Characterization of Risk Profiles for Diabetic Retinopathy Progression
Abstract
:1. Introduction
2. Phenotypes of Diabetic Retinopathy Progression
3. The Early Treatment Diabetic Retinopathy Study and Classification of Diabetic Retinopathy Severity
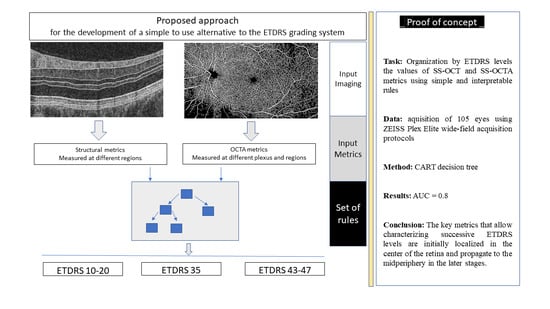
4. Automated Alternative ETDRS Classification
5. Risk Profiles of DR Progression
6. Directions for Future Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IDF. IDF Diabetes Atlas, 9th ed.; IDF: Brussels, Belgium, 2019. [Google Scholar]
- Sato, Y.; Lee, Z.; Hayashi, Y. Subclassification of preproliferative diabetic retinopathy and glycemic control: Relationship between mean hemoglobin A1C value and development of proliferative diabetic retinopathy. Jpn. J. Ophthalmol. 2001, 45, 523–527. [Google Scholar] [CrossRef]
- Marques, I.P.; Madeira, M.H.; Messias, A.L.; Santos, T.; Martinho, A.C.-V.; Figueira, J.; Cunha-Vaz, J. Retinopathy phenotypes in type 2 diabetes with different risks for macular edema and proliferative retinopathy. J. Clin. Med. 2020, 9, 1433. [Google Scholar] [CrossRef]
- Cogan, D.G.; Kuwabara, T. Capillary Shunts in the Pathogenesis of Diabetic Retinopathy. Diabetes 1963, 12, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J.G. Pathophysiology of diabetic retinopathy. Br. J. Ophthalmol. 1978, 62, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Marques, I.P.; Alves, D.; Santos, T.; Mendes, L.; Santos, A.R.; Lobo, C.; Durbin, M.; Cunha-Vaz, J. Multimodal Imaging of the Initial Stages of Diabetic Retinopathy: Different Disease Pathways in Different Patients. Diabetes 2019, 68, 648–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durbin, M.K.; An, L.; Shemonski, N.D.; Soares, M.; Santos, T.; Lopes, M.; Neves, C.; Cunha-Vaz, J. Quantification of Retinal Microvascular Density in Optical Coherence Tomographic Angiography Images in Diabetic Retinopathy. JAMA Ophthalmol. 2017, 135, 370. [Google Scholar] [CrossRef]
- Santos, T.; Warren, L.H.; Santos, A.R.; Marques, I.P.; Kubach, S.; Mendes, L.G.; De Sisternes, L.; Madeira, M.H.; Durbin, M.; Cunha-Vaz, J.G. Swept-source OCTA quantification of capillary closure predicts ETDRS severity staging of NPDR. Br. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Silva, P.S.; Dela Cruz, A.J.; Ledesma, M.G.; Van Hemert, J.; Radwan, A.; Cavallerano, J.D.; Aiello, L.M.; Sun, J.K.; Aiello, L.P. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology 2015, 122, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Marques, I.P.; Alves, D.; Santos, T.; Mendes, L.; Lobo, C.; Santos, A.R.; Durbin, M.; Cunha-Vaz, J. Characterization of Disease Progression in the Initial Stages of Retinopathy in Type 2 Diabetes: A 2-Year Longitudinal Study. Investig. Opthalmol. Vis. Sci. 2020, 61, 20. [Google Scholar] [CrossRef]
- Sohn, E.H.; Van Dijk, H.W.; Jiao, C.; Kok, P.H.B.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; Van Velthoven, M.E.J.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [Green Version]
- Ludovico, J.; Bernardes, R.; Pires, I.; Figueira, J.; Lobo, C.; Cunha-Vaz, J. Alterations of retinal capillary blood flow in preclinical retinopathy in subjects with type 2 diabetes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003, 241, 181–186. [Google Scholar] [CrossRef]
