Poor Prognosis of Diffuse Large B-Cell Lymphoma with Hepatitis C Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Risk Groups and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
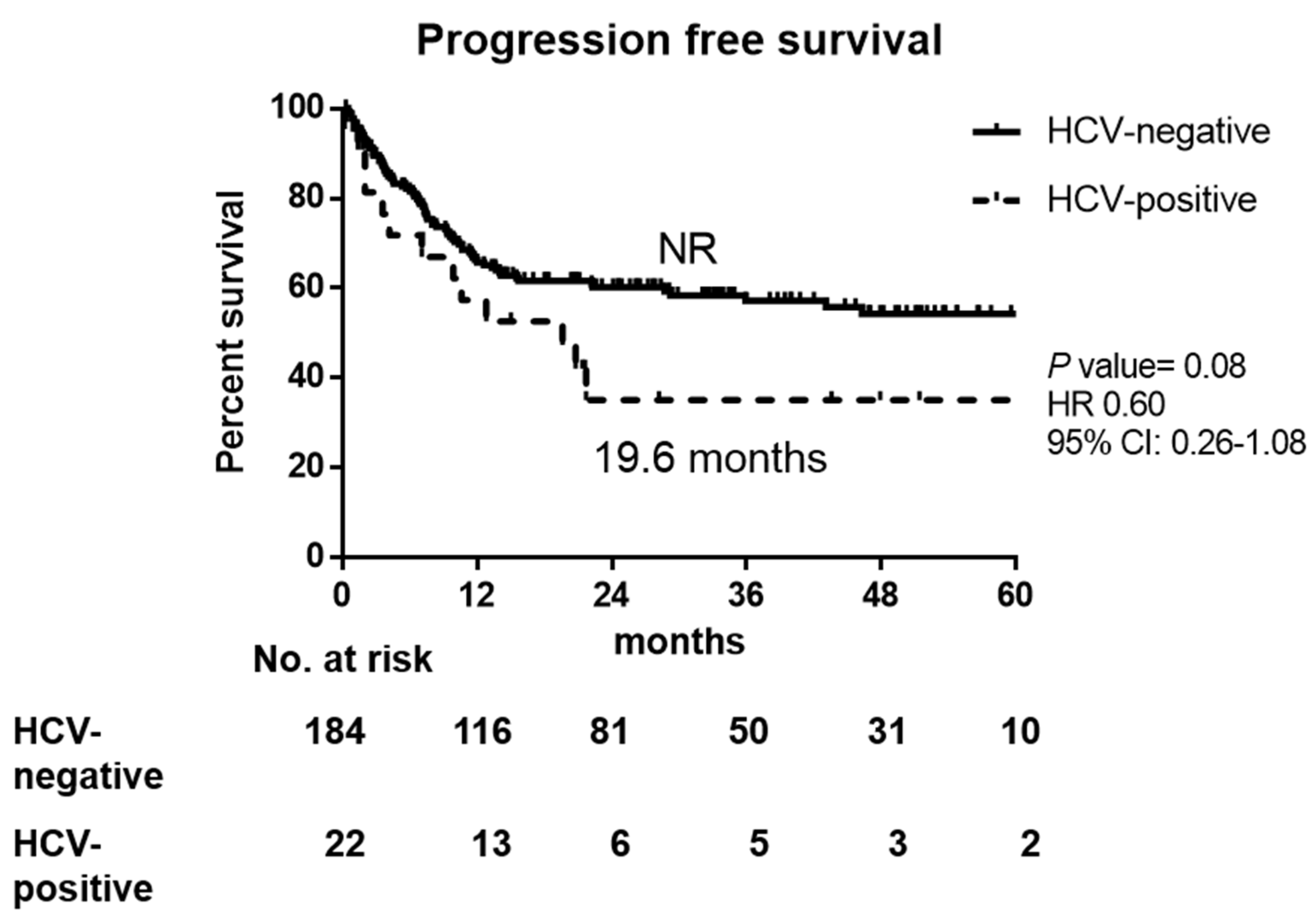
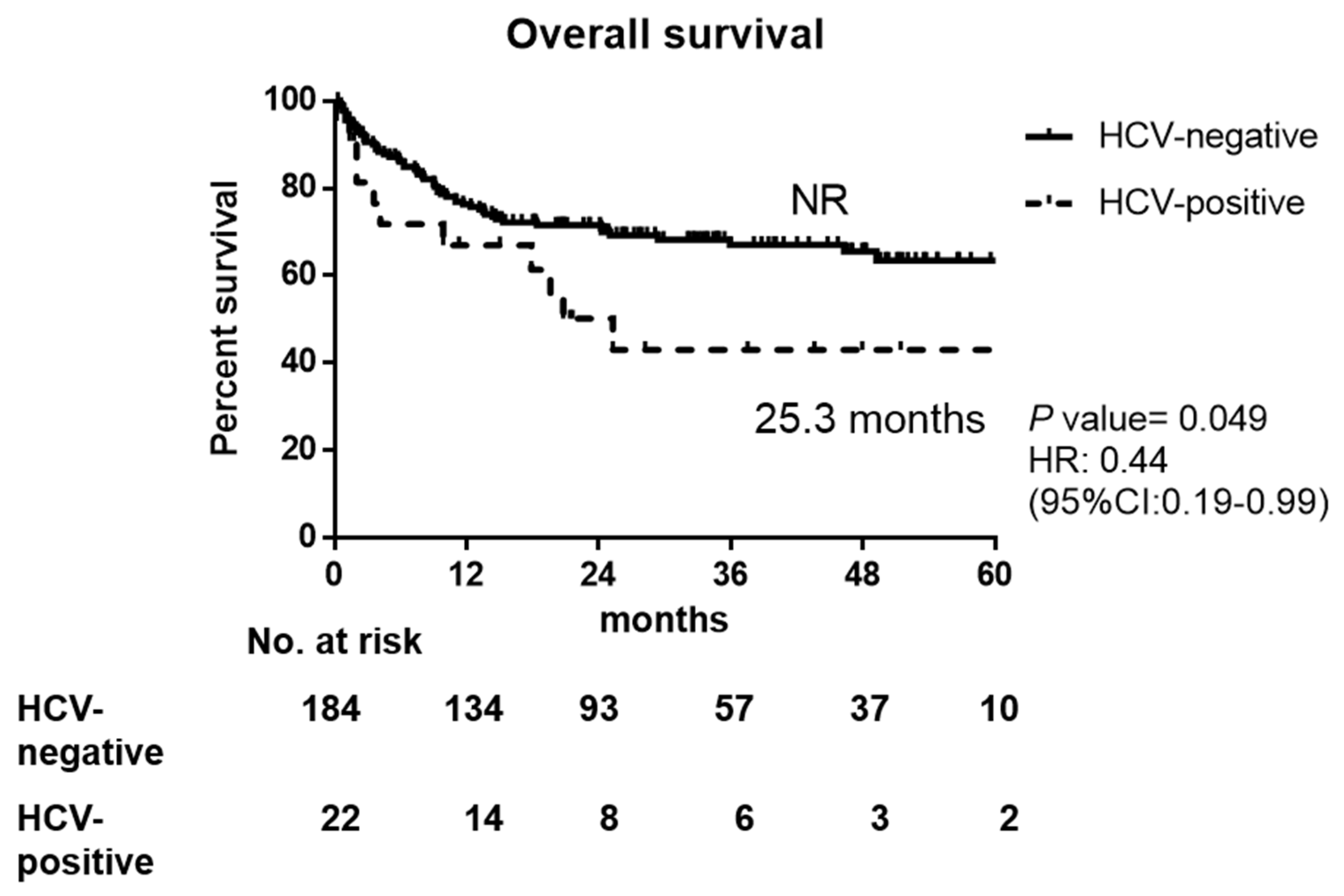
3.2. Response to Therapy and Outcomes
3.3. Prognostic Factors for Overall Survival and Progression Free Survival in DLBCL Patients
3.4. Details of HCV-Positive DLBCL Patients during Follow-Up Period
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Defrancesco, I.; Zerbi, C.; Rattotti, S.; Merli, M.; Bruno, R.; Paulli, M.; Arcaini, L. HCV infection and non-Hodgkin lymphomas: An evolving story. Clin. Exp. Med. 2020, 20, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Negro, F.; Forton, D.; Craxi, A.; Sulkowski, M.S.; Feld, J.J.; Manns, M.P. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology 2015, 149, 1345–1360. [Google Scholar] [CrossRef]
- Dammacco, F.; Lauletta, G.; Russi, S.; Leone, P.; Tucci, M.; Manno, C.; Monaco, S.; Ferrari, S.; Vacca, A.; Racanelli, V. Clinical practice: Hepatitis C virus infection, cryoglobulinemia and cryoglobulinemic vasculitis. Clin. Exp. Med. 2019, 19, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.J.; Lai, A.S.; Krajden, M.; Andonov, A.; Gascoyne, R.D.; Connors, J.M.; Brooks-Wilson, A.R.; Gallagher, R.P. Hepatitis C virus and risk of non-Hodgkin lymphoma in British Columbia, Canada. Int. J. Cancer 2008, 122, 630–633. [Google Scholar] [CrossRef] [PubMed]
- de Sanjose, S.; Benavente, Y.; Vajdic, C.M.; Engels, E.A.; Morton, L.M.; Bracci, P.M.; Spinelli, J.J.; Zheng, T.; Zhang, Y.; Franceschi, S.; et al. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin. Gastroenterol. Hepatol. 2008, 6, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Viswanatha, D.S.; Dogan, A. Hepatitis C virus and lymphoma. J. Clin. Pathol. 2007, 60, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, R.G. Hepatitis C virus—associated B cell non-Hodgkin’s lymphoma. World J. Gastroenterol. 2016, 22, 6214–6223. [Google Scholar] [CrossRef]
- Suarez, F.; Lortholary, O.; Hermine, O.; Lecuit, M. Infection-associated lymphomas derived from marginal zone B cells: A model of antigen-driven lymphoproliferation. Blood 2006, 107, 3034–3044. [Google Scholar] [CrossRef]
