Significant Reduction in Vertebral Artery Dose by Intensity Modulated Proton Therapy: A Pilot Study for Nasopharyngeal Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Simulation
2.3. Target Delineation
2.4. Dose Prescription
2.5. Treatment Planning
2.6. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
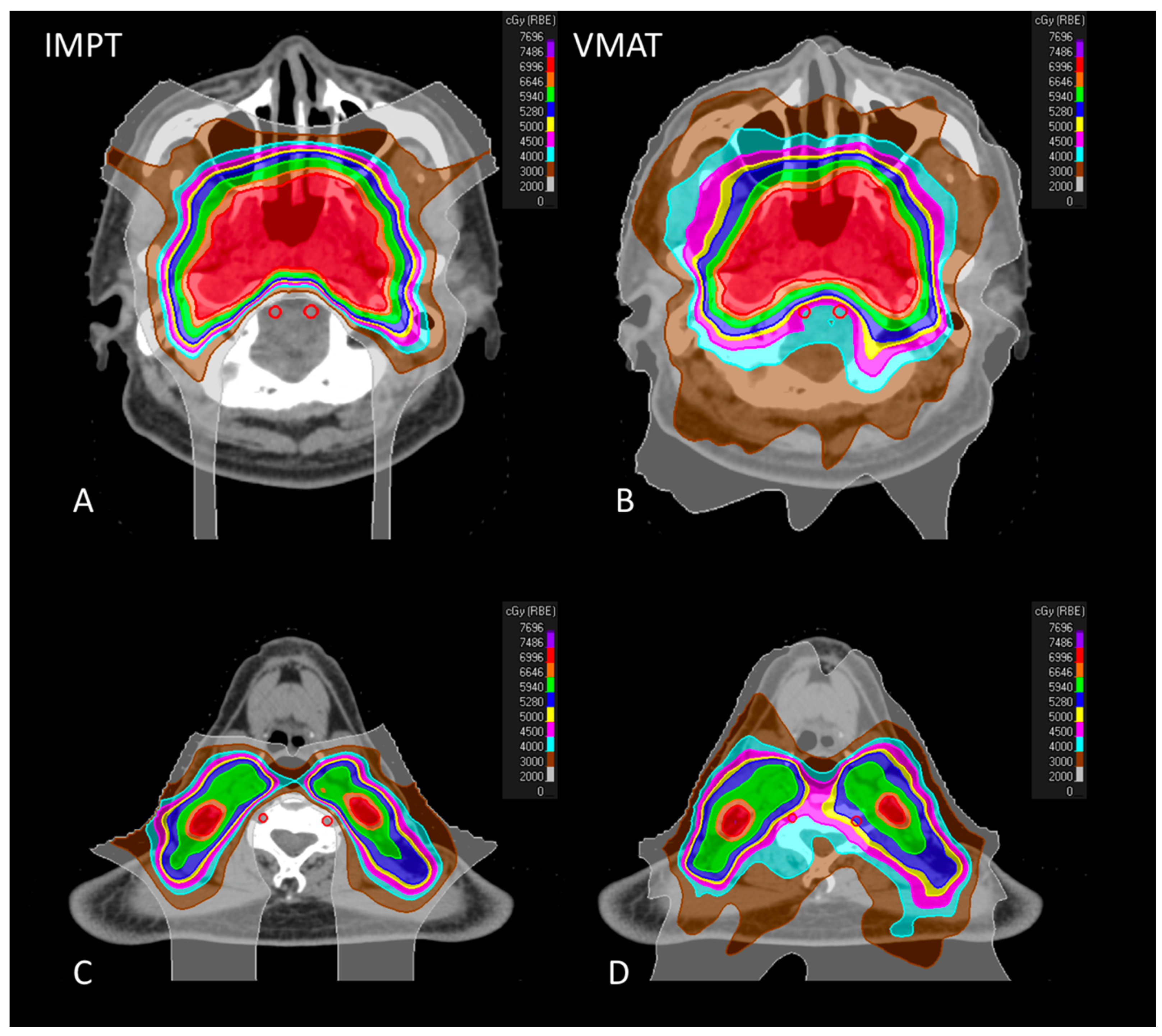
3.2. Treatment Planning Comparison
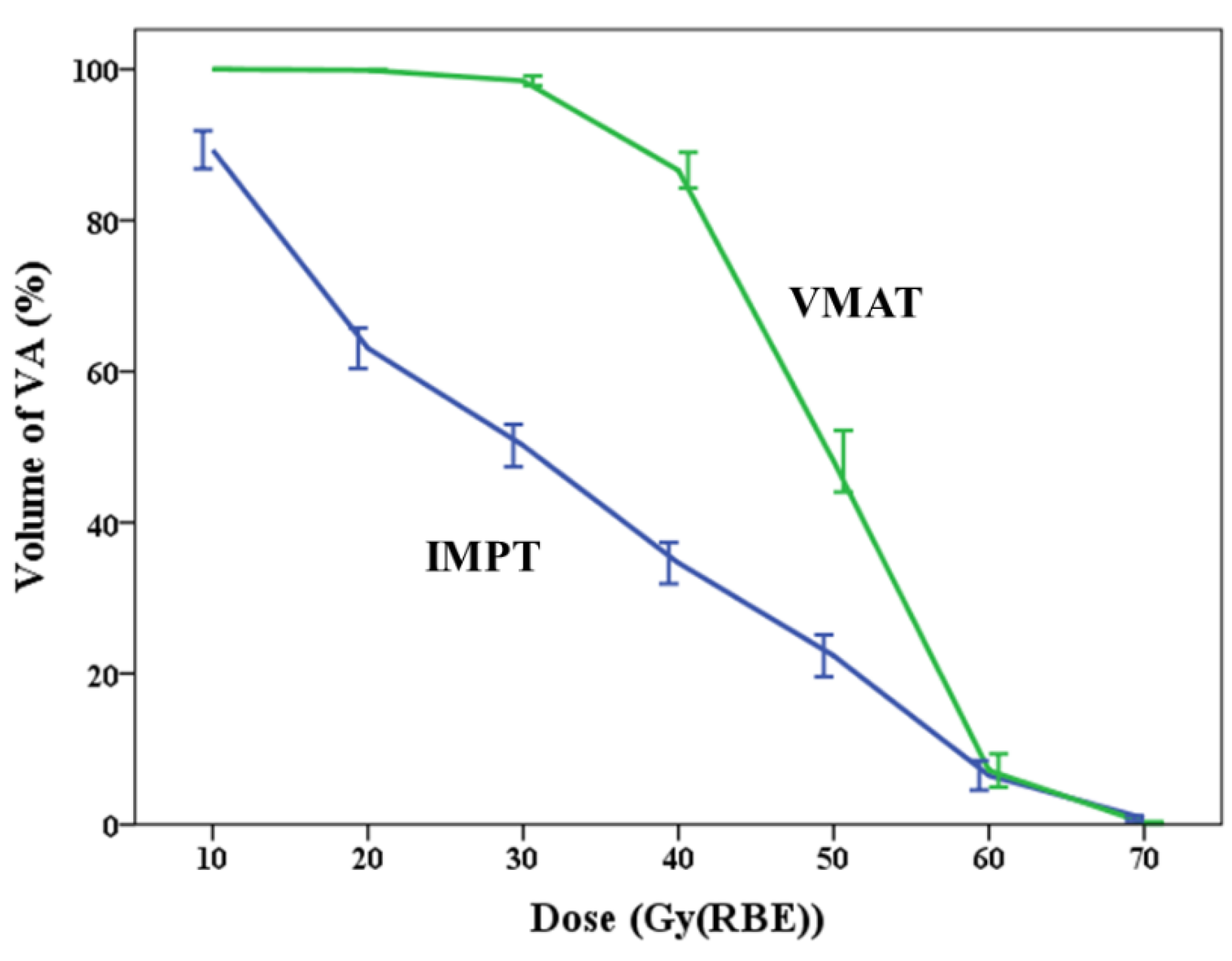
3.2.1. Vertebral Arteries
3.2.2. Target Coverage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wee, J.T.; Ha, T.C.; Loong, S.L.; Qian, C.N. Is nasopharyngeal cancer really a “Cantonese cancer”? Chin. J. Cancer 2010, 29, 517–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Su, S.; Chen, C.; Han, F.; Zhao, C.; Xiao, W.; Deng, X.; Huang, S.; Lin, C.; Lu, T. Long-term outcomes of intensi-ty-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: An analysis of survival and treatment toxicities. Radiother. Oncol. 2014, 110, 398–403. [Google Scholar] [CrossRef]
- Lin, Y.H.; Huang, T.L.; Chien, C.Y.; Chen, H.C.; Hsu, H.C.; Huang, E.Y.; Wang, C.J.; Huang, Y.J.; Wang, Y.M.; Huang, C.C.; et al. Pretreatment prognostic factors of survival and late toxicities for patients with nasopharyngeal carcinoma treated by sim-ultaneous integrated boost intensity-modulated radiotherapy. Radiat. Oncol. 2018, 13, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, W.W.-M.; Leung, S.-F.; So, N.M.-C.; Wong, K.S.L.; Liu, K.-H.; Ku, P.K.-M.; Yuen, H.-Y.; Metreweli, C. Incidence of carotid stenosis in nasopharyngeal carcinoma patients after radiotherapy. Cancer 2001, 92, 2357–2363. [Google Scholar] [CrossRef]
- Plummer, C.; Henderson, R.D.; O’Sullivan, J.D.; Read, S.J. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: A review. Stroke 2011, 42, 2410–2418. [Google Scholar] [CrossRef] [Green Version]
- Gujral, D.; Shah, B.; Chahal, N.; Senior, R.; Harrington, K.; Nutting, C. Clinical Features of Radiation-induced Carotid Atherosclerosis. Clin. Oncol. 2014, 26, 94–102. [Google Scholar] [CrossRef]