- Royle, P.; Mistry, H.; Auguste, P.; Shyangdan, D.; Freeman, K.; Lois, N.; Waugh, N. Pan-retinal photocoagulation and other forms of laser treatment and drug therapies for non-proliferative diabetic retinopathy: Systematic review and economic evaluation. Health Technol. Assess. 2015, 19, 1–247. [Google Scholar] [CrossRef] [Green Version]
- Sivaprasad, S.; Pearce, E. The unmet need for better risk stratification of non-proliferative diabetic retinopathy. Diabet. Med. 2019, 36, 424–433. [Google Scholar] [CrossRef]
- Hubbard, L.D.; Sun, W.; Cleary, P.A.; Danis, R.P.; Hainsworth, D.P.; Peng, Q.; Susman, R.A.; Aiello, L.P.; Davis, M.D. Comparison of digital and film grading of diabetic retinopathy severity in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Arch. Ophthalmol. 2011, 129, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, C.P.; Ferris, F.L.; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- ETDRS. Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 1991, 98, 786–806. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.; Ribeiro, L.; Lobo, C. Phenotypes and biomarkers of diabetic retinopathy. Prog. Retin. Eye Res. 2014, 41, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Nesper, P.L.; Roberts, P.K.; Onishi, A.C.; Chai, H.; Liu, L.; Jampol, L.M.; Fawzi, A.A. Quantifying Microvascular Abnormalities with Increasing Severity of Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Invest. Ophthalmol. Vis. Sci. 2017, 58, BIO307–BIO315. [Google Scholar] [CrossRef] [PubMed]
- Hove, M.N.; Kristensen, J.K.; Lauritzen, T.; Bek, T. The relationships between risk factors and the distribution of retinopathy lesions in type 2 diabetes. Acta Ophthalmol. Scand. 2006, 84, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.R.; Mendes, L.; Madeira, M.H.; Marques, I.P.; Tavares, D.; Figueira, J.; Lobo, C.; Cunha-Vaz, J.G. Microaneurysm Turnover in mild Non-Proliferative Diabetic Retinopathy is Associated with Progression and Development of Vision-threatening Complications: A 5-year longitudinal study. J. Clin. Med. 2021, 10, 2142. [Google Scholar] [CrossRef]
- Wang, F.P.; Saraf, S.S.; Zhang, Q.; Wang, R.K.; Rezaei, K.A. Ultra-Widefield Protocol Enhances Automated Classification of Diabetic Retinopathy Severity with OCT Angiography. Ophthalmol. Retin. 2020, 4, 415–424. [Google Scholar] [CrossRef]
- Li, X.; Xie, J.; Zhang, L.; Cui, Y.; Zhang, G.; Chen, X.; Wang, J.; Zhang, A.; Huang, T.; Meng, Q. Identifying microvascular and neural parameters related to the severity of diabetic retinopathy using optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2020, 61, 39. [Google Scholar] [CrossRef]
- Custo Greig, E.; Brigell, M.; Cao, F.; Levine, E.S.; Peters, K.; Moult, E.M.; Fujimoto, J.G.; Waheed, N.K. Macular and Peripapillary Optical Coherence Tomography Angiography Metrics Predict Progression in Diabetic Retinopathy: A Sub-analysis of TIME-2b Study Data. Am. J. Ophthalmol. 2020, 219, 66–76. [Google Scholar] [CrossRef]
- Martinho, A.C.-V.; Marques, I.P.; Messias, A.L.; Santos, T.; Madeira, M.H.; Sousa, D.C.; Lobo, C.; Cunha-Vaz, J. Ocular and Systemic Risk Markers for Development of Macular Edema and Proliferative Retinopathy in Type 2 Diabetes: A 5-Year Longitudinal Study. Diabetes Care 2021, 44, e12–e14. [Google Scholar] [CrossRef]



| ETDRS Final Scale | |
|---|---|
| Level | Definition |
| 10 | Microaneurysms (MAs) and other characteristics absent |
| 20 | Define presence of Mas and other characteristics absent |