- Provencio, M.; Sanchez, A.; Bonilla, F.; Espana, P. Association between hepatitis C virus and non-Hodgkin’s lymphomas. J. Clin. Oncol. 2006, 24, 3513. [Google Scholar] [CrossRef]
- Zignego, A.L.; Ramos-Casals, M.; Ferri, C.; Saadoun, D.; Arcaini, L.; Roccatello, D.; Antonelli, A.; Desbois, A.C.; Comarmond, C.; Gragnani, L.; et al. International therapeutic guidelines for patients with HCV-related extrahepatic disorders. A multidisciplinary expert statement. Autoimmun. Rev. 2017, 16, 523–541. [Google Scholar] [CrossRef]
- Arcaini, L.; Bruno, R. Hepatitis C virus infection and antiviral treatment in marginal zone lymphomas. Curr. Clin. Pharm. 2010, 5, 74–81. [Google Scholar] [CrossRef]
- Sehn, L.H.; Salles, G. Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef] [PubMed]
- Besson, C.; Canioni, D.; Lepage, E.; Pol, S.; Morel, P.; Lederlin, P.; Van Hoof, A.; Tilly, H.; Gaulard, P.; Coiffier, B.; et al. Characteristics and outcome of diffuse large B-cell lymphoma in hepatitis C virus-positive patients in LNH 93 and LNH 98 Groupe d’Etude des Lymphomes de l’Adulte programs. J. Clin. Oncol. 2006, 24, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Park, B.B.; Kim, J.S.; Lee, Y.Y.; Kang, H.J.; Ryoo, B.Y.; Kang, J.H.; Kim, H.Y.; Kim, B.S.; Oh, S.Y.; Kwon, H.C.; et al. Clinical characteristics and outcome for hepatitis C virus-positive diffuse large B-cell lymphoma. Leuk. Lymphoma 2008, 49, 88–94. [Google Scholar] [CrossRef]
- Visco, C.; Finotto, S. Hepatitis C virus and diffuse large B-cell lymphoma: Pathogenesis, behavior and treatment. World J. Gastroenterol. 2014, 20, 11054–11061. [Google Scholar] [CrossRef]
- Merli, M.; Visco, C.; Spina, M.; Luminari, S.; Ferretti, V.V.; Gotti, M.; Rattotti, S.; Fiaccadori, V.; Rusconi, C.; Targhetta, C.; et al. Outcome prediction of diffuse large B-cell lymphomas associated with hepatitis C virus infection: A study on behalf of the Fondazione Italiana Linfomi. Haematologica 2014, 99, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Ennishi, D.; Maeda, Y.; Niitsu, N.; Kojima, M.; Izutsu, K.; Takizawa, J.; Kusumoto, S.; Okamoto, M.; Yokoyama, M.; Takamatsu, Y.; et al. Hepatic toxicity and prognosis in hepatitis C virus-infected patients with diffuse large B-cell lymphoma treated with rituximab-containing chemotherapy regimens: A Japanese multicenter analysis. Blood 2010, 116, 5119–5125. [Google Scholar] [CrossRef][Green Version]