- Fernández-Alvarez, V.; López, F.; Suárez, C.; Strojan, P.; Eisbruch, A.; Silver, C.E.; Mendenhall, W.M.; Langendijk, J.A.; Rinaldo, A.; Lee, A.W.M.; et al. Radiation-induced carotid artery lesions. Strahlenther. Onkol. 2018, 194, 699–710. [Google Scholar] [CrossRef]
- Liao, W.; Zheng, Y.; Bi, S.; Zhang, B.; Xiong, Y.; Li, Y.; Fang, W.; Xiao, S.; Yang, L.; Thea, A.; et al. Carotid stenosis prevalence after radiotherapy in nasopharyngeal carcinoma: A meta-analysis. Radiother. Oncol. 2019, 133, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, C.; Wu, V.W.C.; Yip, S.P.; Kwong, D.L.W.; Ying, M. Predictors of the Extent of Carotid Atherosclerosis in Patients Treated with Radiotherapy for Nasopharyngeal Carcinoma. PLoS ONE 2014, 9, e116284. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Xing, P.; Chen, Y.; Xu, X.; Shen, J.; Lu, X. Carotid and vertebral artery stenosis evaluated by contrast-enhanced MR angiography in nasopharyngeal carcinoma patients after radiotherapy: A prospective cohort study. Br. J. Radiol. 2015, 88, 20150175. [Google Scholar] [CrossRef] [Green Version]
- Holliday, E.B.; Garden, A.S.; Rosenthal, D.I.; Fuller, C.D.; Morrison, W.H.; Gunn, G.B.; Phan, J.; Beadle, B.M.; Zhu, X.R.; Zhang, X.; et al. Proton Therapy Reduces Treatment-Related Toxicities for Patients with Nasopharyngeal Cancer: A Case-Match Control Study of Intensity-Modulated Proton Therapy and Intensity-Modulated Photon Therapy. Int. J. Part. Ther. 2015, 2, 19–28. [Google Scholar] [CrossRef]
- Lewis, G.D.; Holliday, E.B.; Kocak-Uzel, E.; Hernandez, M.; Garden, A.S.; Rosenthal, D.I.; Frank, S.J. Intensity-modulated proton therapy for nasopharyngeal carcinoma: Decreased radiation dose to normal structures and encouraging clinical out-comes. Head Neck 2016, 38, E1886-95. [Google Scholar] [CrossRef]
- McKeever, M.R.; Sio, T.T.; Gunn, G.B.; Holliday, E.B.; Blanchard, P.; Kies, M.S.; Weber, R.S.; Frank, S.J. Reduced acute toxicity and improved efficacy from intensity-modulated proton therapy (IMPT) for the management of head and neck cancer. Chin. Clin. Oncol. 2016, 5, 54. [Google Scholar] [CrossRef]
