A Cost Decision Model Supporting Treatment Strategy Selection in BRCA1/2 Mutation Carriers in Breast Cancer
Abstract
:1. Introduction
2. Methods
2.1. Reference Case
2.2. The Decision Analysis Model
- -
- Logistical issues: Availability of genetic cancer risk assessment consultative services, adequate time to complete the BRCA genetic testing process (initial counseling, informed consent, obtaining a blood sample, 2–4 weeks for genotyping, disclosure counseling, and genetic-risk-tailored treatment advice), and the timing of the referral with respect to the current diagnostic and therapeutic treatment sequence [34];
- -
- Clinical issues: Tumor size, number of positive axillary lymph nodes, histologic grade, and lymphovascular invasion [2], as well as the patients’ surgical choice.
- -
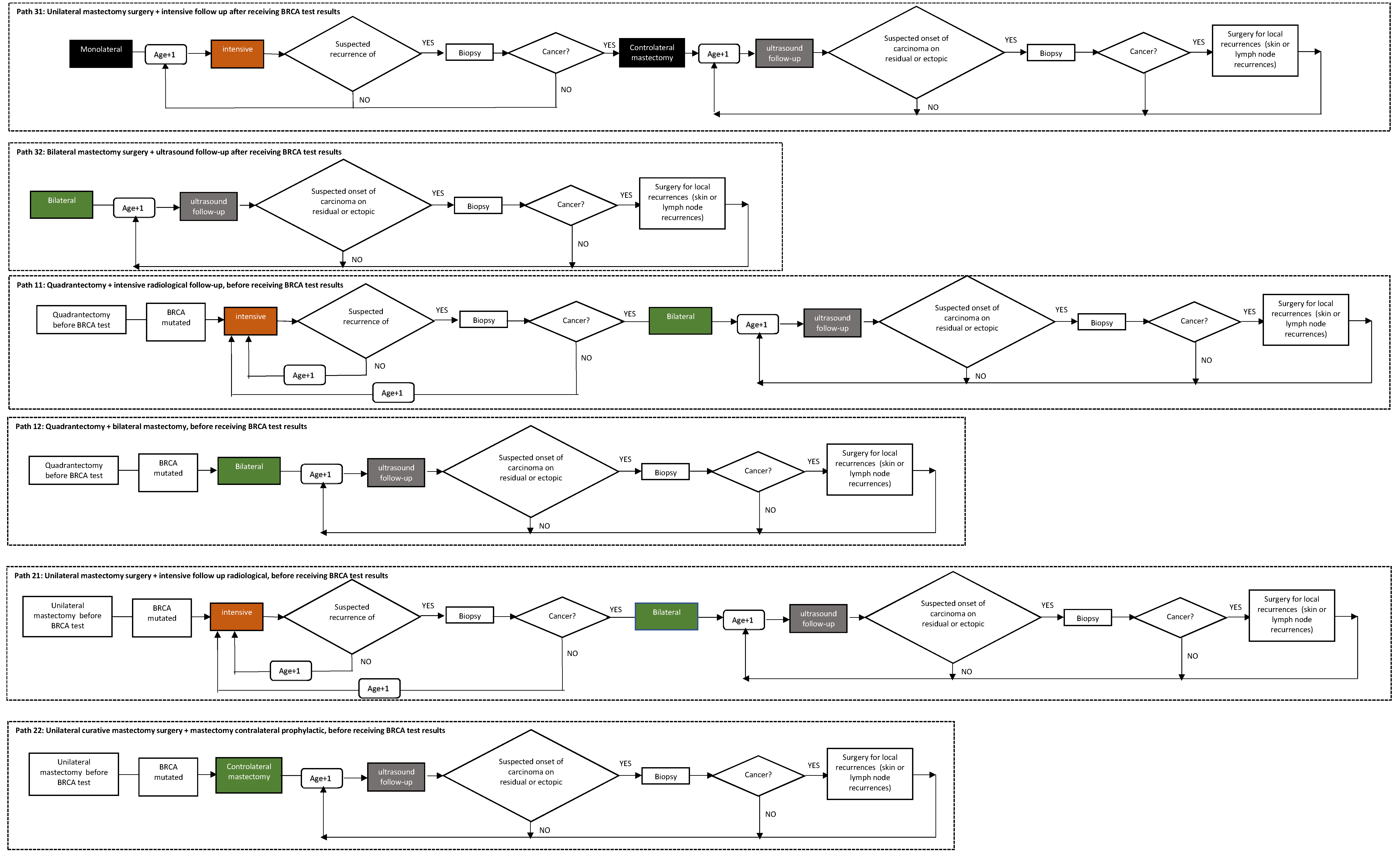
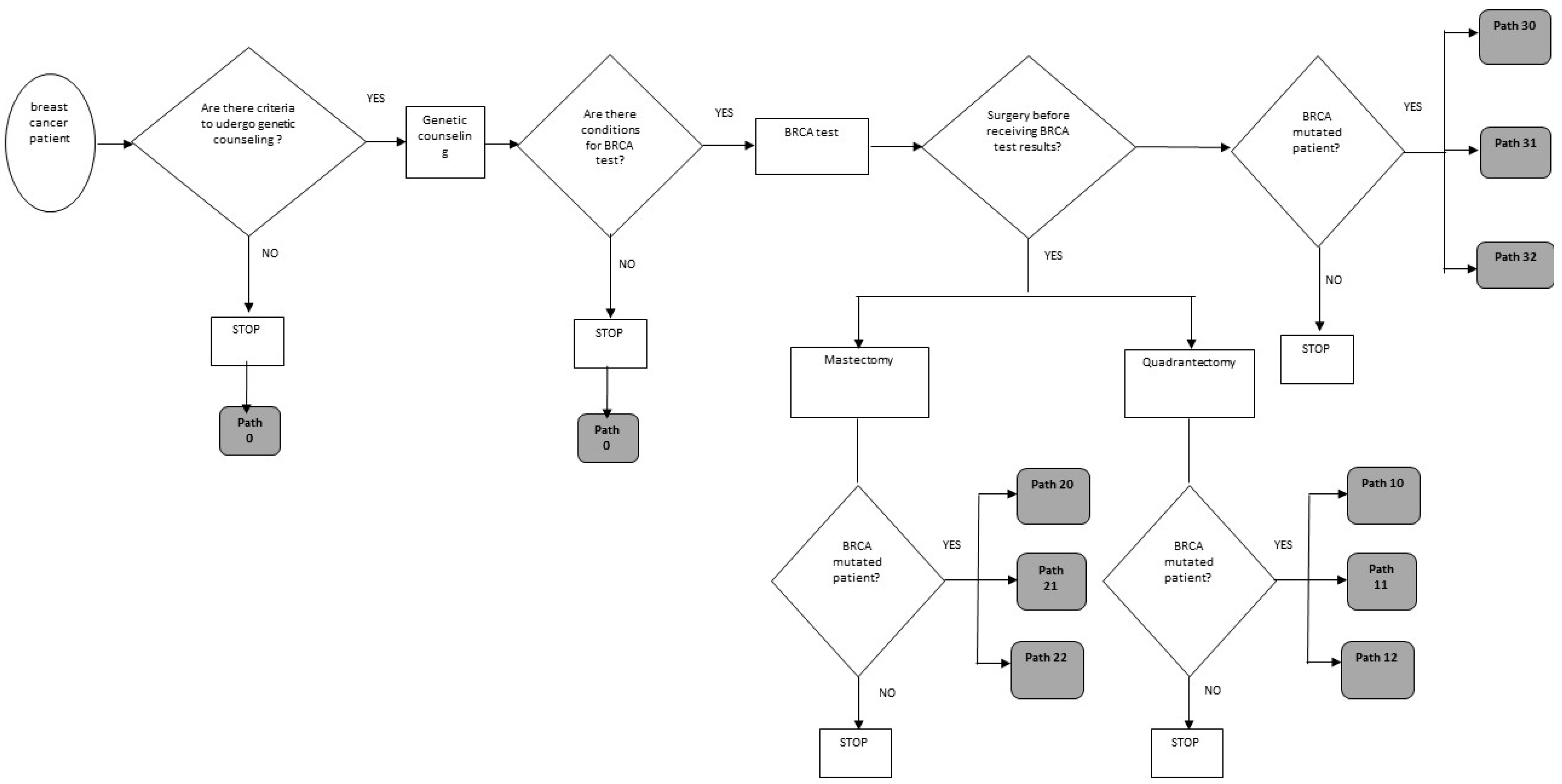
- Path 0: Patient not subjected to genetic counseling and/or BRCA test;
- -
- Path 10: Quadrantectomy surgery without preference for chances (quadrantectomy + intensive follow-up or quadrantectomy + bilateral mastectomy) before receiving BRCA test results;
- -
- Path 11: Quadrantectomy + intensive radiological follow-up, before receiving BRCA test results;
- -
- Path 12: Quadrantectomy + bilateral mastectomy, before receiving BRCA test results;
- -
- Path 20: Mastectomy surgery with no preference for chances (unilateral mastectomy + intensive follow-up or unilateral mastectomy + contralateral prophylactic), before receiving BRCA test results;
- -
- Path 21: Unilateral curative mastectomy surgery + intensive radiological follow-up, before receiving BRCA test results;
- -
- Path 22: Unilateral curative mastectomy surgery + mastectomy contralateral prophylactic, before receiving BRCA test results;
- -
- Path 30: Surgery without preference for therapeutic chance (mastectomy unilateral + radiological follow-up or bilateral mastectomy) after receiving BRCA test results;
- -
- Path 31: Unilateral curative mastectomy surgery + intensive follow-up after receiving BRCA test results;
- -
- Path 32: Bilateral mastectomy surgery + ultrasound follow-up after receiving BRCA test results.
