Resistance to Neuromuscular Blockade by Rocuronium in Surgical Patients with Spastic Cerebral Palsy
Abstract
:1. Introduction
2. Materials and Methods
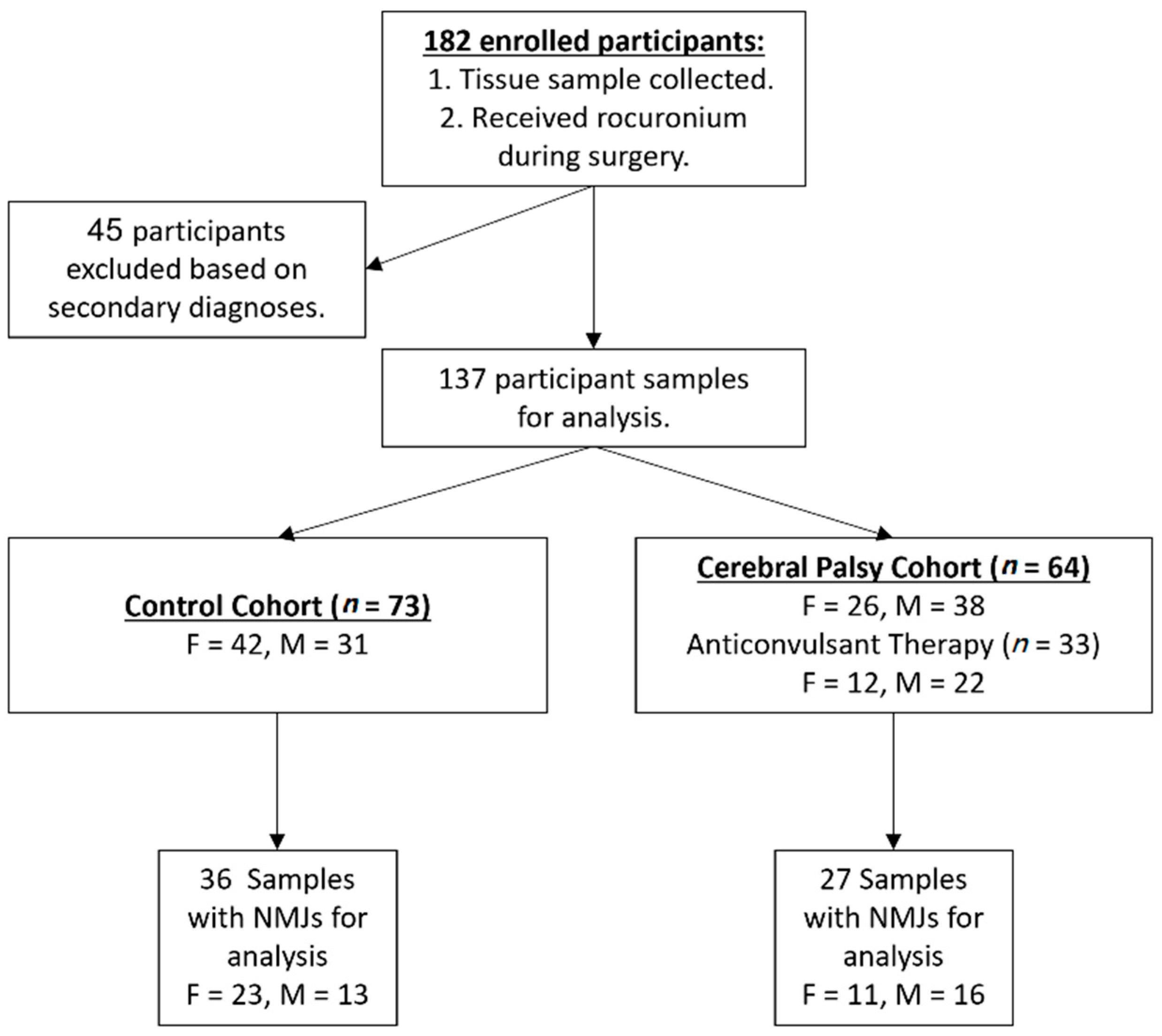
2.1. Participants and Data
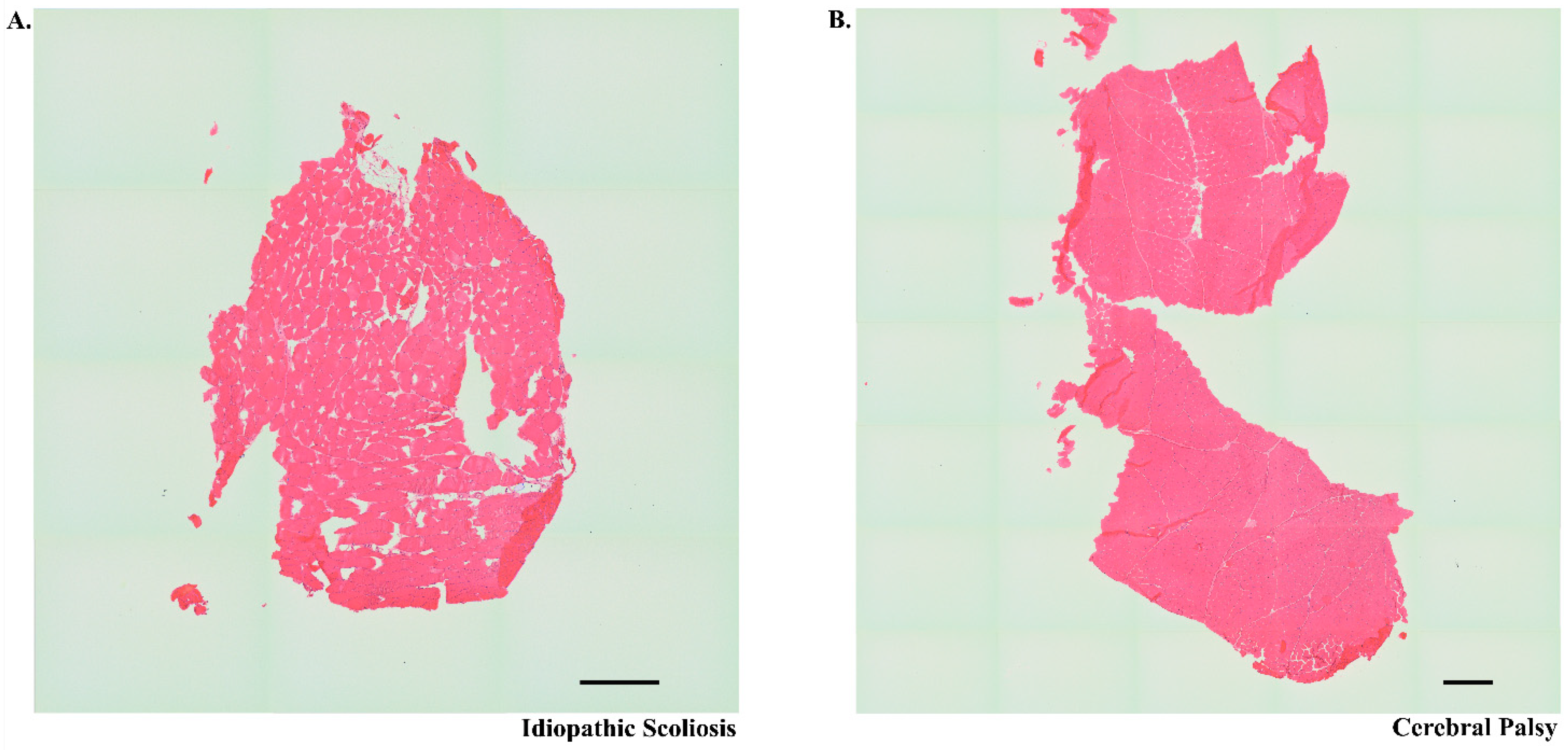
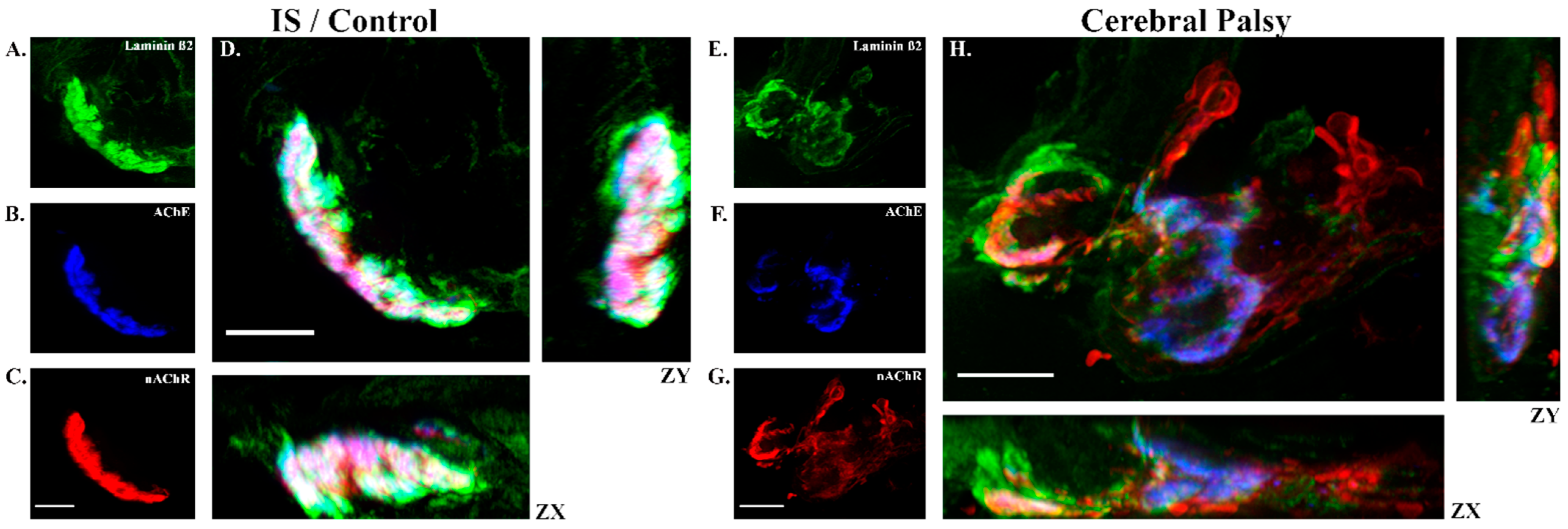
2.2. Tissue Preparation, Staining, and Imaging
2.3. Statistics
3. Results
3.1. Demographics
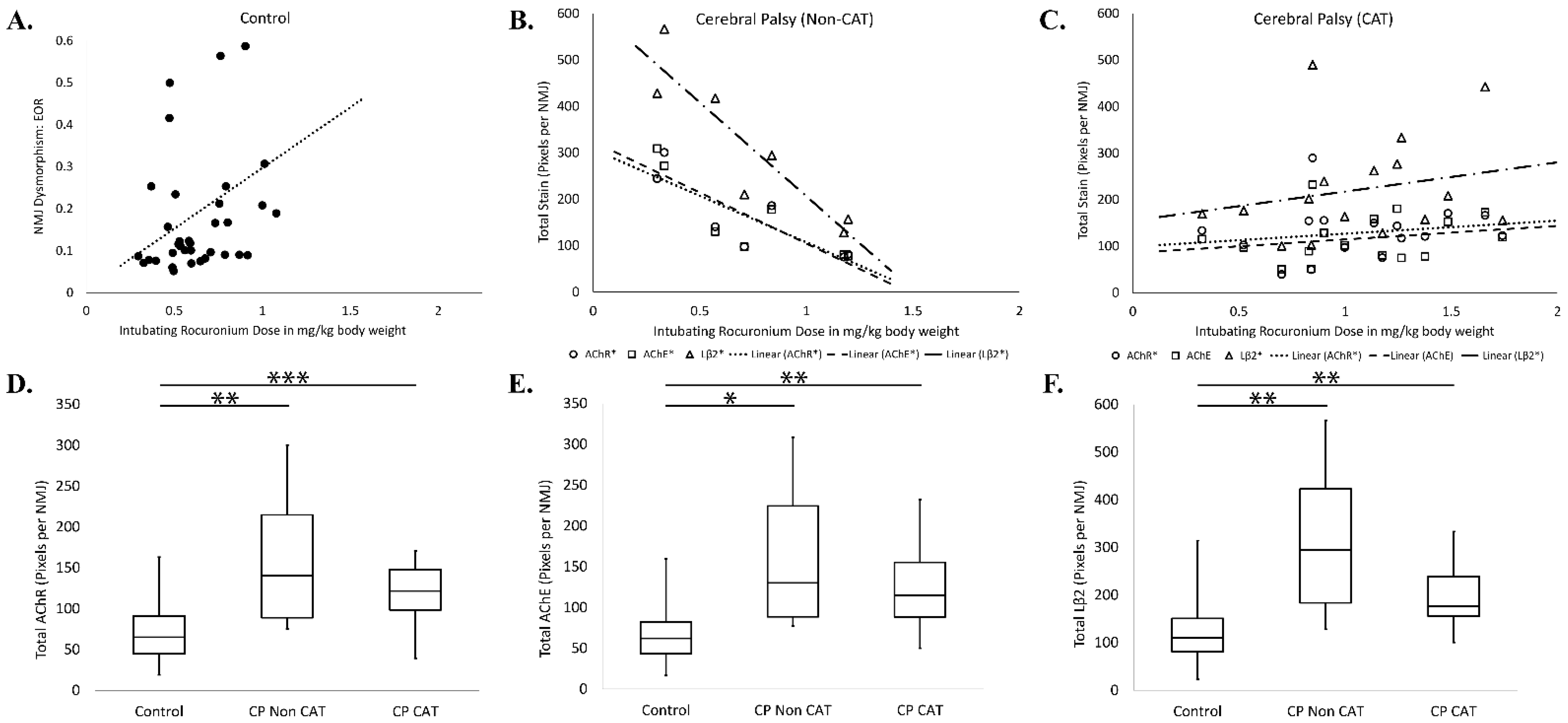
3.2. Rocuronium Dose Determination
3.3. Rocuronium Dose and Degree of Neuromotor Impairment
3.4. Chronic Anticonvulsant Therapy
3.5. Risk Factors Associated with Cerebral Palsy
