Glycemic Gap as a Useful Surrogate Marker for Glucose Variability and Progression of Diabetic Retinopathy
Abstract
:1. Introduction
2. Materials and Methods
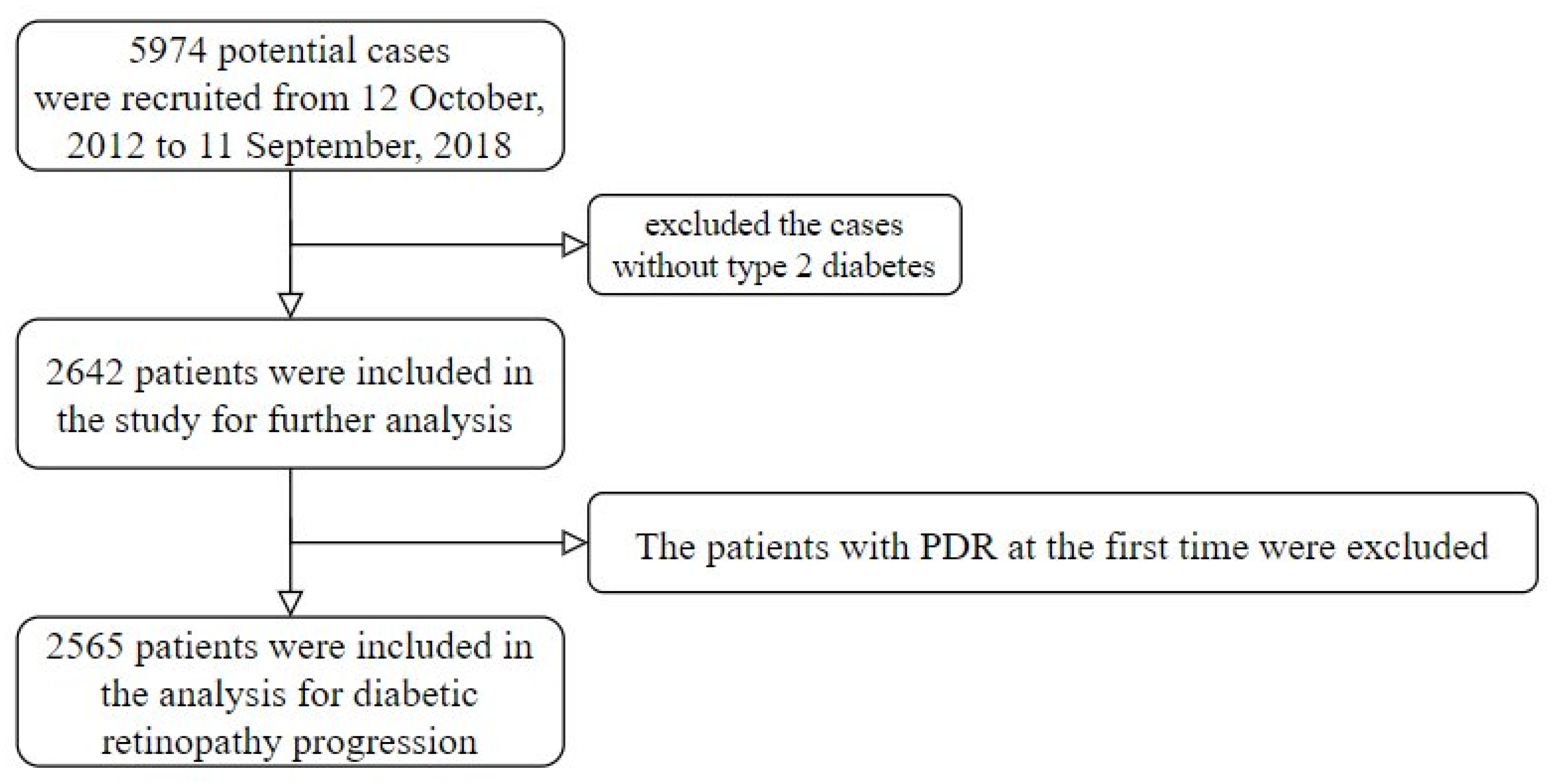
2.1. Population
2.2. Measurements and Variables
2.3. Deep Learning Model for Grading DR
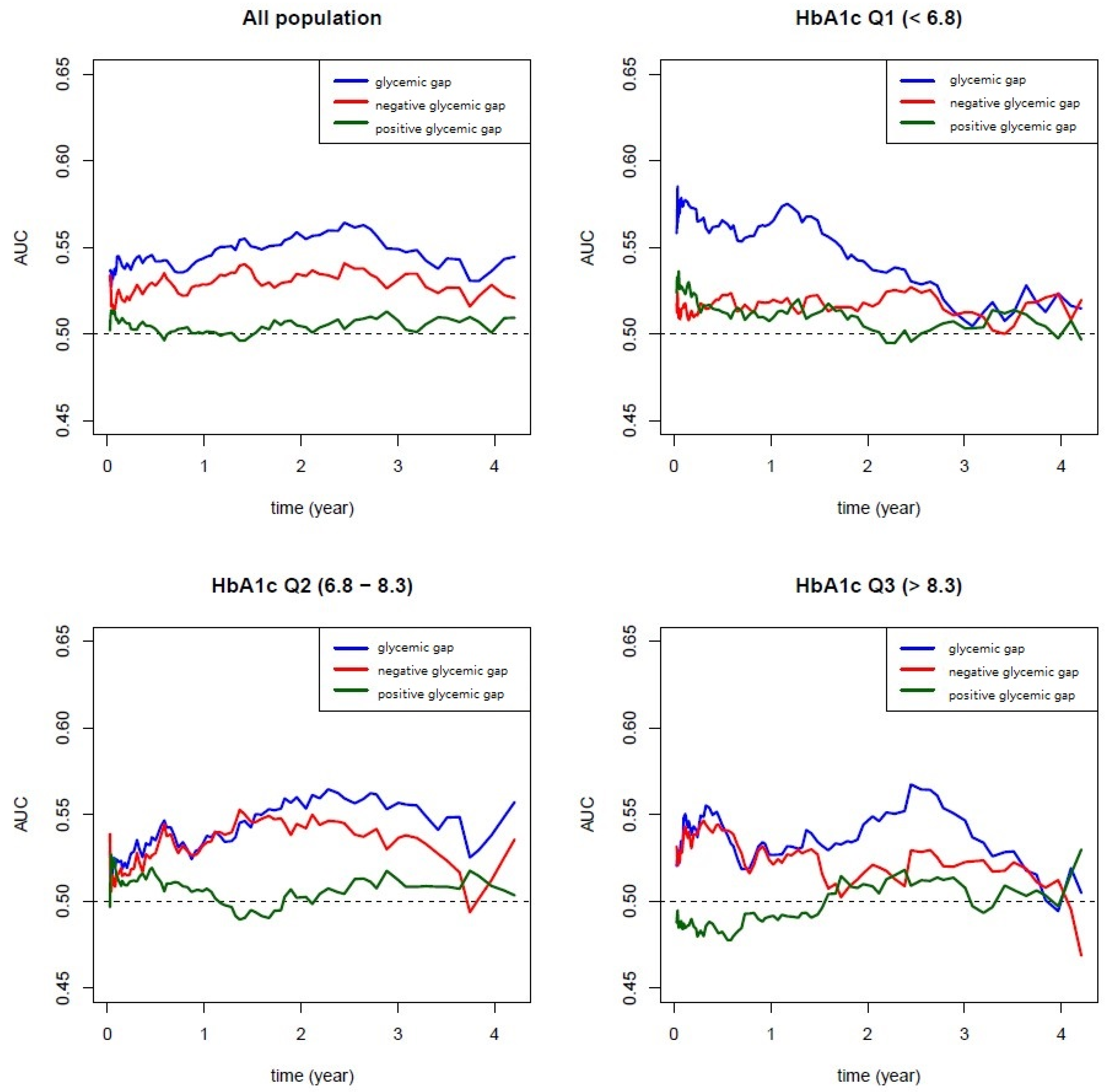
2.4. Statistical Methods
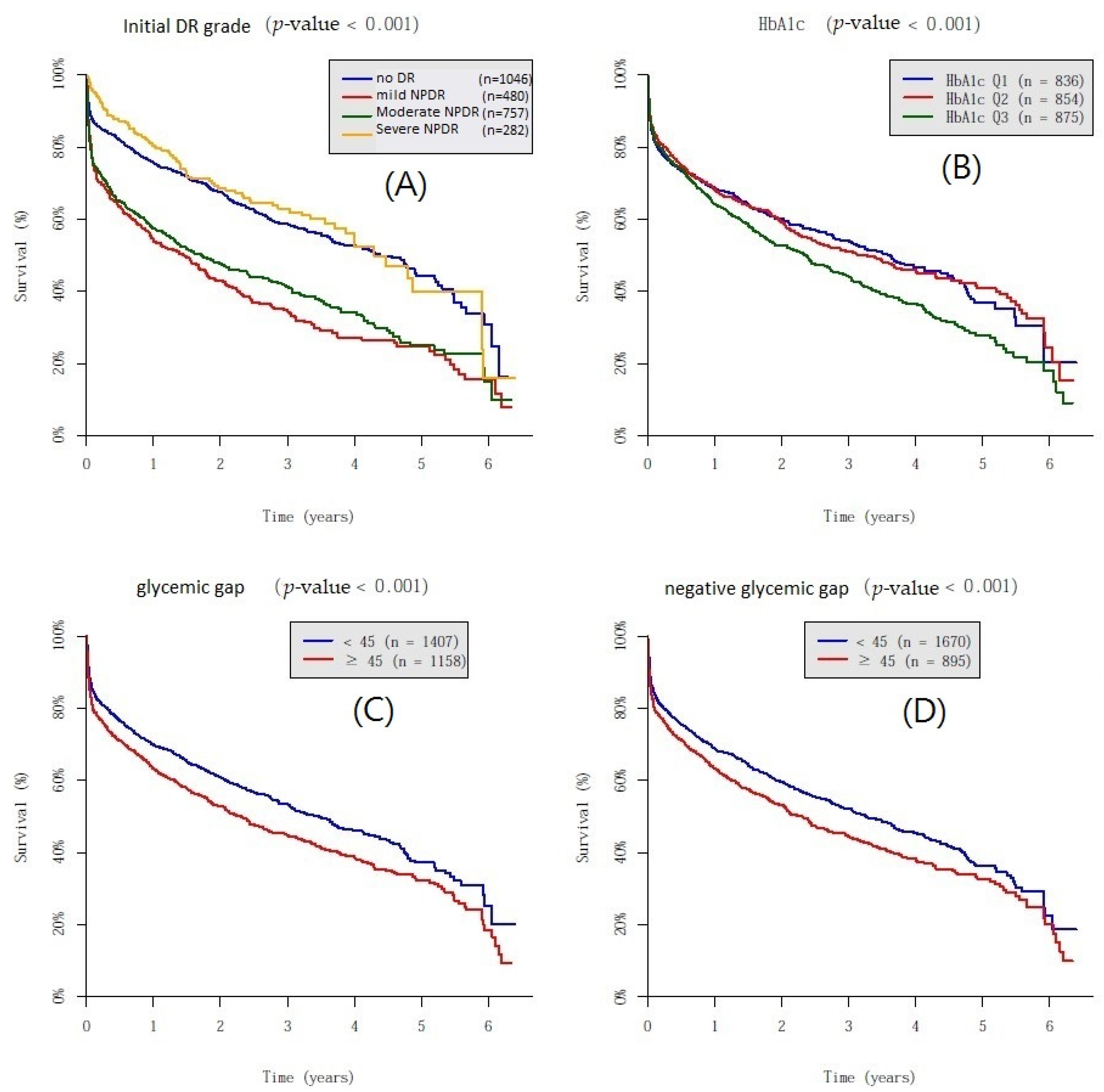
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diabetes Control; Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 1995, 44, 968–983. [Google Scholar] [CrossRef]
- Nathan, D.M.; McGee, P.; Steffes, M.W.; Lachin, J.M.; DCCT/EDIC Research Group. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes 2014, 63, 282–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Currie, C.J.; Peters, J.R.; Tynan, A.; Evans, M.; Heine, R.J.; Bracco, O.L.; Zagar, T.; Poole, C.D. Survival as a function of HbA1c in people with type 2 diabetes: A retrospective cohort study. Lancet 2010, 375, 481–489. [Google Scholar] [CrossRef]
- Huang, E.S.; Liu, J.Y.; Moffet, H.H.; John, P.M.; Karter, A.J. Glycemic control, complications, and death in older diabetic patients: The diabetes and aging study. Diabetes Care 2011, 34, 1329–1336. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Ma, X.; Zhang, L.; Mo, Y.; Ying, L.; Lu, W.; Zhu, W.; Bao, Y.; Zhou, J. Glycemic variability assessed by continuous glucose monitoring and the risk of diabetic retinopathy in latent autoimmune diabetes of the adult and type 2 diabetes. J. Diabetes Investig. 2019, 10, 753–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Ma, X.; Zhou, J.; Zhang, L.; Mo, Y.; Ying, L.; Lu, W.; Zhu, W.; Bao, Y.; Vigersky, R.A.; et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 2018, 41, 2370–2376. [Google Scholar] [CrossRef] [Green Version]
- Škrha, J.; Šoupal, J.; Prázný, M. Glucose variability, HbA1c and microvascular complications. Rev. Endocr. Metab. Disord. 2016, 17, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhou, F.; Zhang, Y.; Zhou, X.; Ying, C.J.D.R.; Practice, C. Fasting plasma glucose variability levels and risk of adverse outcomes among patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2019, 148, 23–31. [Google Scholar] [CrossRef]
- Park, J.Y.; Hwang, J.H.; Kang, M.J.; Sim, H.E.; Kim, J.S.; Ko, K.S.J.R. Effects of glycemic variability on the progression of diabetic retinopathy among patients with type 2 diabetes. Retina 2021, 41, 1487–1495. [Google Scholar] [CrossRef]