- Calculation of the costs associated with the therapeutic possibilities;
- Comparison of the costs of alternative therapeutic possibilities and the choice of the one with the lowest cost (“optimal therapeutic path”);
- Calculation of the cost of the therapeutic path in the “as is” scenario: The therapeutic path that the patient would follow without the optimization model, defined on the basis of historical data;
- Comparison of the cost of the “optimal therapeutic path” with the cost of the “as is” therapeutic path;
- Calculation of the unit savings per affected patient: The cost savings that would be obtained by choosing the optimal therapeutic path, throughout the patient’s entire residual life (over a time horizon of 35 years). It is calculated by considering all the net potential savings (or costs) generated by the optimal path in each year, until the end of the life of the patient, discounted with a predefined discount rate. Specifically, the net present value (NPV) is used to calculate the present value (actual unit of savings per affected patient) of a series of future payments (with a discount rate of 3%) [31].
2.3. Input Data
2.3.1. Probabilities
2.3.2. Costs
2.4. Sensitivity and Scenario Analysis
- Scenario 1: What would happen if genetic counseling were extended to all patients?
- Scenario 2: What would happen if genetic counseling and BRCA testing were extended to all patients? What benefits would be gained by extending genetic counseling and testing BRCA to patients with different starting ages?
- Scenario 3: What would happen if all affected patients were treated after having received the BRCA test result? If performing the surgery after the result of the BRCA test, which therapeutic path is preferable to choose between intensive follow-up and prophylactic surgery?
3. Results
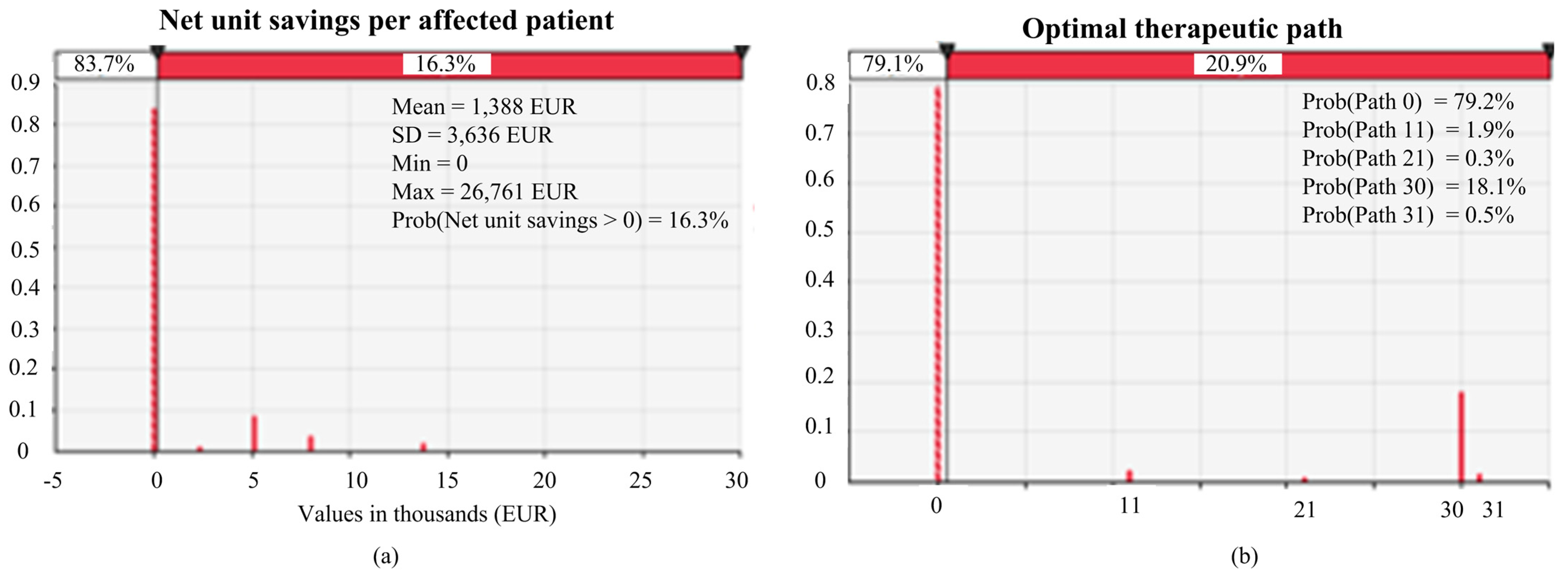
3.1. Results of Base-Case Analysis
3.2. Results of Sensitivity and Scenario Analysis
4. Discussion and Conclusions
Limitations and Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Capri, S.; Russo, A. Cost of breast cancer based on real-world data: A cancer registry study in Italy. BMC Health Serv. Res. 2017, 17, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, P.T.; Olivotto, I.A.; Whelan, T.J.; Levine, M. Clinical practice guidelines for the care and treatment of breast cancer: 16. Locoregional post-mastectomy radiotherapy. Can. Med. Assoc. J. 2004, 170, 1263–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronesi, U.; Marubini, E.; Mariani, L.; Galimberti, V.; Luini, A.; Salvadori, B.; Zucali, R. Radiotherapy after breast-conserving surgery in small breast carcinoma: Long-term results of a randomized trial. Ann. Oncol. 2001, 12, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Tuffaha, H.W.; Mitchell, A.; Ward, R.; Connelly, L.; Butler, J.R.G.; Norris, S.; Scuffham, P. Cost-effectiveness analysis of germ-line BRCA testing in women with breast cancer and cascade testing in family members of mutation carriers. Genet. Med. 2008, 20, 985–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniou, A.; Pharoah, P.D.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, Å. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavaddat, N.; Peock, S.; Frost, D.; Ellis, S.; Platte, R.; Fineberg, E.; Evans, D.G.; Izatt, L.; Eeles, R.A.; Adlard, J.; et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: Results from prospective analysis of EMBRACE. J. Natl. Cancer Inst. 2013, 105, 812–822. [Google Scholar] [CrossRef] [Green Version]