3.6. Rocuronium Dose and Neuromuscular Junction Microanatomy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACL | Anterior cruciate ligament |
| AChE | Acetylcholinesterase |
| ALT | Alanine transaminase |
| ANOVA | Analysis of variance |
| BMI | Body mass index |
| BW | Birth weight |
| CAT | Chronic anticonvulsant therapy |
| CP | Cerebral palsy |
| CYP 3A4 | Cytochrome P450 3A4 |
| EMR | Electronic Medical Record |
| FET | Fisher’s exact test |
| FT | Full-term |
| GA | Gestational age |
| GMFCS | Gross motor function classification system |
| HIE | Hypoxic ischemic encephalopathy |
| IgG | Immunoglobulin G |
| IRB | Institutional review board |
| IVH | Intraventricular hemorrhage |
| KW | Kruskal Wallis |
| Lb2 | Laminin beta 2 |
| LBW | Low birth weight |
| Mg | Magnesium |
| MWU | Mann Whitney U |
| nAChR | Nicotinic acetylcholine receptor |
| NBW | Normal birth weight |
| NMBA | Neuromuscular blocking agent |
| NMJ | Neuromuscular junction |
| PT | Pre-term |
| ROC | Rocuronium bromide |
| SEM | Standard error of the mean |
| Tri | Triglycerides |
| TWA | Two way ANOVA |
References
- Data and Statistics for Cerebral Palsy. Available online: https://www.cdc.gov/ncbddd/cp/data.html (accessed on 31 May 2021).