- Hu, J.; Hsu, H.; Yuan, X.; Lou, K.; Hsue, C.; Miller, J.D.; Lu, J.; Lee, Y.; Lou, Q. HbA1c variability as an independent predictor of diabetes retinopathy in patients with type 2 diabetes. J. Endocrinol. Investig. 2021, 44, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Tsai, S.-H.; Wang, J.-C.; Tzeng, Y.-S.; Wang, Y.-C.; Chu, C.-M.; Chu, S.-J.; Liao, W.-I. An elevated glycemic gap predicts adverse outcomes in diabetic patients with necrotizing fasciitis. PLoS ONE 2019, 14, e0223126. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.-I.; Lin, C.-S.; Lee, C.-H.; Wu, Y.-C.; Chang, W.-C.; Hsu, C.-W.; Wang, J.-C.; Tsai, S.-H. An elevated glycemic gap is associated with adverse outcomes in diabetic patients with acute myocardial infarction. Sci. Rep. 2016, 6, 27770. [Google Scholar] [CrossRef]
- Liao, W.-I.; Sheu, W.H.-H.; Chang, W.-C.; Hsu, C.-W.; Chen, Y.-L.; Tsai, S.-H. An Elevated Gap between Admission and A 1C-Derived Average Glucose Levels Is Associated with Adverse Outcomes in Diabetic Patients with Pyogenic Liver Abscess. PLoS ONE 2013, 8, e64476. [Google Scholar]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.; et al. Guidelines on diabetic eye care: The international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology 2018, 125, 1608–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathan, D.M.; Kuenen, J.; Borg, R.; Zheng, H.; Schoenfeld, D.; Heine, R.J. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008, 31, 1473–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsing, S.-C.; Lee, C.-C.; Lin, C.; Chen, J.-T.; Chen, Y.-H.; Fang, W.-H. The Severity of Diabetic Retinopathy Is an Independent Factor for the Progression of Diabetic Nephropathy. J. Clin. Med. 2021, 10, 3. [Google Scholar] [CrossRef]
- Available online: https://www.kaggle.com/c/diabetic-retinopathy-detection (accessed on 12 June 2020).
- Hu, J.; Shen, L.; Sun, G. Squeeze-and-excitation networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 7132–7141. [Google Scholar]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark. Insights 2016, 11, BMI-S38440. [Google Scholar] [CrossRef]
- Care, D. 6. Glycemic targets: Standards of medical care in diabetes—2019. Diabetes Care 2019, 42, S61–S70. [Google Scholar]
- Bergenstal, R.M. Glycemic variability and diabetes complications: Does it matter? Simply put, there are better glycemic markers! Diabetes Care 2015, 38, 1615–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, I.B. Glycemic variability and diabetes complications: Does it matter? Of course it does! Diabetes Care 2015, 38, 1610–1614. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, C.; Leite, N.; Moram, C.; Salles, G. Long-term visit-to-visit glycemic variability as predictor of micro-and macrovascular complications in patients with type 2 diabetes: The Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc. Diabetol. 2018, 17, 33. [Google Scholar] [CrossRef] [Green Version]