- Haffty, B.G.; Harrold, E.; Khan, A.J.; Pathare, P.; Smith, T.E.; Turner, B.C.; Glazer, P.M.; Ward, B.; Carter, D.; Matloff, E.; et al. Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. Lancet 2002, 359, 1471–1477. [Google Scholar] [CrossRef]
- Schrauder, M.G.; Brunel-Geuder, L.; Häberle, L.; Wunderle, M.; Hoyer, J.; Reis, A.; Schulz-Wendtland, R.; Beckmann, M.W.; Lux, M.P. Cost-effectiveness of risk-reducing surgeries in preventing hereditary breast and ovarian cancer. Breast 2017, 32, 186–191. [Google Scholar] [CrossRef]
- Heemskerk-Gerritsen, B.A.M.; Jager, A.; Koppert, L.B.; Obdeijn, A.I.-M.; Collée, M.; Meijers-Heijboer, H.E.J.; Jenner, D.J.; Oldenburg, H.S.A.; Van Engelen, K.; De Vries, J.; et al. Survival after bilateral risk-reducing mastectomy in healthy BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2019, 177, 723–733. [Google Scholar] [CrossRef] [Green Version]
- Paluch-Shimon, S.; Cardoso, F.; Sessa, C.; Balmaña, J.; Cardoso, M.J.; Gilbert, F.; Senkus, E. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 2016, 27 (Suppl. 5), v103–v110. [Google Scholar] [CrossRef]
- Plevritis, S.K.; Kurian, A.W.; Sigal, B.M.; Daniel, B.L.; Ikeda, D.M.; Stockdale, F.E.; Garber, A.M. Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. JAMA 2006, 295, 2374–2384. [Google Scholar] [CrossRef] [Green Version]
- Lo, G.; McLaughlin, A.; Jacques, A.; Dhillon, R.; Porter, G.; Jayaratne, T.; Bose, S.; Bourke, A. Does Medicare-eligible high-risk breast cancer screening MRI target the right women? J. Med. Imaging Radiat. Oncol. 2020, 64, 220–228. [Google Scholar] [CrossRef]
- Plevritis, S.K.; Ikeda, D.M. Ethical issues in contrastenhanced magnetic resonance imaging screening for breast cancer. Top Magn. Reson. Imaging. 2002, 13, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Forbes, C.; Fayter, D.; de Kock, S.; Quek, R.G. A systematic review of international guidelines and recommendations for the genetic screening, diagnosis, genetic counseling, and treatment of BRCA-mutated breast cancer. Cancer Manag. Res. 2019, 11, 2321–2337. [Google Scholar] [CrossRef] [Green Version]
- Butler, J.R.; Furnival, C.M.; Hart, R.F. Estimating treatment cost functions for progressive diseases: A multiproduct approach with an application to breast cancer. J. Health Econ. 1995, 14, 361–385. [Google Scholar] [CrossRef]
- Kriege, M.; Brekelmans, C.T.; Boetes, C.; Besnard, P.E.; Zonderland, H.M.; Obdeijn, I.M.; Manoliu, R.A.; Kok, T.; Peterse, H.; Tilanus-Linthorst, M.M.A.; et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N. Engl. J. Med. 2004, 351, 427–437. [Google Scholar] [CrossRef] [Green Version]
- MARIBS Study Group. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: A prospective multicentre cohort study (MARIBS). Lancet 2005, 365, 1769–1778. [Google Scholar] [CrossRef]
- Warner, E.; Plewes, D.B.; Hill, K.A.; Causer, P.A.; Zubovits, J.T.; Jong, R.A.; Narod, S.A. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 2004, 292, 1317–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lostumbo, L.; Carbine, N.E.; Wallace, J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst. Rev. 2010, 11, CD002748. [Google Scholar] [CrossRef]