- Sankar, C.; Mundkur, N. Cerebral palsy-definition, classification, etiology and early diagnosis. Indian J. Pediatrics 2005, 72, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Accardo, P.J. (Ed.) Capute & Accardo’s Neurodevelopmental Disabilities in Infancy and Childhood, Third Edition: Volume I: Neurodevelopmental Diagnosis and Treatment; Paul H. Brookes Publishing Co.: Baltimore, MD, USA, 2007. [Google Scholar]
- Peterson, M.D.; Gordon, P.M.; Hurvitz, E.A.; Burant, C.F. Secondary muscle pathology and metabolic dysregulation in adults with cerebral palsy. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1085–E1093. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child. Neurol. Suppl. 2007, 109, 8–14. [Google Scholar] [PubMed]
- Graham, H.K.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.P.; Damiano, D.L.; Becher, J.G.; Gaebler-Spira, D.; Colver, A.; Reddihough, D.S.; et al. Cerebral palsy. Nat. Rev. Dis. Primers 2016, 2, 15082. [Google Scholar] [CrossRef]
- Moster, D.; Wilcox, A.J.; Vollset, S.E.; Markestad, T.; Lie, R.T. Cerebral palsy among term and postterm births. JAMA 2010, 304, 976–982. [Google Scholar] [CrossRef]
- Kuban, K.C.; O’Shea, T.M.; Allred, E.N.; Fichorova, R.N.; Heeren, T.; Paneth, N.; Hirtz, D.; Dammann, O.; Leviton, A.; Investigators, E.S. The breadth and type of systemic inflammation and the risk of adverse neurological outcomes in extremely low gestation newborns. Pediatr. Neurol. 2015, 52, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Leviton, A.; Fichorova, R.N.; O’Shea, T.M.; Kuban, K.; Paneth, N.; Dammann, O.; Allred, E.N.; Investigators, E.S. Two-hit model of brain damage in the very preterm newborn: Small for gestational age and postnatal systemic inflammation. Pediatr. Res. 2013, 73, 362–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLennan, A.H.; Kruer, M.C.; Baynam, G.; Moreno-De-Luca, A.; Wilson, Y.A.; Zhu, C.; Wintle, R.F.; Gecz, J.; members of the International Cerebral Palsy Genomics Consortium. Cerebral palsy and genomics: An international consortium. Dev. Med. Child Neurol. 2018, 60, 209–210. [Google Scholar] [CrossRef] [Green Version]
- MacLennan, A.H.; Thompson, S.C.; Gecz, J. Cerebral palsy: Causes, pathways, and the role of genetic variants. Am. J. Obstet. Gynecol. 2015, 213, 779–788. [Google Scholar] [CrossRef] [Green Version]
- Corbett, M.A.; van Eyk, C.L.; Webber, D.L.; Bent, S.J.; Newman, M.; Harper, K.; Berry, J.G.; Azmanov, D.N.; Woodward, K.J.; Gardner, A.E.; et al. Pathogenic copy number variants that affect gene expression contribute to genomic burden in cerebral palsy. NPJ Genom. Med. 2018, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.A.; Shetty, S.; Wilson, B.A.; Huang, A.J.; Jin, S.C.; Smithers-Sheedy, H.; Fahey, M.C.; Kruer, M.C. Insights From Genetic Studies of Cerebral Palsy. Front. Neurol. 2020, 11, 625428. [Google Scholar] [CrossRef]
- McMichael, G.; Girirajan, S.; Moreno-De-Luca, A.; Gecz, J.; Shard, C.; Nguyen, L.S.; Nicholl, J.; Gibson, C.; Haan, E.; Eichler, E.; et al. Rare copy number variation in cerebral palsy. Eur. J. Hum. Genet. 2014, 22, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Oskoui, M.; Gazzellone, M.J.; Thiruvahindrapuram, B.; Zarrei, M.; Andersen, J.; Wei, J.; Wang, Z.; Wintle, R.F.; Marshall, C.R.; Cohn, R.D.; et al. Clinically relevant copy number variations detected in cerebral palsy. Nat. Commun. 2015, 6, 7949. [Google Scholar] [CrossRef] [PubMed]
- Segel, R.; Ben-Pazi, H.; Zeligson, S.; Fatal-Valevski, A.; Aran, A.; Gross-Tsur, V.; Schneebaum-Sender, N.; Shmueli, D.; Lev, D.; Perlberg, S.; et al. Copy number variations in cryptogenic cerebral palsy. Neurology 2015, 84, 1660–1668. [Google Scholar] [CrossRef]
- Zarrei, M.; Fehlings, D.L.; Mawjee, K.; Switzer, L.; Thiruvahindrapuram, B.; Walker, S.; Merico, D.; Casallo, G.; Uddin, M.; MacDonald, J.R.; et al. De novo and rare inherited copy-number variations in the hemiplegic form of cerebral palsy. Genet. Med. 2018, 20, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Moreno-De-Luca, A.; Millan, F.; Pesacreta, D.R.; Elloumi, H.Z.; Oetjens, M.T.; Teigen, C.; Wain, K.E.; Scuffins, J.; Myers, S.M.; Torene, R.I.; et al. Molecular Diagnostic Yield of Exome Sequencing in Patients With Cerebral Palsy. JAMA 2021, 325, 467–475. [Google Scholar] [CrossRef]
