
| Amitriptyline | CYP2D6, CYP2C19, CYP1A2, CYP2C9, CYP3A4 | [10,15] |
| Aripiprazole | CYP2D6, CYP3A4 | [10,16,17] |
| Atomoxetine | CYP2D6, CYP2C19 | [18,19] |
| Bupropion | CYP2B6 | [10,15] |
| Buspirone | CYP3A4 | [10,20] |
| Carbamazepine | CYP1A2, CYP3A4, CYP3A5, CYP2C19, CYP2C8 | [10,21,22,23] |
| Chlorpromazine | CYP2D6, CYP2C19, CYP1A2, CYP3A4 | [10,24,25,26] |
| Citalopram | CYP2C19, CYP2D6, CYP3A4 | [10,15,27] |
| Clobazam | CYP2C19, CYP3A, CYP2B6 | [28] |
| Clomipramine | CYP2D6, CYP2C19, CYP1A2, CYP3A4 | [10,15] |
| Clonazepam | CYP3A4, CYP3A5 | [10,29] |
| Clopidogrel | CYP2C19 | [30] |
| Clozapine | CYP1A2, CYP2D6, CYP3A4, CYP2C19 | [10,31] |
| Desipramine | CYP1A2, CYP2D6 | [10,32,33] |
| Desvenlafaxine | CYP2C19, CYP3A4 | [15] |
| Doxepin | CYP2D6, CYP2C19, CYP2C9 | [10,15,34] |
| Duloxetine | CYP1A2, CYP2D6 | [10,15,35] |
| Escitalopram | CYP2C19, CYP2D6, CYP3A4 | [10,15,36] |
| Fluoxetine | CYP2D6, CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP3A4, CYP3A5 | [10,37] |
| Fluvoxamine | CYP2D6, CYP1A2 | [10,15,38] |
| Haloperidol | CYP2D6, CYP3A4 | [39] |
| Imipramine | CYP2D6, CYP2C19, CYP1A2, CYP3A4 | [10,15] |
| Lurasidone | CYP3A4 | [10,15] |
| Mirtazapine | CYP1A2, CYP2D6, CYP3A4, CYP2C19 | [10,15,40] |
| Nortriptyline | CYP2D6, CYP1A2, CYP2C19, CYP3A4 | [10,33] |
| Olanzapine | CYP1A2, CYP2D6 | [10,41] |
| Oxcarbazepine | - | [10,42] |
| Paroxetine | CYP2D6, CYP3A4 | [10,15] |
| Perphenazine | CYP2D6 | [10,43] |
| Phenytoin | CYP2C9, CYP2C19 | [44] |
| Pimozide | CYP2D6, CYP3A4, CYP1A2 | [10,45] |
| Quetiapine | CYP2D6, CYP3A4 | [10,15,46] |
| Reboxetine | CYP3A4 | [10,15] |
| Risperidone | CYP2D6 | [10,47,48] |
| Sertraline | CYP2C19, CYP2B6, CYP2C9, CYP2D6, CYP3A4 | [10,15,49] |
| Thioridazine | CYP2D6 | [10,50] |
| Trazodone | CYP2D6, CYP3A4, CYP1A2 | [10,51] |
| Trimipramine | CYP2C19, CYP2C9, CYP2D6, CYP3A4 | [10,15,52] |
| Valproic acid | CYP2A6, CYP2B6, CYP2C9, CYP2C19 | [10,53] |
| Venlafaxine | CYP2C19, CYP2D6, CYP3A4 | [10,15,54] |
| Vortioxetine | CYP3A4, CYP2C9, CYP2D6, CYP2C19 | [10,15,55] |
| Ziprasidone | CYP3A4 | [10,56,57] |
| Zolpidem | CYP1A2, CYP2D6, CYP3A4 | [10,58] |
| Zonisamide | CYP3A4, CYP2C19 | [59,60] |
| Zuclopenthixol | CYP2D6, CYP3A4 | [10,43,61] |
| Genes | RS ID | Alleles | SSA AF | AA/AC AF | Eur AF | NE AF | EA AF | CSA AF | Ame AF | Lat AF | Oce AF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CYP3A5 | rs55817950, rs776746, rs10264272, rs28383479, rs41303343, rs28365083 | (*1 *2 *3 *6 *7 *8 *9) | 0.54 | 0.50 | 0.71 | 0.62 | 0.49 | 0.78 | 0.83 | 0.75 | 0.74 |
| CYP2B6 | rs34223104, rs28399499, rs3211371 | (*1 *5 *7 *16 *18 *22 *34 *35 *36) | 0.44 | 0.52 | 0.64 | 0.52 | 0.66 | 0.61 | n.a. | 0.56 | 0.37 |
| CYP2C9 | rs1799853, rs9332131, rs28371685, rs1057910, rs56165452, rs28371686 | (*1 *2 *3 *4 *5 *6 *11 *18 *35 *61) | 86.42 | 86.70 | 80.01 | 76.91 | 96.57 | 78.90 | 88.92 | n.a. | 96.62 |
| CYP2C19 | rs12248560, rs28399504, rs41291556, rs72552267, rs17884712, rs4986893, rs6413438, rs4244285, rs72558186, rs56337013 | (*1 *2 *3 *4 *5 *6 *7 *8 *9 *10 *17) | 0.74 | 0.75 | 0.78 | 0.81 | 0.98 | 0.83 | 0.89 | 0.82 | 0.94 |
| CYP2D6 | rs1065852, rs5030862, rs28371706, rs5030655, rs5030865, rs3892097, rs35742686, rs5030867, rs28371725, rs59421388, rs1135840, rs16947, rs5030656 | (*1 *2 *3 *4 *5 *6 *7 *8 *9 *10 *11 *12 *14 *17 *19 *20 *21 *28 *29 *30 *31 *32 *34 *35 *36 *37 *39 *40 *41 *42 *45 *46 *47 *49 *51 *52 *54 *55 *56 *57 *58 *59 *64 *65 *69 *70 *72 *73 *82 *83 *84 *85 *87 *88 *91 *94 *95 *98 *99 *100 *101 *102 *103 *104 *105 *109 *111 *114 *115 *117 *119 *121 *123 *125 *126 *128 *129 *132 *133 *135 *136 *138) | ˜1 | ˜1 | ˜1 | ˜1 | ˜1 | ˜1 | ˜1 | ˜1 | 0.85 |