- Domchek, S.M.; Friebel, T.M.; Evans, D.G.; Lynch, H.T.; Isaacs, C. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA J. Am. Med. Assoc. 2010, 304, 967–975. [Google Scholar] [CrossRef] [Green Version]
- La Forgia, D.; Moschetta, M.; Fausto, A.; Cutrignelli, D.; Dentamaro, R.; Losurdo, L.; Portincasa, A. Anaplastic large-cell periprosthetic lymphoma of the breast: Could fibrin be an early radiological indicator of the presence of disease. J. BUON 2019, 24, 1889–1897. [Google Scholar] [PubMed]
- La Forgia, D.; Catino, A.; Fausto, A.; Cutrignelli, D.; Fanizzi, A.; Gatta, G.; Portincasa, A. Diagnostic challenges and potential early indicators of breast periprosthetic anaplastic large cell lymphoma: A case report. Medicine 2020, 99, e21095. [Google Scholar] [CrossRef]
- La Forgia, D.; Moschetta, M.; Fausto, A.; Cutrignelli, D.; Dentamaro, R.; Fanizzi, A.; Portincasa, A. Early indicators in anaplastic large-cell periprosthetic lymphoma of the breast: Clarifications. J. BUON 2020, 25, 2127–2128. [Google Scholar]
- Negrín, M.A.; Vázquez-Polo, F.J. Incorporating model uncertainty in cost-effectiveness analysis: A Bayesian model averaging approach. J. Health Econ. 2008, 27, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Basu, A. A welfare-theoretic model consistent with the practice of cost-effectiveness analysis and its implications. J. Health Econ. 2020, 70, 102287. [Google Scholar] [CrossRef]
- Hao, J.; Hassen, D.; Manickam, K.; Murray, M.F.; Hartzel, D.N.; Hu, Y.; Snyder, S.R. Healthcare utilization and costs after receiving a positive BRCA1/2 result from a genomic screening program. J. Pers. Med. 2020, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Guzauskas, G.F.; Garbett, S.; Zhou, Z.; Spencer, S.J.; Smith, H.S.; Hao, J.; Veenstra, D.L. Cost-effectiveness of Population-Wide Genomic Screening for Hereditary Breast and Ovarian Cancer in the United States. JAMA Netw. Open 2020, 3, e2022874. [Google Scholar] [CrossRef]
- Petelin, L.; Trainer, A.H.; Mitchell, G.; Liew, D.; James, P.A. Cost-effectiveness and comparative effectiveness of cancer risk management strategies in BRCA1/2 mutation carriers: A systematic review. Genet. Med. 2018, 20, 1145–1156. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [Green Version]
- Oeffinger, K.C.; Fontham, E.T.; Etzioni, R.; Herzig, A.; Michaelson, J.S.; Shih, Y.C.T.; Walter, L.C.; Church, T.R.; Flowers, C.R.; LaMonte, S.J.; et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015, 314, 1599–1614. [Google Scholar] [CrossRef]
- Gamble, C.; Havrilesky, L.J.; Myers, E.R.; Chino, J.P.; Hollenbeck, S.; Plichta, J.K.; Marcom, P.K.; Hwang, E.S.; Kauff, N.D.; Greenup, R.A. Cost Effectiveness of Risk-Reducing Mastectomy versus Surveillance in BRCA Mutation Carriers with a History of Ovarian Cancer. Ann. Surg. Oncol. 2017, 24, 3116–3123. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Habibi, M.; Frick, K.D. Cost-Effectiveness of Contralateral Prophylactic Mastectomy for Prevention of Contralateral Breast Cancer. Ann. Surg. Oncol. 2014, 21, 2209–2217. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version 3. 2019. Available online: http://www.nccn.org (accessed on 2 December 2020).