- Sun, C.A.; Chen, H.C.; Lu, C.F.; You, S.L.; Mau, Y.C.; Ho, M.S.; Lin, S.H.; Chen, C.J. Transmission of hepatitis C virus in Taiwan: Prevalence and risk factors based on a nationwide survey. J. Med. Virol. 1999, 59, 290–296. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chen, C.L.; Chen, J.W.; Hsu, N.T.; Wei, S.T.; Hou, S.M.; Lu, S.N.; Chen, P.J. Secular Trends and Geographic Maps of Hepatitis C Virus Infection among 4 Million Blood Donors in Taiwan from 1999 to 2017. Hepatol. Commun. 2020, 4, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.A.; Chen, H.C.; Lu, S.N.; Chen, C.J.; Lu, C.F.; You, S.L.; Lin, S.H. Persistent hyperendemicity of hepatitis C virus infection in Taiwan: The important role of iatrogenic risk factors. J. Med. Virol. 2001, 65, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-S.; Liao, Y.-L.; Chang, S.-T.; Hsieh, Y.-C.; Kuo, S.-Y.; Lu, C.-L.; Hwang, W.-S.; Lin, I.-H.; Tsao, C.-J.; Huang, W.-T. Hepatitis C virus infection is significantly associated with malignant lymphoma in Taiwan, particularly with nodal and splenic marginal zone lymphomas. J. Clin. Pathol. 2010, 63, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Mele, A.; Pulsoni, A.; Bianco, E.; Musto, P.; Szklo, A.; Sanpaolo, M.G.; Iannitto, E.; De Renzo, A.; Martino, B.; Liso, V.; et al. Hepatitis C virus and B-cell non-Hodgkin lymphomas: An Italian multicenter case-control study. Blood 2003, 102, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Giannoulis, E.; Economopoulos, T.; Mandraveli, K.; Giannoulis, K.; Nikolaides, C.; Zervou, E.; Papageorgiou, E.; Zoulas, D.; Tourkantonis, A.; Giannopoulos, G.; et al. The prevalence of hepatitis C and hepatitis G virus infection in patients with B cell non-Hodgkin lymphomas in Greece: A Hellenic Cooperative Oncology Group Study. Acta Haematol. 2004, 112, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A Predictive Model for Aggressive Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 1993, 329, 987–994. [Google Scholar] [CrossRef]