| Drug | Related Genes | Indication in the Guidelines | Label Indication | ||
|---|---|---|---|---|---|
| CPIC | DPWG | FDA | EMA/AIFA | ||
| Agomelatine | noRec | noRec | |||
| Alprazolam | noRec | noRec | |||
| Amitriptyline | CYP2D6 | Y | Y | ||
| CYP2C19 | N | Y | |||
| Aripiprazole | CYP2D6 | Y | Y | ||
| Atomoxetine | CYP2D6 | Y | Y | ||
| Bupropion | noRec | noRec | |||
| Buspirone | noRec | noRec | NA | ||
| Carbamazepine | noRec | noRec | |||
| Chlorpromazine | noRec | noRec | |||
| Citalopram | CYP2C19 | Y | Y | ||
| Clobazam | CYP2C19 | Y | Y | ||
| Clomipramine | CYP2D6 | Y | Y | ||
| Clonazepam | noRec | noRec | |||
| Clopidogrel | CYP2C19 | Y | Y | ||
| Clozapine | noRec | noRec | |||
| Desipramine | noRec | noRec | NA | ||
| Desvenlafaxine | noRec | noRec | NA | ||
| Doxepin | CYP2D6 | Y | NA | ||
| CYP2C19 | N | NA | |||
| Duloxetine | noRec | noRec | |||
| Escitalopram | CYP2C19 | Y | Y | ||
| Fluoxetine | CYP2D6 | Y | Y | ||
| Fluvoxamine | CYP2D6 | Y | N | ||
| Haloperidol | CYP2D6 | N | N | ||
| Imipramine | CYP2D6 | Y | NA | ||
| CYP2C19 | N | NA | |||
| Lurasidone | noRec | noRec | |||
| Mirtazapine | noRec | noRec | |||
| Nortriptyline | CYP2D6 | Y | N | ||
| Olanzapine | noRec | noRec | |||
| Oxcarbazepine | noRec | noRec | |||
| Paroxetine | CYP2D6 | Y | Y | ||
| Perphenazine | noRec | noRec | |||
| Phenytoin | CYP2C9 | Y | Y | ||
| Pimozide | CYP2D6 | N | N | ||
| Quetiapine | noRec | noRec | |||
| Reboxetine | noRec | noRec | |||
| Risperidone | noRec | noRec | |||
| Sertraline | CYP2C19 | N | Y | ||
| Thioridazine | noRec | noRec | NA | ||
| Trazodone | noRec | noRec | |||
| Trimipramine | CYP2C19 | Y | Y | ||
| Valproic acid | noRec | noRec | |||
| Venlafaxine | noRec | noRec | |||
| Vortioxetine | noRec | noRec | NA | ||
| Ziprasidone | noRec | noRec | |||
| Zolpidem | noRec | noRec | |||
| Zonisamide | noRec | noRec | |||
| Zuclopenthixol | noRec | noRec | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zampatti, S.; Fabrizio, C.; Ragazzo, M.; Campoli, G.; Caputo, V.; Strafella, C.; Pellicano, C.; Cascella, R.; Spalletta, G.; Petrosini, L.; et al. Precision Medicine into Clinical Practice: A Web-Based Tool Enables Real-Time Pharmacogenetic Assessment of Tailored Treatments in Psychiatric Disorders. J. Pers. Med. 2021, 11, 851. https://doi.org/10.3390/jpm11090851
Zampatti S, Fabrizio C, Ragazzo M, Campoli G, Caputo V, Strafella C, Pellicano C, Cascella R, Spalletta G, Petrosini L, et al. Precision Medicine into Clinical Practice: A Web-Based Tool Enables Real-Time Pharmacogenetic Assessment of Tailored Treatments in Psychiatric Disorders. Journal of Personalized Medicine. 2021; 11(9):851. https://doi.org/10.3390/jpm11090851
Chicago/Turabian StyleZampatti, Stefania, Carlo Fabrizio, Michele Ragazzo, Giulia Campoli, Valerio Caputo, Claudia Strafella, Clelia Pellicano, Raffaella Cascella, Gianfranco Spalletta, Laura Petrosini, and et al. 2021. "Precision Medicine into Clinical Practice: A Web-Based Tool Enables Real-Time Pharmacogenetic Assessment of Tailored Treatments in Psychiatric Disorders" Journal of Personalized Medicine 11, no. 9: 851. https://doi.org/10.3390/jpm11090851
APA StyleZampatti, S., Fabrizio, C., Ragazzo, M., Campoli, G., Caputo, V., Strafella, C., Pellicano, C., Cascella, R., Spalletta, G., Petrosini, L., Caltagirone, C., Termine, A., & Giardina, E. (2021). Precision Medicine into Clinical Practice: A Web-Based Tool Enables Real-Time Pharmacogenetic Assessment of Tailored Treatments in Psychiatric Disorders. Journal of Personalized Medicine, 11(9), 851. https://doi.org/10.3390/jpm11090851