- Lee, N.; Harris, J.; Garden, A.S.; Straube, W.; Glisson, B.; Xia, P.; Bosch, W.; Morrison, W.H.; Quivey, J.; Thorstad, W.; et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: Radiation therapy on-cology group phase II trial 0225. J. Clin. Oncol. 2009, 27, 3684–3690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubec, J.J.; Munk, P.L.; Tsang, V.; Lee, M.J.; Janzen, D.L.; Buckley, J.; Seal, M.; Taylor, D. Carotid artery stenosis in patients who have undergone radiation therapy for head and neck malignancy. Br. J. Radiol. 1998, 71, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.W.; Wu, L.L.; Ting, A.C.; Lau, H.; Lam, L.K.; Wei, W. Irradiation-induced extracranial carotid stenosis in patients with head and neck malignancies. Am. J. Surg. 1999, 178, 323–328. [Google Scholar] [CrossRef]
- Dorresteijn, L.D.; Kappelle, A.C.; Boogerd, W.; Klokman, W.J.; Balm, A.J.; Keus, R.B.; van Leeuwen, F.E.; Bartelink, H. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J. Clin. Oncol. 2002, 20, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, D.I.; Fuller, C.; Barker, J.L.; Mason, B.; Garcia, J.A.; Lewin, J.; Holsinger, F.C.; Stasney, C.R.; Frank, S.J.; Schwartz, D.L.; et al. Simple Carotid-Sparing Intensity-Modulated Radiotherapy Technique and Preliminary Experience for T1–2 Glottic Cancer. Int. J. Radiat. Oncol. 2010, 77, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Lee, J.; Park, J.I.; Sung, W.; Lee, S.M.; Kim, G.E. Volumetric modulated arc therapy for carotid sparing in the management of early glottic cancer. Radiat. Oncol. J. 2016, 34, 18–25. [Google Scholar] [CrossRef]
- Lee, A.W.; Ng, W.T.; Pan, J.; Poh, S.S.; Ahn, Y.C.; AlHussain, H.; Corry, J.; Grau, C.; Grégoire, V.; Harrington, K.; et al. International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiother. Oncol. 2018, 126, 25–36. [Google Scholar] [CrossRef]