- Sehn, L.H.; Berry, B.; Chhanabhai, M.; Fitzgerald, C.; Gill, K.; Hoskins, P.; Klasa, R.; Savage, K.J.; Shenkier, T.; Sutherland, J.; et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007, 109, 1857–1861. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Chen, C.-H.; Yang, P.-M.; Huang, G.-T.; Lee, H.-S.; Sung, J.-L.; Sheu, J.-C. Estimation of Seroprevalence of Hepatitis B Virus and Hepatitis C Virus in Taiwan from a Large-scale Survey of Free Hepatitis Screening Participants. J. Formos. Med. Assoc. 2007, 106, 148–155. [Google Scholar] [CrossRef]
- Machida, K.; Cheng, K.T.; Sung, V.M.; Shimodaira, S.; Lindsay, K.L.; Levine, A.M.; Lai, M.Y.; Lai, M.M. Hepatitis C virus induces a mutator phenotype: Enhanced mutations of immunoglobulin and protooncogenes. Proc. Natl. Acad. Sci. USA 2004, 101, 4262–4267. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, D.; Landau, D.A.; Calabrese, L.H.; Cacoub, P.P. Hepatitis C-associated mixed cryoglobulinaemia: A crossroad between autoimmunity and lymphoproliferation. Rheumatology 2007, 46, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Dlouhy, I.; Torrente, M.; Lens, S.; Rovira, J.; Magnano, L.; Giné, E.; Delgado, J.; Balagué, O.; Martínez, A.; Campo, E.; et al. Clinico-biological characteristics and outcome of hepatitis C virus-positive patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Ann. Hematol. 2017, 96, 405–410. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Huang, C.E.; Liang, F.W.; Lu, C.H.; Chen, P.T.; Lee, K.D.; Chen, C.C. Prognostic impact of hepatitis C virus infection in patients with diffuse large B-cell lymphoma treated with immunochemotherapy in the context of a novel prognostic index. Cancer Epidemiol. 2015, 39, 382–387. [Google Scholar] [CrossRef]
- Visco, C.; Arcaini, L.; Brusamolino, E.; Burcheri, S.; Ambrosetti, A.; Merli, M.; Bonoldi, E.; Chilosi, M.; Viglio, A.; Lazzarino, M.; et al. Distinctive natural history in hepatitis C virus positive diffuse large B-cell lymphoma: Analysis of 156 patients from northern Italy. Ann. Oncol. 2006, 17, 1434–1440. [Google Scholar] [CrossRef]