- Park, S.P. Long-term HbA1c variability and the progression of diabetic retinopathy in patients with type 2 diabetes. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1073. [Google Scholar]
- Yang, C.-Y.; Su, P.-F.; Hung, J.-Y.; Ou, H.-T.; Kuo, S. Comparative predictive ability of visit-to-visit HbA1c variability measures for microvascular disease risk in type 2 diabetes. Cardiovasc. Diabetol. 2020, 19, 105. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Arima, H.; Zoungas, S.; Ninomiya, T.; Cooper, M.; Hamet, P.; Mancia, G.; Poulter, N.; Harrap, S.; Woodward, M.; et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: The ADVANCE trial. Diabetes Care 2014, 37, 2359–2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penno, G.; Solini, A.; Bonora, E.; Fondelli, C.; Orsi, E.; Zerbini, G.; Morano, S.; Cavalot, F.; Lamacchia, O.; Laviola, L.; et al. HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: The Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care 2013, 36, 2301–2310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorst, C.; Kwok, C.S.; Aslam, S.; Buchan, I.; Kontopantelis, E.; Myint, P.K.; Heatlie, G.; Loke, Y.; Rutter, M.K.; Mamas, M.A. Long-term glycemic variability and risk of adverse outcomes: A systematic review and meta-analysis. Diabetes Care 2015, 38, 2354–2369. [Google Scholar] [CrossRef] [Green Version]
- Sartore, G.; Chilelli, N.C.; Burlina, S.; Lapolla, A. Association between glucose variability as assessed by continuous glucose monitoring (CGM) and diabetic retinopathy in type 1 and type 2 diabetes. Acta Diabetol. 2013, 50, 437–442. [Google Scholar] [CrossRef]
- Donagaon, S.; Dharmalingam, M. Association between Glycemic Gap and adverse outcomes in critically ill patients with diabetes. Indian J. Endocrinol. Metab. 2018, 22, 208. [Google Scholar] [PubMed]
- Chen, P.-C.; Liao, W.-I.; Wang, Y.-C.; Chang, W.-C.; Hsu, C.-W.; Chen, Y.-H.; Tsai, S.-H. An elevated glycemic gap is associated with adverse outcomes in diabetic patients with community-acquired pneumonia. Medicine 2015, 94, e1456. [Google Scholar] [CrossRef]