- Zarrinkoob, L.; Ambarki, K.; Wåhlin, A.; Birgander, R.; Eklund, A.; Malm, J. Blood Flow Distribution in Cerebral Arteries. Br. J. Pharmacol. 2015, 35, 648–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, S. Posterior Circulation Ischemic Stroke. Mo. Med. 2015, 112, 192–196. [Google Scholar]
- Martin, J.D.; Buckley, A.R.; Graeb, D.; Walman, B.; Salvian, A.; Hay, J.H. Carotid artery stenosis in asymptomatic patients who have received unilateral head-and-neck irradiation. Int. J. Radiat. Oncol. 2005, 63, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.S.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, L.S.; Watson, K.E.; Barac, A.; Beckie, T.M.; Bittner, V.; Cruz-Flores, S.; Dent, S.; Kondapalli, L.; Ky, B.; Okwuosa, T.; et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e30–e66. [Google Scholar] [CrossRef]
- Fang, H.; Song, B.; Cheng, B.; Wong, K.S.; Xu, Y.M.; Ho, S.S.Y.; Chen, X.Y. Compensatory patterns of collateral flow in stroke patients with unilateral and bilateral carotid stenosis. BMC Neurol. 2016, 16, 39. [Google Scholar] [CrossRef] [Green Version]
- Elwertowski, M.; Leszczyński, J.; Kaszczewski, P.; Lamparski, K.; Yee, H.S.S.; Gałązka, Z. The importance of blood flow volume in the brain-supplying arteries for the clinical management—The impact of collateral circulation. J. Ultrason. 2018, 18, 112–119. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, C.; Hou, B.; Huang, A.; Zhang, X.; Du, B. Four collateral circulation pathways were observed after common carotid artery occlusion. BMC Neurol. 2019, 19, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bacci, D.; Valecchi, D.; Sgambati, E.; Gulisano, M.; Conti, A.A.; Molino-Lova, R.; Macchi, C. Compensatory collateral circles in vertebral and carotid artery occlusion. Ital. J. Anat. Embryol. 2008, 113, 265–271. [Google Scholar] [PubMed]
| Patient | Age | Sex | WHO Type | T Stage | N Stage | Clinical Stage | Tumor Laterality | Lymph Node Distribution |
|---|---|---|---|---|---|---|---|---|
| 1 | 26 | Male | 3 | 3 | 1 | III | Right | Right RP, level Va |
| 2 | 56 | Male | 3 | 1 | 1 | II | Left | Left level IB |