- Nooka, A.; Shenoy, P.J.; Sinha, R.; Lonial, S.; Flowers, C.R. Hepatitis C reactivation in patients who have diffuse large B-cell lymphoma treated with rituximab: A case report and review of literature. Clin. Lymphoma Myeloma Leuk. 2011, 11, 379–384. [Google Scholar] [CrossRef]
- Krassenburg, L.A.P.; Maan, R.; Ramji, A.; Manns, M.P.; Cornberg, M.; Wedemeyer, H.; de Knegt, R.J.; Hansen, B.E.; Janssen, H.L.A.; de Man, R.A.; et al. Clinical outcomes following DAA therapy in patients with HCV-related cirrhosis depend on disease severity. J. Hepatol. 2021, 74, 1053–1063. [Google Scholar] [CrossRef]
- Hsu, W.F.; Lai, H.C.; Su, W.P.; Lin, C.H.; Chuang, P.H.; Chen, S.H.; Chen, H.Y.; Wang, H.W.; Huang, G.T.; Peng, C.Y. Rapid decline of noninvasive fibrosis index values in patients with hepatitis C receiving treatment with direct-acting antiviral agents. BMC Gastroenterol. 2019, 19, 63. [Google Scholar] [CrossRef]
- Cheng, C.H.; Chu, C.Y.; Chen, H.L.; Lin, I.T.; Wu, C.H.; Lee, Y.K.; Hu, P.J.; Bair, M.J. Direct-acting antiviral therapy of chronic hepatitis C improves liver fibrosis, assessed by histological examination and laboratory markers. J. Med. Assoc. 2021, 120, 1259–1268. [Google Scholar] [CrossRef]
- Occhipinti, V.; Farina, L.; Vigano, M.; Capecchi, M.; Labanca, S.; Fanetti, I.; Corradini, P.; Rumi, M. Concomitant therapy with direct-acting antivirals and chemoimmunotherapy in HCV-associated diffuse large B-cell lymphoma. Dig. Liver Dis. 2019, 51, 719–723. [Google Scholar] [CrossRef] [PubMed]
| Variables | HCV-Negative DLBCL | HCV-Positive DLBCL | |
|---|---|---|---|
| N = 184 | N = 22 | p Value | |
| Age, (Mean ± SD) | 61.24 ± 15.27 | 67.23 ± 17.13 | 0.088 |
| Gender, N(%) | 0.618 | ||
| Male | 94(51.1%) | 10(45.5%) | |
| Female | 90(48.9%) | 12(54.5%) | |
| ECOG, N(%) | 0.011 * | ||
| 0 | 79(42.9%) | 6(27.3%) | |
| 1 | 78(42.4%) | 12(54.5%) | |
| 2 | 13(7.1%) | 1(4.5%) | |
| 3 | 11(6.0%) | 0(0%) | |
| 4 | 3(1.6%) | 3(13.6%) | |
| Ann Arbor stage, N(%) | 0.957 | ||
| I-II | 68(37.0%) | 8(36.4%) | |
| III-IV | 116(63.0%) | 14(63.6%) | |
| B symptoms, N(%) | 74(40.2%) | 13(59.1%) | 0.090 |
| Liver involvement, N(%) | 8(4.3%) | 4 4(18.2%) | 0.027 * |
| Spleen involvement, N(%) | 25(13.6%) | 7(31.8%) | 0.026 * |
| Bone marrow involvement, N(%) | 38(20.7%) | 4(18.2%) | 1.000 |
| R-IPI score, N(%) | 0.202 | ||