- Weitzel, J.N.; McCaffrey, S.M.; Nedelcu, R.; MacDonald, D.J.; Blazer, K.R.; Cullinane, C.A. Effect of genetic cancer risk assessment on surgical decisions at breast cancer diagnosis. Arch. Surg. 2003, 138, 1323–1328. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, K.; Gershman, S.; Ghadirian, P.; Lynch, H.T.; Snyder, C.; Tung, N.; Kim-Sing, C.; Eisen, A.; Foulkes, W.; Rosen, B.; et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: Retrospective anal. BMJ 2014, 348, g226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, M.B.; West, C.N.; Liu, I.L.A.; Harris, E.L.; Rolnick, S.J.; Elmore, J.G.; Geiger, A.M. Complications following bilateral prophylactic mastectomy. JNCI Monogr. 2005, 2005, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donker, M.; Hage, J.J.; Woerdeman, L.A.E.; Rutgers, E.T.; Sonke, G.S.; Peeters, M.J.V. Surgical complications of skin sparing mastectomy and immediate prosthetic reconstruction after neoadjuvant chemotherapy for invasive breast cancer. Eur. J. Surg. Oncol. 2012, 38, 25–30. [Google Scholar] [CrossRef]
- Lee, K.T.; Kim, S.; Jeon, B.J.; Pyon, J.K.; Mun, G.H.; Ryu, J.M.; Bang, S.I. Association of the implant surface texture used in reconstruction with breast cancer recurrence. JAMA Surg. 2020, 155, 1132–1140. [Google Scholar] [CrossRef]
- Woitek, R.; Pfeiler, G.; Farr, A.; Kapetas, P.; Furtner, J.; Bernathova, M.; Helbich, T.H. MRI-based quantification of residual fibroglandular tissue of the breast after conservative mastectomies. Eur. J. Radiol. 2018, 104, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mun, J. Modeling Risk: Applying Monte Carlo Simulation, Real Options Analysis, Forecasting, and Optimization Techniques; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 347. [Google Scholar]
- Vose, D. Quantitative Risk Analysis: A Guide to Monte Carlo Simulation Modelling; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Penn, C.A.; Wong, M.S.; Lee, J.; Walsh, C. Cost-minimization analysis of germline and somatic testing strategies for BRCA mutations in women with newly diagnosed epithelial ovarian cancer. Gynecol. Oncol. 2020, 159, 127. [Google Scholar] [CrossRef]
- Asban, A.; Homsy, C.; Chen, L.; Fisher, C.; Losken, A.; Chatterjee, A. A cost-utility analysis comparing large volume displacement oncoplastic surgery to mastectomy with single stage implant reconstruction in the treatment of breast cancer. Breast 2018, 41, 159–164. [Google Scholar] [CrossRef]
- Müller, D.; Danner, M.; Rhiem, K.; Stollenwerk, B.; Engel, C.; Rasche, L.; Borsi, L.; Schmutzler, R.; Stock, S. Cost-effectiveness of different strategies to prevent breast and ovarian cancer in German women with a BRCA 1 or 2 mutation. Eur. J. Health Econ. 2018, 19, 341–353. [Google Scholar] [CrossRef]
- Collins, I.M.; Milne, R.L.; Weideman, P.C. Preventing breast and ovarian cancers in high-risk BRCA1 and BRCA2 mutation carriers. Med. J. Aust. 2013, 199, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.; Weston, R.; Qu, L. Families Then and Now: 1980–2010; Australian Institute of Family Studies: Melbourne, Australia, 2010. [Google Scholar]
- Konstantopoulou, I.; Rampias, T.; Ladopoulou, A. Greek BRCA1 and BRCA2 mutation spectrum: Two BRCA1 mutations account for half the carriers found among high-risk breast/ovarian cancer patients. Breast Cancer Res. Treat. 2008, 107, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Ristori, E.; Giannini, G.; Gulino, A. BRCA1 and BRCA2: The genetic testing and the current management options for mutation carriers. Crit. Rev. Oncol. Hematol. 2006, 57, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Malone, K.E.; Daling, J.R.; Doody, D.R. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res. 2006, 66, 8297–8308. [Google Scholar] [CrossRef] [Green Version]
- Eccleston, A.; Bentley, A.; Dyer, M.; Strydom, A.; Vereecken, W.; George, A.; Rahman, N. A Cost-Effectiveness Evaluation of Germline BRCA1 and BRCA2 Testing in UK Women with Ovarian Cancer. Value Health 2017, 5, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Fattore, G.; Torbica, A. Inpatient reimbursement system in Italy: How do tariffs relate to costs? Health Care Manag. Sci. 2006, 9, 251–258. [Google Scholar] [CrossRef]