| 3 | 45 | Female | 3 | 1 | 2 | III | Central | Bilateral level II |
| 4 | 40 | Male | 2 | 1 | 0 | I | Right | NA |
| 5 | 76 | Male | 3 | 3 | 1 | III | Left | Left RP, level II |
| 6 | 55 | Male | 3 | 1 | 1 | II | Right | Right level II |
| IMPT | VMAT | Paired Differences | p Value | |
|---|---|---|---|---|
| Mean dose, Gy (RBE) | <0.001 | |||
| Mean ± SD | 31.6 ± 4.29 | 48.79 ± 2.5 | 17.19 ± 4.04 | |
| Median (min to max) | 31.99 (23.55 to 37.47) | 48.91 (44.32 to 52.62) | 17.27 (11.33 to 23.49) | |
| V10 (%) | 0.001 | |||
| Mean ± SD | 89.3 ± 8.7 | 100.0 ± 0.0 | 10.7 ± 8.7 | |
| Median (min to max) | 90.6 (70.5 to 99.4) | 100.0 (100.0 to 100.0) | 9.4 (0.7 to 29.5) | |
| V20 (%) | <0.001 | |||
| Mean ± SD | 63.1 ± 9.3 | 99.9 ± 0.3 | 36.8 ± 9.5 | |
| Median (min to max) | 61.5 (47.9 to 77.9) | 100.0 (98.9 to 100.0) | 38.5 (21.0 to 52.1) | |
| V30 (%) | <0.001 | |||
| Mean ± SD | 50.2 ± 9.7 | 98.5 ± 2.3 | 48.3 ± 10.3 | |
| Median (min to max) | 51.3 (29.9 to 61.5) | 99.9 (93.4 to 100.0) | 45.4 (34.6 to 69.1) | |
| V40 (%) | <0.001 | |||
| Mean ± SD | 34.6 ± 9.5 | 86.6 ± 8.2 | 52.0 ± 12.6 | |
| Median (min to max) | 37.3 (14.6 to 48.9) | 85.9 (75.1 to 100.0) | 54.5 (32.8 to 70.6) | |
| V50 (%) | <0.001 | |||
| Mean ± SD | 22.4 ± 9.6 | 48.1 ± 14.1 | 25.7 ± 16.0 | |
| Median (min to max) | 21.0 (10.9 to 41.9) | 53.4 (22.5 to 69.4) | 31.0 (3.0 to 44.3) | |
| V60 (%) | 0.608 | |||
| Mean ± SD | 6.5 ± 6.8 | 7.2 ± 7.6 | 0.7 ± 4.4 | |
| Median (min to max) | 4.6 (0 to 21.22) | 5.2 (0 to 27.3) | 0.3 (−9.0 to 6.0) | |
| V70 (%) | 0.049 | |||
| Mean ± SD | 0.8 ± 1.2 | 0.2 ± 0.5 | −0.6 ± 0.9 | |
| Median (min to max) | 0 (0 to 2.8) | 0 (0 to1.6) | 0 (−2.3 to 0.1) |
| IMPT, Median (Range) | VMAT, Median (Range) | p Value | |
|---|---|---|---|
| CTV6996 | |||
| Coverage (%) | 99.5 (99.2−100.0) | 99.8 (98.9−100.0) | 0.776 |
| Mean dose, Gy (RBE) | 72.29 (66.75−73.39) | 72.54 (68.84−72.84) | 0.422 |
| D5%, Gy (RBE) | 73.90 (72.50−75.72) | 74.41 (73.97−74.93) | 0.370 |
| D95%, Gy (RBE) | 71.19 (70.69−71.36) | 70.97 (70.6−71.33) | 0.366 |