| Very good | 15(8.2%) | 0(0%) | |
| Good | 90(48.9%) | 9(40.9%) | |
| Poor | 79(42.9%) | 13(59.1%) | |
| IPI score, N(%) | 0.448 | ||
| Low | 57(31.0%) | 6(27.3%) | |
| Low-intermediate | 48(26.1%) | 3(13.6%) | |
| High-intermediate | 41(22.3%) | 6(27.3%) | |
| High | 38(20.7%) | 7(31.8%) | |
| HBsAg positive, N(%) | 38(20.7%) | 2(9.1%) | 0.261 |
| Abnormal liver funtion at baseline, N(%) | 30(16.3%) | 7(31.8%) | 0.073 |
| WBC (Mean ± SD) | 7554.6 ± 3474.0 | 7480.0 ± 2823.8 | 0.923 |
| Hgb (Mean ± SD) | 11.8 ± 2.3 | 11.6 ± 1.7 | 0.687 |
| Platelet (×103, Mean ± SD) | 236.2 ± 102.9 | 186.7 ± 68.8 | 0.029 * |
| Albumin (Mean ± SD) | 3.6 ± 0.6 | 3.6 ± 0.6 | 0.969 |
| LDH (Mean ± SD) | 366.9 ± 453.2 | 430.9 ± 450.3 | 0.532 |
| GOT (Mean ± SD) | 48.2 ± 134.5 | 58.1 ± 77.5 | 0.734 |
| GPT (Mean ± SD) | 43.7 ± 135.0 | 34.9 ± 28.6 | 0.763 |
| Beta-2 microglobulin (Mean ± SD) | 303.5 ± 273.2 | 332.9 ± 116.1 | 0.627 |
| Variables | HCV-Negative DLBCL | HCV-Positive DLBCL | |
|---|---|---|---|
| N = 184 | N = 22 | p Value | |
| Receiving treatment (Yes), N(%) | 171(92.9%) | 19(86.4%) | 0.388 |
| First-line treatment regimen in receiving treatment population | 0.354 | ||
| RCHOP | 127(74.3%) | 13(68.4%) | |
| RCOP | 30(17.5%) | 6(31.6%) | |
| Other regimen (REPOCH, RMTX) | 11(6.4%) | 0(0.0%) | |
| R with prednisolone | 3(1.8%) | 0(0.0%) | |
| Chemotherapy cycles (Mean ± SD) | 6.3 ± 3.4 | 4.8 ± 3.5 | 0.048 * |
| Treatment response, N(%) | |||
| Complete response/Partial response | 105(57.1%) | 9(40.9%) | 0.150 |
| Progression or recurrent | 52(28.3%) | 6(27.3%) | 0.922 |
| Unable to evaluate response | 27(14.7%) | 7(31.8%) | 0.041 * |
| Liver toxicity | 83(45.1%) | 19(86.4%) | <0.001 * |
| ≥Grade 3 liver toxicity | 18(9.8%) | 8(36.4%) | <0.001 * |
| Treatment delay or discontinue | 23(12.5%) | 9(40.9%) | 0.001 * |
| Univariate Analysis | Multivariate Analysis a | |||
|---|---|---|---|---|
| Variables | HR (95%CI) | p-Value | HR (95%CI) | p-Value |
| Age | 1.03(1.01–1.04) | 0.004 * | 1.00(0.98–1.02) | 0.733 |
| Gender | 0.479 | |||
| Male | 1.0 | |||
| Female | 0.84(0.52–1.36) | |||
| Hepatitis C infection | 0.054 | 0.574 | ||
| Negative | 1.0 | 1.00 | ||
| Positive | 1.89 (0.99–3.60) | 0.82(0.41–1.64) | ||