- Ceriello, A.; Novials, A.; Ortega, E.; Canivell, S.; La Sala, L.; Pujadas, G.; Bucciarelli, L.; Rondinelli, M.; Genovese, S. Vitamin C further improves the protective effect of glucagon-like peptide-1 on acute hypoglycemia-induced oxidative stress, inflammation, and endothelial dysfunction in type 1 diabetes. Diabetes Care 2013, 36, 4104–4108. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Alexanian, A.; Ying, R.; Kizhakekuttu, T.J.; Dharmashankar, K.; Vasquez-Vivar, J.; Gutterman, D.D.; Widlansky, M.E. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: Role for AMP kinase. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 712–720. [Google Scholar] [CrossRef] [Green Version]
- Yousefzade, G.; Nakhaee, A. Insulin-induced hypoglycemia and stress oxidative state in healthy people. Acta Diabetol. 2012, 49, 81–85. [Google Scholar] [CrossRef]
- Gerhardinger, C.; Costa, M.B.; Coulombe, M.C.; Toth, I.; Hoehn, T.; Grosu, P. Expression of acute-phase response proteins in retinal Muller cells in diabetes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Kusner, L.L.; Sarthy, V.P.; Mohr, S. Nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase: A role in high glucose-induced apoptosis in retinal Muller cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1553–1561. [Google Scholar]
- Puro, D.G. Diabetes-induced dysfunction of retinal Müller cells. Trans. Am. Ophthalmol. Soc. 2002, 100, 339. [Google Scholar] [PubMed]
- Picconi, F.; Parravano, M.; Sciarretta, F.; Fulci, C.; Nali, M.; Frontoni, S.; Varano, M.; Caccuri, A.M. Activation of retinal Müller cells in response to glucose variability. Endocrine 2019, 65, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Cryer, P.E. Glycemic goals in diabetes: Trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes 2014, 63, 2188–2195. [Google Scholar] [CrossRef] [Green Version]
- FLAT-SUGAR Trial Investigators. Design of FLAT-SUGAR: Randomized trial of prandial insulin versus prandial GLP-1 receptor agonist together with basal insulin and metformin for high-risk type 2 diabetes. Diabetes Care 2015, 38, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
| Initial Grade of DR | p-Value | ||||
|---|---|---|---|---|---|
| No DR n = 1046 | Mild NPDR n = 480 | Moderate NPDR n = 757 | Severe NPDR n = 282 | ||
| Basic characteristics | |||||
| Gender | 0.201 | ||||
| Female | 506(48.4%) | 215(44.8%) | 329(43.5%) | 129(45.7%) | |
| Male | 540(51.6%) | 265(55.2%) | 428(56.5%) | 153(54.3%) | |
| Age (years) | 63.76 ± 12.99 | 63.07 ± 12.26 | 59.59 ± 11.11 | 57.15 ± 11.71 | <0.001 |
| SBP (mmHg) | 139.06 ± 20.30 | 140.37 ± 23.46 | 141.14 ± 21.70 | 141.83 ± 23.19 | 0.116 |