- Maio, M.; Ascierto, P.; Testori, A.; Ridolfi, R.; Bajetta, E.; Queirolo, P.; Guida, M.; Romanini, A.; Chiarion-Sileni, V.; Pigozzo, J.; et al. The cost of unresectable stage III or stage IV melanoma in Italy. J. Exp. Clin. Cancer Res. 2012, 31, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mullins, C.D.; Sikirica, M.; Seneviratne, V.; Ahn, J.; Akhras, K.S. Comparisons of Hypertension-Related Costs from Multinational Clinical Studies. Pharmacoeconomics 2004, 22, 1001–1014. [Google Scholar] [CrossRef]
- Slade, I.; Hanson, H.; George, A.; Kohut, K.; Strydom, A.; Wordsworth, S.; Rahman, N. A cost analysis of a cancer genetic service model in the UK. J. Community Genet. 2016, 7, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Bernstein-Molho, R.; Kaufman, B.; David, M.A.B.; Sklair-Levy, M.; Feldman, D.M.; Zippel, D.; Friedman, E. Breast cancer surveillance for BRCA1/2 mutation carriers–is “early detection” early enough? Breast 2020, 49, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, E.; Zabor, E.C.; Stempel, M.; Mangino, D.; Heerdt, A.; Pilewskie, M. Differences among a modern cohort of BRCA mutation carriers choosing bilateral prophylactic mastectomies compared to breast surveillance. Ann. Surg. Oncol. 2017, 24, 3048–3054. [Google Scholar] [CrossRef]
- La Forgia, D.; Fanizzi, A.; Campobasso, F.; Bellotti, R.; Didonna, V.; Lorusso, V.; Moschetta, M.; Massafra, R.; Tamborra, P.; Tangaro, S.; et al. Radiomic Analysis in Contrast-Enhanced Spectral Mammography for Predicting Breast Cancer Histological Outcome. Diagnostics 2020, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Fanizzi, A.; Basile, T.M.A.; Losurdo, L.; Amoroso, N.; Bellotti, R.; Bottigli, U.; Dentamaro, R.; Didonna, V.; Fausto, A.; Massafra, R.; et al. Hough transform for clustered microcalcifications detection in full-field digital mammograms. In Proceedings of the Conference on Applications of Digital Image Processing XL, San Diego, CA, USA, 7–10 August 2017; International Society for Optics and Photonics: Bellingham, WA, USA, 2017; Volume 10396, p. 1039616. [Google Scholar] [CrossRef]
- Fanizzi, A.; Basile, T.M.; Losurdo, L.; Bellotti, R.; Bottigli, U.; Campobasso, F.; Didonna, V.; Fausto, A.; Massafra, R.; Tagliafico, A.; et al. Ensemble discrete wavelet transform and gray-level co-occurrence matrix for microcalcification cluster classification in digital mammography. Appl. Sci. 2019, 9, 5388. [Google Scholar] [CrossRef]
- Losurdo, L.; Fanizzi, A.; Basile, T.M.A.; Bellotti, R.; Bottigli, U.; Dentamaro, R.; Tagliafico, A. Radiomics Analysis on Contrast-Enhanced Spectral Mammography Images for Breast Cancer Diagnosis: A Pilot Study. Entropy 2019, 21, 1110. [Google Scholar] [CrossRef] [Green Version]
| Variable | Distribution | Source | |
|---|---|---|---|
| Starting age (affected) | Normal | Mean = 40 SD = 2.5 | [4,45,46] |
| Probability of being BRCA-mutation-positive in affected individuals | Uniform | Min = 10% Max = 20% | [4,47,48,49] |
| Annual risk of new incidence of breast cancer if BRCA-positive | 20–29 years | 0.005 | [4] |
| 30–39 | 0.015 | ||
| 40–49 | 0.03 | ||
| 50–59 | 0.026 | ||
| 60–69 | 0.012 | ||
| 70–79 | 0.012 | ||
| Annual risk of contralateral breast cancer if BRCA-positive | 20–29 years | 0 | [4] |
| 30–39 | 0.05 | ||
| 40–49 | 0.04 | ||
| 50–59 | 0.03 | ||
| 60–69 | 0.03 | ||
| 70–79 | 0.03 | ||
| Probability that patient is treated with radiotherapy after mastectomy | 40% | Historical data | |
| Probability that patient is treated with radiotherapy after quadrantectomy | 95% | Historical data | |
| Probability of undergoing genetic counseling | Bernoulli | 45% | Historical data |
| Probability of undergoing BRCA genetic testing | Bernoulli | 45% | Historical data |
| Probability of detecting suspected local recurrence (skin or lymph node recurrences) | Bernoulli | 5% | Historical data |
| Risk of surgery complications | Uniform | min = 10% max = 20% | Historical data |
| Positive biopsy rate | Bernoulli | 60% | Historical data |
| Therapeutic Options | Data Inputs |
|---|---|
| % affected patients undergoing surgery after receiving BRCA test results (Path 30) | 26% |
| % affected patients undergoing quadrantectomy before receiving BRCA test results (Path 10) | 70% |
| % affected patients, BRCA-positive, choosing intensive breast screening (intensive follow-up) after quadrantectomy (Path 11) | 20% |
| % affected BRCA-positive patients choosing bilateral mastectomy (RRM) and ultrasound follow-up after quadrantectomy (Path 12) | 80% |