| Hepatitis B infection | 0.069 | 0.202 | ||
| Negative | 1 | 1.00 | ||
| Positive | 0.50(0.24–1.06) | 0.61(0.28–1.31) | ||
| ECOG | <0.001 * | 0.003 * | ||
| <2 | 1.00 | 1.00 | ||
| ≥2 | 6.22(3.66–10.56) | 2.64(1.39–4.99) | ||
| Stage | <0.001 * | <0.001 * | ||
| I/II | 1.0 | 1.00 | ||
| III/IV | 4.36(2.23–8.55) | 4.28(2.12–8.63) | ||
| Chemotherapy cycles | 0.77(0.70–0.84) | <0.001 * | 0.78(0.70–0.86) | <0.001 * |
| Liver toxicity | 0.001 * | <0.001 * | ||
| Yes | 2.46(1.47–4.12) | 2.73(1.62–4.60) | ||
| No | 1.00 | 1.00 | ||
| Univariate Analysis | Multivariate Analysis a | |||
|---|---|---|---|---|
| Variables | HR (95%CI) | p-Value | HR (95%CI) | p-Value |
| Age | 1.02(1.00–1.03) | 0.033 * | 1.00(0.98–1.01) | 0.838 |
| Gender | 0.184 | |||
| Male | 1.0 | |||
| Female | 0.75(0.49–1.15) | |||
| Hepatitis C infection | 0.084 | 0.827 | ||
| Negative | 1.0 | 1.00 | ||
| Positive | 1.68 (0.93–3.03) | 0.93(0.50–1.74) | ||
| Hepatitis B infection | 0.025 * | 0.041 * | ||
| Negative | 1 | 1.00 | ||
| Positive | 0.47(0.24–0.91) | 0.49(0.25–0.97) | ||
| ECOG | <0.001 * | 0.001 * | ||
| <2 | 1.00 | 1.00 | ||
| ≥2 | 4.60(2.81–7.53) | 2.82(1.56–5.09) | ||
| Stage | <0.001 * | <0.001 * | ||
| I/II | 1.0 | 1.00 | ||
| III/IV | 3.59(2.09–6.18) | 3.18(1.81–5.58) | ||
| Chemotherapy cycles | 0.89(0.82–0.96) | 0.003 * | 0.90(0.83–0.98) | 0.015 * |
| Liver toxicity | 0.001 * | <0.001* | ||
| Yes | 2.56(1.63–4.02) | 2.67(1.69–4.22) | ||
| No | 1.00 | 1.00 | ||
| Variables | HCV-Positive DLBCL | ||
|---|---|---|---|
| Dead (n = 11) | Alive (n = 11) | p-Value | |
| Age, (Mean ± SD) | 69.00 ± 14.11 | 65.45 ± 20.25 | 0.639 |
| Sex | 0.198 | ||
| Male | 3(27.3%) | 7(63.6%) | |
| Female | 8(72.7%) | 4(36.4%) | |
| ECOG | 1.000 | ||
| <2 | 9(81.8%) | 9(81.8%) | |
| ≥2 | 2(18.2%) | 2(18.2%) | |
| Ann Arbor stage | 1.000 | ||
| I–II | 4(36.4%) | 4(36.4%) | |
| III–IV | 7(63.6%) | 7(63.6%) | |
| IPI score | 0.924 | ||
| Low | 3(27.3%) | 3(27.3%) | |
| Low-intermediate | 1(9.1%) | 2(18.2%) | |
| High-intermediate | 3(27.3%) | 3(27.3%) | |
| High | 4(36.4%) | 3(27.3%) | |
| GPT at diagnosis (Mean ± SD) | 42.82 ± 36.68 | 27.00 ± 15.29 | 0.209 |
| PLT at diagnosis (×103, Mean ± SD) | 184.45 ± 72.12 | 188.91 ± 68.80 | 0.884 |