| DBP (mmHg) | 78.84 ± 11.98 | 78.66 ± 11.73 | 79.87 ± 12.21 | 80.62 ± 12.25 | 0.046 |
| Comorbidity | |||||
| Hypertension | 389(37.2%) | 211(44.0%) | 319(42.1%) | 128(45.4%) | 0.014 |
| Lipidemia | 318(30.4%) | 140(29.2%) | 225(29.7%) | 87(30.9%) | 0.948 |
| Ischemic heart disease | 222(21.2%) | 125(26.0%) | 169(22.3%) | 69(24.5%) | 0.180 |
| Heart failure | 54(5.2%) | 34(7.1%) | 67(8.9%) | 30(10.6%) | 0.002 |
| COPD | 38(3.6%) | 21(4.4%) | 12(1.6%) | 2(0.7%) | 0.001 |
| Stroke | 145(13.9%) | 66(13.8%) | 90(11.9%) | 36(12.8%) | 0.635 |
| Diabetic neuropathy | 67(6.4%) | 29(6.0%) | 75(9.9%) | 19(6.7%) | 0.019 |
| Laboratory test | |||||
| HbA1c (%) | 7.63 ± 1.91 | 7.91 ± 1.91 | 8.20 ± 2.03 | 8.40 ± 2.16 | <0.001 |
| Triglyceride (mg/dL) | 152.89 ± 104.63 | 154.97 ± 118.99 | 158.71 ± 118.46 | 151.34 ± 88.38 | 0.671 |
| Total cholesterol (mg/dL) | 170.82 ± 40.39 | 167.83 ± 38.99 | 173.07 ± 43.54 | 172.47 ± 44.15 | 0.169 |
| LDL cholesterol (mg/dL) | 99.07 ± 33.42 | 98.40 ± 31.41 | 100.16 ± 35.22 | 100.33 ± 34.48 | 0.771 |
| HDL cholesterol (mg/dL) | 46.83 ± 13.34 | 46.13 ± 12.95 | 45.99 ± 12.90 | 46.65 ± 13.08 | 0.537 |
| Creatinine (mg/dL) | 1.37 ± 1.75 | 1.70 ± 2.17 | 1.65 ± 2.00 | 1.63 ± 2.01 | 0.002 |
| ALT | 24.94 ± 27.24 | 24.39 ± 31.69 | 22.94 ± 37.79 | 25.10 ± 61.13 | 0.677 |
| Total bilirubin | 0.64 ± 0.41 | 0.66 ± 0.61 | 0.63 ± 0.53 | 0.59 ± 0.35 | 0.200 |
| WBC | 7.40 ± 5.58 | 7.39 ± 2.58 | 7.28 ± 4.72 | 7.86 ± 7.21 | 0.444 |
| PLT | 213.06 ± 76.15 | 216.90 ± 74.50 | 221.20 ± 81.48 | 227.85 ± 84.12 | 0.019 |
| Neutrophil | 63.63 ± 11.29 | 64.49 ± 11.30 | 63.81 ± 10.73 | 64.29 ± 11.07 | 0.497 |
| Lymphocyte | 27.43 ± 10.33 | 26.56 ± 10.38 | 27.03 ± 9.85 | 26.72 ± 9.72 | 0.409 |
| Uric acid (mg/dL) | 5.88 ± 1.78 | 6.08 ± 1.85 | 6.10 ± 1.89 | 6.32 ± 1.81 | 0.001 |
| Hb (g/dL) | 12.95 ± 1.95 | 12.70 ± 2.01 | 12.59 ± 2.19 | 12.58 ± 2.07 | 0.001 |
| Albumin (g/dL) | 3.91 ± 0.53 | 3.88 ± 0.56 | 3.83 ± 0.59 | 3.76 ± 0.61 | <0.001 |
| Result | |||||
| Glycemic gap | 49.54 ± 59.55 | 52.82 ± 68.25 | 59.75 ± 74.43 | 65.29 ± 94.93 | 0.001 |
| Positive glycemic gap | 46.43 ± 52.36 | 47.63 ± 40.13 | 52.90 ± 44.44 | 54.184 ± 44.18 | 0.008 |
| Negative glycemic gap | 45.72 ± 52.23 | 50.06 ± 67.47 | 56.70 ± 73.08 | 62.184 ± 93.49 | <0.001 |
| Into 3 Groups Equally Based on HbA1c Level | p-Value | |||
|---|---|---|---|---|
| HbA1c Q1 (<6.8) n = 836 | HbA1c Q2 (6.8−8.3) n = 854 | HbA1c Q3 (>8.3) n = 875 | ||
| Basic characteristics | ||||
| DR severity | <0.001 | |||
| No DR | 394(47.1%) | 377(44.1%) | 275(31.4%) | |