| % affected patients undergoing mastectomy before receiving BRCA test results (Path 20) | 30% |
| % affected BRCA-positive patients choosing intensive breast screening (intensive follow-up) after mastectomy (Path 21) | 20% |
| % affected BRCA-positive patients choosing contralateral mastectomy (RRM) and ultrasound follow-up after mastectomy (Path 22) | 80% |
| % affected patients undergoing monolateral mastectomy after receiving BRCA test results, if BRCA-positive (Path 31) | 70% |
| % affected patients undergoing bilateral mastectomy after receiving BRCA test results, if BRCA-positive (Path 32) | 30% |
| Activity | Cost (EUR) | Notes | Reference |
|---|---|---|---|
| Quadrantectomy | 2,354 | Without complications | NHS: DRG code 259 |
| 2,717 | With complications | NHS: DRG code 260 | |
| Intensive breast screening (intensive follow-up) | 263.31 | mammography and breast magnetic resonance imaging (MRI) | NHS: DRG codes 87371-88929-897 |
| Biopsy | 52.08 | core-biopsy | NHS: DRG code 85111 |
| Mastectomy including reconstructive surgery | 8,265 | Without complications | NHS: DRG codes 258-461 |
| 8,872 | With complications | NHS: DRG codes 257-461 | |
| Bilateral mastectomy including reconstructive surgery | 16,530 | Without complications | NHS: DRG codes 258-461 |
| 17,744 | With complications | NHS: DRG codes 257-461 | |
| Ultrasound follow-up | 56.55 | Breast examination and ultrasound | NHS: DRG codes 88731-897 |
| Surgery for local recurrences (skin or lymph node recurrences) | 4,583 | NHS: DRG code 19881 | |
| Plastic surgery after complications or for breast implant replacement after 15 years | 4,924 | NHS: DRG code 461 | |
| Radiotherapy | 2,936 | cost per regimen in combination with systemic therapy | NHS: DRG code 409 |
| Genetic counseling | 20.01 | NHS: DRG code 897B1 | |
| BRCA testing | 1,107 | Primary data collection |
| Net Unit Savings (EUR) | Optimal Therapeutic Path | |||||||
|---|---|---|---|---|---|---|---|---|
| Scenario 1 | (A) | Mean | 2,982 | Prob(Path 0) | 55.00% | |||
| SD | 5,564 | Prob(Path 11) | 3.40% | |||||
| Min | 0 | Prob(Path 21) | 1.60% | |||||
| Max | 36,398 | Prob(Path 30) | 38.70% | |||||
| Prob(Net unit savings > 0) | 34.20% | Prob(Path 31) | 1.30% | |||||
| Scenario 2 | (B) | Mean | 6,360 | Prob(Path 11) | 8.70% | |||
| SD | 6,458 | Prob(Path 21) | 4.20% | |||||
| Min | 0 | Prob(Path 30) | 84.40% | |||||
| Max | 39,000 | Prob(Path 31) | 2.70% | |||||
| Prob(Net unit savings > 0) | 75.70% | |||||||
| Net unit savings (EUR) | ||||||||
| Starting age (years) | 20 | 30 | 40 | 50 | 60 | 70 | ||
| (C) | Mean | 6,526 | 6,623 | 6,360 | 6,719 | 6,583 | 6,447 | |
| SD | 6,404 | 6,755 | 6,458 | 6,968 | 6,603 | 6,115 | ||
| Min | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Max | 36,669 | 45,561 | 39,000 | 37,923 | 37,308 | 40,083 | ||
| Prob(Net unit savings > 0) | 76.90% | 76.20% | 75.70% | 75.70% | 76.10% | 76.60% | ||
| Costs throughout the life of the patient of the most probable “as is” scenarios in BRCA-mutated patients (values in thousands (EUR)) | ||||||||
| Cost of Path 21 | Cost of Path 32 | |||||||
| Scenario 3 | (D) | Mean | 3,034 | 4,718 | ||||
| SD | 7,329 | 11,199 | ||||||
| Min | 0 | 0 | ||||||
| Max | 32,204 | 44,105 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbonara, N.; La Forgia, D.; Pellegrino, R.; Ressa, C.; Tommasi, S. A Cost Decision Model Supporting Treatment Strategy Selection in BRCA1/2 Mutation Carriers in Breast Cancer. J. Pers. Med. 2021, 11, 847. https://doi.org/10.3390/jpm11090847
Carbonara N, La Forgia D, Pellegrino R, Ressa C, Tommasi S. A Cost Decision Model Supporting Treatment Strategy Selection in BRCA1/2 Mutation Carriers in Breast Cancer. Journal of Personalized Medicine. 2021; 11(9):847. https://doi.org/10.3390/jpm11090847
Chicago/Turabian StyleCarbonara, Nunzia, Daniele La Forgia, Roberta Pellegrino, Cosmo Ressa, and Stefania Tommasi. 2021. "A Cost Decision Model Supporting Treatment Strategy Selection in BRCA1/2 Mutation Carriers in Breast Cancer" Journal of Personalized Medicine 11, no. 9: 847. https://doi.org/10.3390/jpm11090847
APA StyleCarbonara, N., La Forgia, D., Pellegrino, R., Ressa, C., & Tommasi, S. (2021). A Cost Decision Model Supporting Treatment Strategy Selection in BRCA1/2 Mutation Carriers in Breast Cancer. Journal of Personalized Medicine, 11(9), 847. https://doi.org/10.3390/jpm11090847