| Albumin at diagnosis (Mean ± SD) | 3.53 ± 0.74 | 3.63 ± 0.58 | 0.737 |
| PT(INR) at diagnosis (Mean ± SD) | 1.05 ± 0.78 | 1.04 ± 0.53 | 0.707 |
| LDH at diagnosis (Mean ± SD) | 531.45 ± 615.90 | 330.45 ± 155.97 | 0.307 |
| Abnormal liver funtion at baseline, N(%) | 5(45.5%) | 2(18.2%) | 0.361 |
| Liver cirrhosis | 0.035 * | ||
| No | 6(54.5%) | 11(100%) | |
| Yes | 5(45.5%) | 0(0%) | |
| FIB-4 index | 0.032 * | ||
| <1.45 | 0(0%) | 4(36.4%) | |
| 1.45–3.25 | 4(36.4%) | 5(45.5%) | |
| >3.25 | 7(63.6%) | 2(18.2%) | |
| Receiving treatment | 1.000 | ||
| Yes | 9(81.8%) | 10(90.9%) | |
| No | 2(18.2%) | 1(9.1%) | |
| Chemotherapy cycles | 3.91 ± 4.04 | 5.64 ± 2.77 | 0.255 |
| ≥Grade 3 Liver toxicity | 0.183 | ||
| Yes | 6(54.5%) | 2(18.2%) | |
| No | 5(45.5%) | 9(81.8%) | |
| Treatment delay or discontinuation | 0.387 | ||
| Yes | 6(54.5%) | 3(27.3%) | |
| No | 5(45.5%) | 8(72.7%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-F.; Liu, Y.-C.; Yang, C.-I.; Chuang, T.-M.; Ke, Y.-L.; Yeh, T.-J.; Gau, Y.-C.; Du, J.-S.; Wang, H.-C.; Cho, S.-F.; et al. Poor Prognosis of Diffuse Large B-Cell Lymphoma with Hepatitis C Infection. J. Pers. Med. 2021, 11, 844. https://doi.org/10.3390/jpm11090844
Tsai Y-F, Liu Y-C, Yang C-I, Chuang T-M, Ke Y-L, Yeh T-J, Gau Y-C, Du J-S, Wang H-C, Cho S-F, et al. Poor Prognosis of Diffuse Large B-Cell Lymphoma with Hepatitis C Infection. Journal of Personalized Medicine. 2021; 11(9):844. https://doi.org/10.3390/jpm11090844
Chicago/Turabian StyleTsai, Yu-Fen, Yi-Chang Liu, Ching-I Yang, Tzer-Ming Chuang, Ya-Lun Ke, Tsung-Jang Yeh, Yuh-Ching Gau, Jeng-Shiun Du, Hui-Ching Wang, Shih-Feng Cho, and et al. 2021. "Poor Prognosis of Diffuse Large B-Cell Lymphoma with Hepatitis C Infection" Journal of Personalized Medicine 11, no. 9: 844. https://doi.org/10.3390/jpm11090844
APA StyleTsai, Y.-F., Liu, Y.-C., Yang, C.-I., Chuang, T.-M., Ke, Y.-L., Yeh, T.-J., Gau, Y.-C., Du, J.-S., Wang, H.-C., Cho, S.-F., Hsu, C.-M., Wu, P.-F., Huang, C.-I., Huang, C.-F., Yu, M.-L., Dai, C.-Y., & Hsiao, H.-H. (2021). Poor Prognosis of Diffuse Large B-Cell Lymphoma with Hepatitis C Infection. Journal of Personalized Medicine, 11(9), 844. https://doi.org/10.3390/jpm11090844