| Mild NPDR | 150(17.9%) | 161(18.9%) | 169(19.3%) | |
| Moderate NPDR | 217(26.0%) | 232(27.2%) | 308(35.2%) | |
| Severe NPDR | 75(9.0%) | 84(9.8%) | 123(14.1%) | |
| Gender | 0.377 | |||
| Female | 368(44.0%) | 398(46.6%) | 413(47.2%) | |
| Male | 468(56.0%) | 456(53.4%) | 462(52.8%) | |
| Age (years) | 62.12 ± 12.45 | 63.62 ± 11.87 | 59.34 ± 12.52 | <0.001 |
| SBP (mmHg) | 139.00 ± 20.39 | 140.85 ± 21.13 | 140.78 ± 23.30 | 0.140 |
| DBP (mmHg) | 78.44 ± 11.53 | 78.72 ± 11.41 | 80.72 ± 12.98 | <0.001 |
| Comorbidity | ||||
| Hypertension | 345(41.3%) | 369(43.2%) | 333(38.1%) | 0.088 |
| Lipidemia | 237(28.3%) | 255(29.9%) | 278(31.8%) | 0.301 |
| Ischemic heart disease | 189(22.6%) | 204(23.9%) | 192(21.9%) | 0.62 |
| Heart failure | 52(6.2%) | 57(6.7%) | 76(8.7%) | 0.109 |
| COPD | 18(2.2%) | 29(3.4%) | 26(3.0%) | 0.296 |
| Stroke | 126(15.1%) | 106(12.4%) | 105(12.0%) | 0.127 |
| Diabetic neuropathy | 42(5.0%) | 49(5.7%) | 99(11.3%) | <0.001 |
| Laboratory test | ||||
| Triglyceride (mg/dL) | 132.54 ± 73.00 | 142.32 ± 91.21 | 188.31 ± 143.38 | <0.001 |
| Total cholesterol (mg/dL) | 164.75 ± 39.84 | 166.52 ± 37.60 | 181.65 ± 44.64 | <0.001 |
| LDL cholesterol (mg/dL) | 95.05 ± 33.14 | 96.30 ± 31.20 | 106.59 ± 35.41 | <0.001 |
| HDL cholesterol (mg/dL) | 47.39 ± 13.04 | 47.05 ± 13.36 | 44.92 ± 12.81 | <0.001 |
| Creatinine (mg/dL) | 1.69 ± 2.21 | 1.51 ± 1.84 | 1.43 ± 1.75 | 0.015 |
| Uric acid (mg/dL) | 6.07 ± 1.86 | 5.97 ± 1.78 | 6.06 ± 1.86 | 0.469 |
| Hb (g/dL) | 12.54 ± 2.17 | 12.70 ± 1.96 | 13.02 ± 2.00 | <0.001 |
| Albumin (g/dL) | 3.87 ± 0.57 | 3.91 ± 0.56 | 3.82 ± 0.56 | 0.004 |
| Result | ||||
| Glycemic gap | 31.92 ± 51.45 | 47.39 ± 52.99 | 84.18 ± 88.80 | <0.001 |
| Positive glycemic gap | 28.87 ± 26.18 | 44.48 ± 32.84 | 73.87 ± 61.59 | <0.001 |
| Negative glycemic gap | 29.62 ± 50.92 | 45.44 ± 52.43 | 78.58 ± 83.07 | <0.001 |
| Crude-HR (95% CI) | p-Value | Adjusted-HR (95% CI) | p-Value | |
|---|---|---|---|---|
| Initial DR grade | <0.001 | <0.001 | ||
| No DR | 1.00 | 1.00 | ||
| Mild NPDR | 2.00 (1.72–2.32) | <0.001 | 1.98 (1.70–2.31) | <0.001 |
| Moderate NPDR | 1.82 (1.58–2.09) | <0.001 | 1.78 (1.54–2.04) | <0.001 |
| Servere NPDR | 0.87 (0.69–1.09) | 0.223 | 0.86 (0.69–1.08) | 0.191 |
| Glycemic control | 0.003 | 0.060 | ||
| HbA1c Q1 (<6.8) | 1.00 | 1.00 | ||
| HbA1c Q2 (6.8−8.3) | 1.00 (0.86–1.15) | 0.976 | 0.97 (0.84–1.12) | 0.668 |
| HbA1c Q3 (>8.3) | 1.23 (1.07–1.41) | 0.004 | 1.13 (0.98–1.31) | 0.083 |
| Glycemic gap | <0.001 | 0.001 | ||