- Jin, S.C.; Lewis, S.A.; Bakhtiari, S.; Zeng, X.; Sierant, M.C.; Shetty, S.; Nordlie, S.M.; Elie, A.; Corbett, M.A.; Norton, B.Y.; et al. Mutations disrupting neuritogenesis genes confer risk for cerebral palsy. Nat. Genet. 2020, 52, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Bahado-Singh, R.O.; Vishweswaraiah, S.; Aydas, B.; Mishra, N.K.; Guda, C.; Radhakrishna, U. Deep Learning/Artificial Intelligence and Blood-Based DNA Epigenomic Prediction of Cerebral Palsy. Int. J. Mol. Sci. 2019, 20, 2075. [Google Scholar] [CrossRef] [Green Version]
- Crowgey, E.L.; Marsh, A.G.; Robinson, K.G.; Yeager, S.K.; Akins, R.E. Epigenetic machine learning: Utilizing DNA methylation patterns to predict spastic cerebral palsy. BMC Bioinform. 2018, 19, 225. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Jiang, Z.; Wang, J.; Xu, H.; Zhang, Q.; Liu, S.; Du, N.; Zhang, Y.; Qiu, H. Wholegenome scale identification of methylation markers specific for cerebral palsy in monozygotic discordant twins. Mol. Med. Rep. 2017, 16, 9423–9430. [Google Scholar] [CrossRef]
- Mohandas, N.; Bass-Stringer, S.; Maksimovic, J.; Crompton, K.; Loke, Y.J.; Walstab, J.; Reid, S.M.; Amor, D.J.; Reddihough, D.; Craig, J.M. Epigenome-wide analysis in newborn blood spots from monozygotic twins discordant for cerebral palsy reveals consistent regional differences in DNA methylation. Clin. Epigenetics 2018, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Graham, H.K. Painful hip dislocation in cerebral palsy. Lancet 2002, 359, 907–908. [Google Scholar] [CrossRef]
- Graham, H.K.; Thomason, P.; Willoughby, K.; Hastings-Ison, T.; Stralen, R.V.; Dala-Ali, B.; Wong, P.; Rutz, E. Musculoskeletal Pathology in Cerebral Palsy: A Classification System and Reliability Study. Children 2021, 8, 252. [Google Scholar] [CrossRef]
- Rehbein, I.; Teske, V.; Pagano, I.; Cuneo, A.; Perez, M.E.; von Heideken, J. Analysis of orthopedic surgical procedures in children with cerebral palsy. World J. Orthop. 2020, 11, 222–231. [Google Scholar] [CrossRef]
- Persson-Bunke, M.; Hagglund, G.; Lauge-Pedersen, H.; Wagner, P.; Westbom, L. Scoliosis in a total population of children with cerebral palsy. Spine 2012, 37, E708–E713. [Google Scholar] [CrossRef] [PubMed]
- Sharan, D. Cerebral Palsy—Challenges for the Future; Svraka, E., Ed.; InTech: London, UK, 2014. [Google Scholar]
- Raw, D.A.; Beattie, J.K.; Hunter, J.M. Anaesthesia for spinal surgery in adults. Br. J. Anaesth. 2003, 91, 886–904. [Google Scholar] [CrossRef] [Green Version]
- Appiah-Ankam, J.; Hunter, J.M. Pharmacology of neuromuscular blocking drugs. Contin. Educ. Anaesth. Crit. Care Pain 2004, 4, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Clinical Use of Neuromuscular Blocking Agents in Anesthesia—Up to Date. Available online: https://www.uptodate.com/contents/clinical-use-of-neuromuscular-blocking-agents-in-anesthesia (accessed on 31 May 2021).
- Hepaguslar, H.; Ozzeybek, D.; Elar, Z. The effect of cerebral palsy on the action of vecuronium with or without anticonvulsants. Anaesthesia 1999, 54, 593–596. [Google Scholar] [CrossRef]
- Moorthy, S.S.; Krishna, G.; Dierdorf, S.F. Resistance to vecuronium in patients with cerebral palsy. Anesth. Analg. 1991, 73, 275–277. [Google Scholar] [CrossRef]
- Na, H.S.; Lee, J.H.; Hwang, J.Y.; Ryu, J.H.; Han, S.H.; Jeon, Y.T.; Do, S.H. Effects of magnesium sulphate on intraoperative neuromuscular blocking agent requirements and postoperative analgesia in children with cerebral palsy. Br. J. Anaesth. 2010, 104, 344–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Nakamura, T.; Saeki, S.; Ogawa, S. Vecuronium-induced neuromuscular blockade in a patient with cerebral palsy and hemiplegia. Anesth. Analg. 2000, 91, 492–493. [Google Scholar]
- Theroux, M.C.; Brandom, B.W.; Zagnoev, M.; Kettrick, R.G.; Miller, F.; Ponce, C. Dose response of succinylcholine at the adductor pollicis of children with cerebral palsy during propofol and nitrous oxide anesthesia. Anesth. Analg. 1994, 79, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Theroux, M.C.; Akins, R.E.; Barone, C.; Boyce, B.; Miller, F.; Dabney, K.W. Neuromuscular junctions in cerebral palsy: Presence of extrajunctional acetylcholine receptors. Anesthesiology 2002, 96, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Lumsden, D.E.; Crowe, B.; Basu, A.; Amin, S.; Devlin, A.; DeAlwis, Y.; Kumar, R.; Lodh, R.; Lundy, C.T.; Mordekar, S.R.; et al. Pharmacological management of abnormal tone and movement in cerebral palsy. Arch. Dis. Child 2019, 104, 775–780. [Google Scholar] [CrossRef] [Green Version]