| 35 A | Definite presence of venous loops in 1 field |
| 35 B | Questionable soft exudates, Intraretinal Microvascular Abnormality (IrMA), or venous beading |
| 35 C | Presence of Hemorrhage |
| 35 D | Definite presence of hemorrhage in 1–5 field |
| 35 E | Moderately severe hemorrhages in 1 field |
| 35 F | Definite presence soft exudates in 1 field |
| 43 A | Moderately severe hemorrhages in 4–5 fields or severe hemorrhages in 1 field |
| 43 B | Definite presence of IrMA in 1–3 fields |
| 47 A | Both 43 A and 43 B definitions |
| 47 B | Definite presence of IrMA in 4–5 fields |
| 47 C | Severe hemorrhages in 2–3 fields |
| 47 D | Definite venous beading in 1 field |
| 53 A | ≥2 level 47 definition |
| 53 B | Severe hemorrhages in 4–5 fields |
| 53 C | Moderately severe presence of IrMA in 1 field |
| 53 D | Definite presence of venous beading in 2–3 fields |
| 53 D | Two or more level 53 definitions |
| Lesion Type (Fundus Photos) | Description | Alternative Metric | Examination |
|---|---|---|---|
| Microaneurysms and dot hemorrhages | Capillary wall outpouching (adjacent to capillary closure) | Capillary closure metrics: 1. Vessel density 2. Intercapillary spaces 3. Foveal avascular zone (FAZ) metrics | Optical Coherence Tomography (Angiography (OCT-A) |
| Venous beading Venous loops | Shunt vessels (?), tortuosity of venula (Adjacent to capillary closure) | ||
| Intraretinal Microvascular Abnormalities (IrMA) | Remodeling new capillary “buds” (Adjacent to capillary closure) | Measurements performed at: 1. Perifovea 2. Mid-periphery 3. Number of quadrants involved | |
| Cotton wool spots | Site of ischemia, poor perfusion | Capillary closure metrics: 1. Vessel density 2. Intercapillary spaces Number of Quadrants | OCTA |
| Hard Exudates | Lipid and lipoproteins deposits | Central retinal thickness | Optical coherence tomography (OCT) |
| Neurodegenerative changes | Ganglion cell + Inner Plexiform layers thinning |
| Alternative DR Grading | Perifovea | Mid-Periphery | |
|---|---|---|---|
| 1. Skeletonized vessel density SCP, DCP, FR | Capillary closure | ✓ * | ✓ * |
| 2. FAZ area and circularity | Capillary closure | ✓ | |
| 3. Thinning of GCL | Neurodegeneration | ✓ * | ✓ * |
| Control vs. ETDRS 10–20 | ETDRS 10–20 vs. ETDRS 35 | ETDRS 35 vs. ETDRS 43–47 |
|---|---|---|
| Faz_Circularity | GCL_ExtC3 | VD_OuterNasal_SCP |
| VD_CSF_Retina | VD_InnerRing_SCP | VD_OuterTemp_SCP |
| VD_ExtC3_DCP | GCL_ExtC3TempInf | VD_ExtC2NasInf_Deep |
| GCL_ExtC2NasSup | VD_OutCircle_DCP | VD_InnerNas_SCP |
| VD_ExtC2NasalInf_DCP | VD_EXTC1InfTemp_SCP | VD_ExtC1NasalInf_SCP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha-Vaz, J.; Mendes, L. Characterization of Risk Profiles for Diabetic Retinopathy Progression. J. Pers. Med. 2021, 11, 826. https://doi.org/10.3390/jpm11080826
Cunha-Vaz J, Mendes L. Characterization of Risk Profiles for Diabetic Retinopathy Progression. Journal of Personalized Medicine. 2021; 11(8):826. https://doi.org/10.3390/jpm11080826
Chicago/Turabian StyleCunha-Vaz, José, and Luís Mendes. 2021. "Characterization of Risk Profiles for Diabetic Retinopathy Progression" Journal of Personalized Medicine 11, no. 8: 826. https://doi.org/10.3390/jpm11080826
APA StyleCunha-Vaz, J., & Mendes, L. (2021). Characterization of Risk Profiles for Diabetic Retinopathy Progression. Journal of Personalized Medicine, 11(8), 826. https://doi.org/10.3390/jpm11080826