| <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.26 (1.13–1.42) | <0.001 | 1.22 (1.09–1.37) | 0.001 |
| Positive glycemic gap | 0.065 | 0.076 | ||
| <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.21 (0.99–1.49) | 0.065 | 1.21 (0.98–1.48) | 0.076 |
| Negative glycemic gap | 0.001 | 0.005 | ||
| <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.22 (1.08–1.37) | 0.001 | 1.18 (1.05–1.33) | 0.005 |
| Gender | 0.019 | 0.033 | ||
| Female | 1.00 | 1.00 | ||
| Male | 1.15 (1.02–1.29) | 0.019 | 1.13 (1.01–1.27) | 0.033 |
| Age | 1.00 (1.00–1.00) | 0.962 | 1.00 (1.00–1.01) | 0.945 |
| Height | 1.18 (0.92–1.51) | 0.184 | 0.96 (0.71–1.30) | 0.804 |
| Weight | 0.95 (0.74–1.21) | 0.669 | 0.88 (0.50–1.57) | 0.676 |
| Systolic blood pressure | 1.00 (1.00–1.00) | 0.269 | 1.00 (1.00–1.00) | 0.639 |
| Diastolic blood pressure | 1.00 (1.00–1.01) | 0.214 | 1.00 (1.00–1.01) | 0.300 |
| Comorbidity | ||||
| Hypertension | 1.14 (1.02–1.28) | 0.027 | 1.07 (0.95–1.21) | 0.253 |
| Lipidemia | 0.97 (0.85–1.10) | 0.609 | 0.94 (0.82–1.07) | 0.335 |
| Ischemic heart disease | 1.09 (0.96–1.25) | 0.184 | 1.03 (0.89–1.19) | 0.682 |
| Stroke | 1.10 (0.93–1.30) | 0.249 | 1.07 (0.91–1.27) | 0.415 |
| Diabetic neuropathy | 1.30 (1.07–1.59) | 0.009 | 1.21 (0.99–1.49) | 0.066 |
| Heart failure | 1.16 (0.95–1.43) | 0.152 | 1.08 (0.87–1.34) | 0.472 |
| Laboratory test | ||||
| Triglyceride | 1.00 (1.00–1.00) | 0.488 | 1.00 (1.00–1.00) | 0.602 |
| Total cholesterol | 1.00 (1.00–1.00) | 0.483 | 1.00 (1.00–1.00) | 0.420 |
| Low-density lipoprotein | 1.00 (1.00–1.00) | 0.488 | 1.00 (1.00–1.00) | 0.602 |
| High-density lipoprotein | 1.00 (1.00–1.00) | 0.983 | 1.00 (1.00–1.01) | 0.630 |
| Hemoglobin | 0.99 (0.97–1.02) | 0.699 | 1.00 (0.97–1.03) | 0.835 |
| Crude-HR (95% CI) | p-Value | Adjusted-HR (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| Initial DR grade | 0.164 (interaction) | 0.129 (interaction) | |||
| No DR | <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.30 (1.06–1.59) | 0.012 | 1.30 (1.06–1.59) | 0.013 | |
| Mild NPDR | <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.03 (0.81–1.32) | 0.790 | 1.01 (0.79–1.29) | 0.952 | |
| Moderate NPDR | <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.14 (0.93–1.40) | 0.200 | 1.14 (0.93–1.39) | 0.221 | |
| Servere NPDR | <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.71 (1.13–2.57) | 0.010 | 1.76 (1.16–2.66) | 0.007 | |
| Glycemic control | 0.709 (interaction) | 0.686 (interaction) | |||