- Soriano, S.G.; Kaus, S.J.; Sullivan, L.J.; Martyn, J.A. Onset and duration of action of rocuronium in children receiving chronic anticonvulsant therapy. Paediatr. Anaesth. 2000, 10, 133–136. [Google Scholar] [CrossRef]
- Sheshadri, V.; Radhakrishnan, A.; Halemani, K.; Keshavan, V.H. Infusion dose requirement of rocuronium in patients on phenytoin therapy—A prospective comparative study. Indian J. Anaesth. 2017, 61, 793–797. [Google Scholar] [CrossRef]
- Pirttiaho, H.I.; Sotaniemi, E.A.; Pelkonen, R.O.; Pitkanen, U. Hepatic blood flow and drug metabolism in patients on enzyme-inducing anticonvulsants. Eur. J. Clin. Pharmacol. 1982, 22, 441–445. [Google Scholar] [CrossRef]
- Anzenbacherova, E.; Spicakova, A.; Jourova, L.; Ulrichova, J.; Adamus, M.; Bachleda, P.; Anzenbacher, P. Interaction of rocuronium with human liver cytochromes P450. J. Pharmacol. Sci. 2015, 127, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Robinson, K.G.; Mendonca, J.L.; Militar, J.L.; Theroux, M.C.; Dabney, K.W.; Shah, S.A.; Miller, F.; Akins, R.E. Disruption of basal lamina components in neuromotor synapses of children with spastic quadriplegic cerebral palsy. PLoS ONE 2013, 8, e70288. [Google Scholar] [CrossRef]
- Theroux, M.C.; Oberman, K.G.; Lahaye, J.; Boyce, B.A.; Duhadaway, D.; Miller, F.; Akins, R.E. Dysmorphic neuromuscular junctions associated with motor ability in cerebral palsy. Muscle Nerve 2005, 32, 626–632. [Google Scholar] [CrossRef]
- Murphy, G.S. Neuromuscular Monitoring in the Perioperative Period. Anesth. Analg. 2018, 126, 464–468. [Google Scholar] [CrossRef]
- Viby-Mogensen, J. Neuromuscular monitoring. Curr. Opin. Anaesthesiol. 2001, 14, 655–659. [Google Scholar] [CrossRef]
- Brandom, B.W.; Yellon, R.F.; Lloyd, M.E.; Gronert, B.J.; Theroux, M.C.; Simhi, E.; Chakravorti, S.; Venkataraman, S.; Dohar, J.E.; Shapiro, A.M.; et al. Recovery from doxacurium infusion administered to produce immobility for more than four days in pediatric patients in the intensive care unit. Anesth. Analg. 1997, 84, 307–314. [Google Scholar] [CrossRef]
- Gilbertson, L.E.; Fiedorek, M.C.; Fiedorek, C.S.; Trinh, T.A.; Lam, H.; Austin, T.M. Prolonged neuromuscular block after rocuronium administration in laparoscopic pyloromyotomy patients: A retrospective bayesian regression analysis. Paediatr. Anaesth. 2021, 31, 290–297. [Google Scholar] [CrossRef]
- Iwasaki, H.; Renew, J.R.; Kunisawa, T.; Brull, S.J. Preparing for the unexpected: Special considerations and complications after sugammadex administration. BMC Anesthesiol. 2017, 17, 140. [Google Scholar] [CrossRef] [Green Version]
- Blobner, M.; Frick, C.G.; Stauble, R.B.; Feussner, H.; Schaller, S.J.; Unterbuchner, C.; Lingg, C.; Geisler, M.; Fink, H. Neuromuscular blockade improves surgical conditions (NISCO). Surg. Endosc. 2015, 29, 627–636. [Google Scholar] [CrossRef]
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content validity of the expanded and revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, A.; Hadjichristodoulou, C.; Youroukos, S. Epilepsy in patients with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 659–663. [Google Scholar] [CrossRef]
- Sellier, E.; Uldall, P.; Calado, E.; Sigurdardottir, S.; Torrioli, M.G.; Platt, M.J.; Cans, C. Epilepsy and cerebral palsy: Characteristics and trends in children born in 1976–1998. Eur. J. Paediatr. Neurol. 2012, 16, 48–55. [Google Scholar] [CrossRef]
- Singhi, P.; Jagirdar, S.; Khandelwal, N.; Malhi, P. Epilepsy in children with cerebral palsy. J. Child Neurol. 2003, 18, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.J. Epilepsy in cerebral palsy. Dev. Med. Child Neurol. 2001, 43, 713–717. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Li, Y.; Ruan, J. Serum alanine transaminase total bilirubin concentrations predict CYP3A activity as measured by midazolam and 1’-hydroxylation. Med. Sci. Monit. 2015, 21, 396–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Anticonvulsants. [Updated 2019 Apr 18]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548365/ (accessed on 19 April 2019).