| CTV5940 | |||
| Coverage (%) | 99.8 (99.2−100.0) | 99.9 (99.3−100.0) | 0.213 |
| Mean dose, Gy (RBE) | 69.68 (68.99−71.13) | 69.04 (68.72−70.2) | 0.008 |
| D5%, Gy (RBE) | 73.54 (72.53−75.35) | 73.95 (73.57−74.55) | 0.423 |
| D95%, Gy (RBE) | 61.75 (60.86−63.21) | 61.25 (61.16−61.73) | 0.167 |
| CTV5280 | |||
| Coverage (%) | 99.9 (99.8−100.0) | 99.9 (99.8−100.0) | 0.091 |
| Mean dose, Gy (RBE) | 66.69 (65.13−67.89) | 66.14 (64.16−66.81) | 0.022 |
| D5%, Gy (RBE) | 73.39 (72.29−75.16) | 73.74 (73.16−74.32) | 0.596 |
| D95%, Gy (RBE) | 54.58 (53.66−55.01) | 54.28 (54.09−54.54) | 0.615 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H.; Cheng, J.-Y.; Huang, B.-S.; Luo, S.-D.; Lin, W.-C.; Chou, S.-Y.; Juang, P.-J.; Li, S.-H.; Huang, E.-Y.; Wang, Y.-M. Significant Reduction in Vertebral Artery Dose by Intensity Modulated Proton Therapy: A Pilot Study for Nasopharyngeal Carcinoma. J. Pers. Med. 2021, 11, 822. https://doi.org/10.3390/jpm11080822
Lin Y-H, Cheng J-Y, Huang B-S, Luo S-D, Lin W-C, Chou S-Y, Juang P-J, Li S-H, Huang E-Y, Wang Y-M. Significant Reduction in Vertebral Artery Dose by Intensity Modulated Proton Therapy: A Pilot Study for Nasopharyngeal Carcinoma. Journal of Personalized Medicine. 2021; 11(8):822. https://doi.org/10.3390/jpm11080822
Chicago/Turabian StyleLin, Yun-Hsuan, Jen-Yu Cheng, Bing-Shen Huang, Sheng-Dean Luo, Wei-Che Lin, Shang-Yu Chou, Pei-Jiuan Juang, Shen-Hao Li, Eng-Yen Huang, and Yu-Ming Wang. 2021. "Significant Reduction in Vertebral Artery Dose by Intensity Modulated Proton Therapy: A Pilot Study for Nasopharyngeal Carcinoma" Journal of Personalized Medicine 11, no. 8: 822. https://doi.org/10.3390/jpm11080822
APA StyleLin, Y.-H., Cheng, J.-Y., Huang, B.-S., Luo, S.-D., Lin, W.-C., Chou, S.-Y., Juang, P.-J., Li, S.-H., Huang, E.-Y., & Wang, Y.-M. (2021). Significant Reduction in Vertebral Artery Dose by Intensity Modulated Proton Therapy: A Pilot Study for Nasopharyngeal Carcinoma. Journal of Personalized Medicine, 11(8), 822. https://doi.org/10.3390/jpm11080822