| HbA1c Q1 (<6.8) | <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.25 (0.88–1.78) | 0.217 | 1.23 (0.87–1.76) | 0.244 | |
| HbA1c Q2(6.8−8.3) | <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.22 (1.00–1.50) | 0.053 | 1.21 (0.98–1.48) | 0.073 | |
| HbA1c Q3 (>8.3) | <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.11 (0.92–1.34) | 0.296 | 1.10 (0.91–1.33) | 0.339 |
| Crude-HR (95% CI) | p-Value | Adjuseted-HR(95% CI) | p-Value | ||
|---|---|---|---|---|---|
| Initial DR grade | 0.374 (interaction) | 0.336 (interaction) | |||
| No DR | <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.28 (1.05–1.56) | 0.014 | 1.26 (1.03–1.53) | 0.023 | |
| Mild NPDR | <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.07 (0.85–1.35) | 0.565 | 1.06 (0.84–1.33) | 0.647 | |
| Moderate NPDR | <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.25 (1.03–1.53) | 0.025 | 1.25 (1.03–1.53) | 0.026 | |
| Servere NPDR | <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.56 (1.03–2.36) | 0.036 | 1.56 (1.02–2.36) | 0.038 | |
| Glycemic control | 0.787 (interaction) | 0.806 (interaction) | |||
| HbA1c Q1 (<6.8) | <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.15 (0.89–1.49) | 0.277 | 1.15 (0.89–1.48) | 0.300 | |
| HbA1c Q2(6.8−8.3) | <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.28 (1.05–1.57) | 0.016 | 1.26 (1.03–1.54) | 0.025 | |
| HbA1c Q3 (>8.3) | <45 mg/dL | 1.00 | 1.00 | ||
| >45 mg/dL | 1.20 (0.98–1.48) | 0.079 | 1.19 (0.97–1.47) | 0.099 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsing, S.-C.; Lin, C.; Chen, J.-T.; Chen, Y.-H.; Fang, W.-H. Glycemic Gap as a Useful Surrogate Marker for Glucose Variability and Progression of Diabetic Retinopathy. J. Pers. Med. 2021, 11, 799. https://doi.org/10.3390/jpm11080799
Hsing S-C, Lin C, Chen J-T, Chen Y-H, Fang W-H. Glycemic Gap as a Useful Surrogate Marker for Glucose Variability and Progression of Diabetic Retinopathy. Journal of Personalized Medicine. 2021; 11(8):799. https://doi.org/10.3390/jpm11080799
Chicago/Turabian StyleHsing, Shi-Chue, Chin Lin, Jiann-Torng Chen, Yi-Hao Chen, and Wen-Hui Fang. 2021. "Glycemic Gap as a Useful Surrogate Marker for Glucose Variability and Progression of Diabetic Retinopathy" Journal of Personalized Medicine 11, no. 8: 799. https://doi.org/10.3390/jpm11080799
APA StyleHsing, S.-C., Lin, C., Chen, J.-T., Chen, Y.-H., & Fang, W.-H. (2021). Glycemic Gap as a Useful Surrogate Marker for Glucose Variability and Progression of Diabetic Retinopathy. Journal of Personalized Medicine, 11(8), 799. https://doi.org/10.3390/jpm11080799