- Van Naarden Braun, K.; Doernberg, N.; Schieve, L.; Christensen, D.; Goodman, A.; Yeargin-Allsopp, M. Birth Prevalence of Cerebral Palsy: A Population-Based Study. Pediatrics 2016, 137, e20152872. [Google Scholar] [CrossRef] [Green Version]
- Camp, S.; De Jaco, A.; Zhang, L.; Marquez, M.; De la Torre, B.; Taylor, P. Acetylcholinesterase expression in muscle is specifically controlled by a promoter-selective enhancesome in the first intron. J. Neurosci. 2008, 28, 2459–2470. [Google Scholar] [CrossRef]
- Rogers, R.S.; Nishimune, H. The role of laminins in the organization and function of neuromuscular junctions. Matrix Biol. 2017, 57–58, 86–105. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Hwang, J.W.; Jeon, Y.T.; Do, S.H. Effects of valproic acid and magnesium sulphate on rocuronium requirement in patients undergoing craniotomy for cerebrovascular surgery. Br. J. Anaesth. 2012, 109, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitoh, Y.; Oshima, T.; Nakata, Y. Recovery of neuromuscular blockade caused by vecuronium is delayed in patients with hypertriglyceridemia. J. Anesth. 2008, 22, 89–92. [Google Scholar] [CrossRef]
- Nelson, K.B.; Blair, E. Prenatal Factors in Singletons with Cerebral Palsy Born at or near Term. N. Engl. J. Med. 2015, 373, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Redline, R.W. Cerebral palsy in term infants: A clinicopathologic analysis of 158 medicolegal case reviews. Pediatr. Dev. Pathol. 2008, 11, 456–464. [Google Scholar] [CrossRef]
- Ahlin, K.; Himmelmann, K.; Hagberg, G.; Kacerovsky, M.; Cobo, T.; Wennerholm, U.B.; Jacobsson, B. Cerebral palsy and perinatal infection in children born at term. Obstet. Gynecol. 2013, 122, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Tsimis, M.E.; Johnson, C.T.; Raghunathan, R.S.; Northington, F.J.; Burd, I.; Graham, E.M. Risk factors for periventricular white matter injury in very low birthweight neonates. Am. J. Obstet. Gynecol. 2016, 214, 380.e1–380.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himpens, E.; Van den Broeck, C.; Oostra, A.; Calders, P.; Vanhaesebrouck, P. Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: A meta-analytic review. Dev. Med. Child Neurol. 2008, 50, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Hemming, K.; Colver, A.; Hutton, J.L.; Kurinczuk, J.J.; Pharoah, P.O. The influence of gestational age on severity of impairment in spastic cerebral palsy. J. Pediatr. 2008, 153, 203–208.e4. [Google Scholar] [CrossRef] [PubMed]
- Jauhari, P.; Singhi, P.; Sankhyan, N.; Malhi, P.; Vyas, S.; Khandelwal, N. A Comparison of Spastic Diplegia in Term and Preterm-Born Children. J. Child Neurol. 2018, 33, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xing, Y.; Wang, H.; Chen, L.; Li, S. Differences in pharmacodynamic responses to rocuronium in normal or injured orbicularis oris are associated with expression of acetylcholine receptor subunits. Sci. Rep. 2017, 7, 3238. [Google Scholar] [CrossRef] [Green Version]
- Theroux, M.C.; Akins, R.E. Surgery and anesthesia for children who have cerebral palsy. Anesthesiol. Clin. N. Am. 2005, 23, 733–743. [Google Scholar] [CrossRef]
- Blotnick-Rubin, E.; Anglister, L. Fine Localization of Acetylcholinesterase in the Synaptic Cleft of the Vertebrate Neuromuscular Junction. Front. Mol. Neurosci. 2018, 11, 123. [Google Scholar] [CrossRef] [Green Version]

| Control | GMFCS I | GMFCS II | GMFCS III | GMFCS IV | GMFCS V | Total CP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | 73 | 3 | 6 | 6 | 14 | 35 | 64 | |||||||
| No. per Gender | M:31 | F:42 | M:2 | F:1 | M:2 | F:4 | M:1 | F:5 | M:9 | F:5 | M:24 | F:11 | M:38 | F:26 |
| Mean Age ** | 14.9 ± 2.55 | 11.3 ± 0.58 | 12.7 ± 3.50 | 13.7 ± 2.80 | 11.4 ± 4.07 | 13.1 ± 4.19 | 12.7 ± 3.90 | |||||||
| Mean BMI ** | 23.4 ± 6.43 | 15.6 ± 1.89 | 19.7 ± 4.76 | 21.3 ± 8.67 | 17.1 ± 3.57 | 18.4 ± 335 | 18.0 ± 4.78 | |||||||
| Mean Surgery Duration (hrs) ** | 7.42 ± 2.19 | 4.8 ± 1.04 | 5.40 ± 1.71 | 5.38 ± 1.73 | 7.50 ± 3.27 | 6.67 ± 2.54 | 6.58 ± 2.61 | |||||||
| Patients on CAT | 0 | 1 | 2 | 0 | 7 | 23 | 33 | |||||||
| Patients not on CAT | 0 | 2 | 4 | 6 | 7 | 12 | 31 | |||||||
| Hypoxia | 0 | 0 | 1 | 3 | 2 | 10 | 16 | |||||||
| IVH/Hemorrhage | 0 | 0 | 0 | 0 | 1 | 1 | 2 | |||||||
| HIE | 0 | 0 | 0 | 0 | 3 | 6 | 9 | |||||||
| Perinatal Infection | 0 | 0 | 0 | 0 | 1 | 1 | 2 | |||||||
| Encephalitis | 0 | 0 | 0 | 1 | 0 | 1 | 2 | |||||||
| Peri/Postnatal Trauma | 0 | 0 | 0 | 1 | 1 | 2 | 4 | |||||||
| Combination of Above | 0 | 2 | 2 | 1 | 2 | 4 | 11 | |||||||
| Other or Not Recorded | 0 | 1 | 3 | 0 | 4 | 10 | 18 | |||||||
| Preterm (GA < 37 weeks) *** | 5 | 3 | 2 | 5 | 12 | 15 | 37 | |||||||
| Full Term (GA ≥ 37 weeks) | 52 | 0 | 4 | 1 | 2 | 18 | 25 | |||||||
| GA Not Recorded | 16 | 0 | 0 | 0 | 0 | 2 | 2 | |||||||
| Intubating Rocuronium Dose | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Reference Range | Control | Cerebral Palsy Not on CAT | Cerebral Palsy on CAT | |||||||||
| n | Mean ± SEM | Pearson r | p Value | n | Mean ± SEM | Pearson r | p Value | n | Mean ± SEM | Pearson r | p Value | ||
| BW (kg) 1 | 2.5–4.0 | 44 | 3.53 ± 0.10 | −0.166 | 0.281 | 24 | 1.58 ± 0.18 | 0.544 | 0.006 ** | 29 | 2.58 ± 0.22 | 0.160 | 0.408 |
| GA (weeks) 1 | 37–40 | 58 | 39.3 ± 0.31 | −0.142 | 0.289 | 30 | 32.0 ± 0.90 | 0.416 | 0.022 * | 32 | 35.3 ± 1.13 | 0.247 | 0.173 |
| BW-by-GA | 0.07–0.10 | 42 | 0.09 ± 0.00 | −0.148 | 0.35 | 24 | 0.05 ± 0.00 | 0.510 | 0.011 * | 29 | 0.07 ± 0.00 | 0.117 | 0.545 |
| Serum ALT 2 | 0–35 U/L | 0 | NA | NA | NA | 14 | 25.4 ± 2.10 | 0.286 | 0.322 | 29 | 26.8 ± 2.49 | 0.283 | 0.136 |
| Serum Bilirubin 2 | 0.3–1.2 mg/dL | 0 | NA | NA | NA | 14 | 0.54 ± 0.08 | −0.062 | 0.833 | 29 | 0.27 ± 0.03 | −0.153 | 0.427 |
| Serum Mg 2 | 1.5–2.4 mg/dL | 19 | 2.92 ± 0.19 | 0.255 | 0.291 | 4 | 3.27 ± 0.25 | 0.117 | 0.883 | 13 | 3.23 ± 0.30 | −0.219 | 0.473 |
| Serum Tri 2 | <250 mg/dL | 19 | 56.9 ± 11.1 | −0.042 | 0.864 | 4 | 19.6 ± 10.3 | −0.768 | 0.232 | 13 | 24.6 ± 6.60 | 0.032 | 0.918 |
| Total Rocuronium Dose | |||||||||||||
| Measure | Reference Range | Control | Cerebral Palsy Not on CAT | Cerebral Palsy on CAT | |||||||||
| n | Mean ± SEM | Pearson r | p Value | n | Mean ± SEM | Pearson r | p Value | n | Mean ± SEM | Pearson r | p Value | ||
| BW (kg) 1 | 2.5–4.0 | 44 | 3.53 ± 0.10 | −0.166 | 0.281 | 24 | 1.58 ± 0.18 | 0.581 | 0.003 ** | 29 | 2.58 ± 0.22 | 0.160 | 0.408 |
| GA (weeks) 1 | 37–40 | 58 | 39.3 ± 0.31 | −0.142 | 0.289 | 30 | 32.0 ± 0.90 | 0.432 | 0.017 * | 32 | 35.3 ± 1.13 | 0.247 | 0.173 |
| BW-by-GA | 0.07–0.10 | 42 | 0.09 ± 0.00 | −0.148 | 0.35 | 24 | 0.05 ± 0.00 | 0.555 | 0.005 ** | 29 | 0.07 ± 0.00 | 0.117 | 0.545 |
| Serum ALT 2 | 0–35 U/L | 0 | NA | NA | NA | 14 | 25.4 ± 2.10 | 0.287 | 0.320 | 29 | 26.8 ± 2.49 | 0.454 | 0.013 * |
| Serum Bilirubin 2 | 0.3–1.2 mg/dL | 0 | NA | NA | NA | 14 | 0.54 ± 0.08 | −0.154 | 0.600 | 29 | 0.27 ± 0.03 | 0.040 | 0.837 |
| Serum Mg 2 | 1.5–2.4 mg/dL | 19 | 2.92 ± 0.19 | 0.259 | 0.285 | 4 | 3.27 ± 0.25 | −0.573 | 0.427 | 13 | 3.23 ± 0.30 | −0.051 | 0.869 |
| Serum Tri 2 | <250 mg/dL | 19 | 56.9 ± 11.1 | 0.016 | 0.948 | 4 | 19.6 ± 10.3 | −0.260 | 0.740 | 13 | 24.6 ± 6.60 | −0.324 | 0.279 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Robinson, K.; Lodge, M.; Theroux, M.; Miller, F.; Akins, R., Jr. Resistance to Neuromuscular Blockade by Rocuronium in Surgical Patients with Spastic Cerebral Palsy. J. Pers. Med. 2021, 11, 765. https://doi.org/10.3390/jpm11080765
Lee S, Robinson K, Lodge M, Theroux M, Miller F, Akins R Jr. Resistance to Neuromuscular Blockade by Rocuronium in Surgical Patients with Spastic Cerebral Palsy. Journal of Personalized Medicine. 2021; 11(8):765. https://doi.org/10.3390/jpm11080765
Chicago/Turabian StyleLee, Stephanie, Karyn Robinson, Madison Lodge, Mary Theroux, Freeman Miller, and Robert Akins, Jr. 2021. "Resistance to Neuromuscular Blockade by Rocuronium in Surgical Patients with Spastic Cerebral Palsy" Journal of Personalized Medicine 11, no. 8: 765. https://doi.org/10.3390/jpm11080765
APA StyleLee, S., Robinson, K., Lodge, M., Theroux, M., Miller, F., & Akins, R., Jr. (2021). Resistance to Neuromuscular Blockade by Rocuronium in Surgical Patients with Spastic Cerebral Palsy. Journal of Personalized Medicine, 11(8), 765. https://doi.org/10.3390/jpm11080765






