Treatment Resistance: A Time-Based Approach for Early Identification in First Episode Psychosis
Abstract
:1. Introduction
- (1)
- To identify the symptom domains and thresholds that define a “probable TR” subgroup in a prospective manner in FES samples as early as 6 months after presentation. We studied the utility of a 20% response threshold (as defined by TRIPP; [5]) as well as a more stringent 50% response threshold (identified as a “good response” cut-off for clinical trials by Aboraya et al., (2017) [19] in the domains of positive, negative, and total symptoms 6 months following FES. In keeping with previous literature [1,2], and the single-site data from a 5-year follow-up of FES, we hypothesized that the most valid criteria would categorize approximately 33% of the sample as probable TR.
- (2)
- To test the predictive and clinical validity of the various “probable TR” definitions at 6 months by assessing whether global functioning over 5 years and clozapine use at the 5th year could be reliably predicted on the basis of these definitions. Given the high response rates for positive symptoms expected in FES, we hypothesized that the use of 50% threshold as well as the inclusion of negative symptoms would be important in characterizing probable TR in a FES sample. Nevertheless, given the historical focus on positive symptoms when prescribing clozapine [11], we expected probable-TR defined as per positive rather than negative symptom thresholds to relate to eventual clozapine use.
2. Materials and Methods
2.1. Sample Recruitment
2.2. Clinical Assessment
2.3. Assessment of Adherence
2.4. Probable Treatment Resistance Criteria
2.5. Statistical Analysis
3. Results
3.1. Final Sample
3.2. Goodness of Fit for Various Definitions for Probable TR
3.3. Ability to Predict Poor Global Functioning over the Next 5 Years
3.4. Ability to Predict Clozapine Use by 5 Years
3.5. Patterns of Antipsychotic Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Kane, J.; Honigfeld, G.; Singer, J.; Meltzer, H. Clozapine for the Treatment-Resistant Schizophrenic. A Double-Blind Comparison with Chlorpromazine. Arch. Gen. Psychiatry 1988, 45, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y. Treatment-Resistant Schizophrenia—The Role of Clozapine. Curr. Med. Res. Opin. 1997, 14, 1–20. [Google Scholar] [CrossRef]
- Agid, O.; Arenovich, T.; Sajeev, G.; Zipursky, R.B.; Kapur, S.; Foussias, G.; Remington, G. An Algorithm-Based Approach to First-Episode Schizophrenia: Response Rates over 3 Prospective Antipsychotic Trials with a Retrospective Data Analysis. J. Clin. Psychiatry 2011, 72, 1439–1444. [Google Scholar] [CrossRef]
- Sutterland, A.L.; van der Pluijm, M.; Becker, H.E.; van de Giessen, E.; de Haan, L. Shortening Duration of Treatment Resistance: The Next Step in the Treatment of Schizophrenia. Schizophr. Bull. Open 2020, 1. [Google Scholar] [CrossRef]
- Howes, O.D.; McCutcheon, R.; Agid, O.; de Bartolomeis, A.; van Beveren, N.J.M.; Birnbaum, M.L.; Bloomfield, M.A.P.; Bressan, R.A.; Buchanan, R.W.; Carpenter, W.T.; et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am. J. Psychiatry 2017, 174, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Lally, J.; Ajnakina, O.; Di Forti, M.; Trotta, A.; Demjaha, A.; Kolliakou, A.; Mondelli, V.; Reis Marques, T.; Pariante, C.; Dazzan, P.; et al. Two Distinct Patterns of Treatment Resistance: Clinical Predictors of Treatment Resistance in First-Episode Schizophrenia Spectrum Psychoses. Psychol. Med. 2016, 46, 3231–3240. [Google Scholar] [CrossRef] [Green Version]
- Demjaha, A.; Lappin, J.M.; Stahl, D.; Patel, M.X.; MacCabe, J.H.; Howes, O.D.; Heslin, M.; Reininghaus, U.A.; Donoghue, K.; Lomas, B.; et al. Antipsychotic Treatment Resistance in First-Episode Psychosis: Prevalence, Subtypes and Predictors. Psychol. Med. 2017, 47, 1981–1989. [Google Scholar] [CrossRef] [Green Version]
- Demjaha, A.; Egerton, A.; Murray, R.M.; Kapur, S.; Howes, O.D.; Stone, J.M.; McGuire, P.K. Antipsychotic Treatment Resistance in Schizophrenia Associated with Elevated Glutamate Levels but Normal Dopamine Function. Biol. Psychiatry 2014, 75, e11–e13. [Google Scholar] [CrossRef] [PubMed]
- Derks, E.M.; Fleischhacker, W.W.; Boter, H.; Peuskens, J.; Kahn, R.S. Antipsychotic Drug Treatment in First-Episode Psychosis Should Patients Be Switched to a Different Antipsychotic Drug After 2, 4, or 6 Weeks of Nonresponse? J. Clin. Psychopharmacol. 2010. [Google Scholar] [CrossRef]
- Takeuchi, H.; Siu, C.; Remington, G.; Fervaha, G.; Zipursky, R.B.; Foussias, G.; Agid, O. Does Relapse Contribute to Treatment Resistance? Antipsychotic Response in First- vs. Second-Episode Schizophrenia. Neuropsychopharmacology 2018, 1. [Google Scholar] [CrossRef]
- Lee, J.; Takeuchi, H.; Fervaha, G.; Sin, G.L.; Foussias, G.; Agid, O.; Farooq, S.; Remington, G. Subtyping Schizophrenia by Treatment Response: Antipsychotic Development and the Central Role of Positive Symptoms. Can. J. Psychiatry Rev. Can. Psychiatr. 2015, 60, 515–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, C.L.M.; Lam, B.S.T.; Lee, E.H.M.; Chan, S.K.W.; Chang, W.C.; Suen, Y.N.; Chen, E.Y.H. A Systematic Review of Clinical Guidelines on Choice, Dose, and Duration of Antipsychotics Treatment in First- and Multi-Episode Schizophrenia. Int. Rev. Psychiatry 2019, 31, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, R.; Chue, P.; Malla, A.; Tibbo, P.; Roy, M.-A.; Williams, R.; Iyer, S.; Lutgens, D.; Banks, N. Long-Acting Injectable Antipsychotics: Evidence of Effectiveness and Use. Can. J. Psychiatry Rev. Can. Psychiatr. 2013, 58, 5S–13S. [Google Scholar] [CrossRef]
- Alessi-Severini, S.; Dorze, J.-A.L.; Nguyen, D.; Honcharik, P.; Eleff, M. Clozapine Prescribing in a Canadian Outpatient Population. PLoS ONE 2013, 8, e83539. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Vergunst, F.; Gee, S.; McGuire, P.; Kapur, S.; Taylor, D. Adherence to Treatment Guidelines in Clinical Practice: Study of Antipsychotic Treatment Prior to Clozapine Initiation. Br. J. Psychiatry J. Ment. Sci. 2012, 201, 481–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Üçok, A.; Çikrikçili, U.; Karabulut, S.; Salaj, A.; Öztürk, M.; Tabak, Ö.; Durak, R. Delayed Initiation of Clozapine May Be Related to Poor Response in Treatment-Resistant Schizophrenia. Int. Clin. Psychopharmacol. 2015, 30, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Yada, Y.; Yoshimura, B.; Kishi, Y. Correlation between Delay in Initiating Clozapine and Symptomatic Improvement. Schizophr. Res. 2015, 168, 585–586. [Google Scholar] [CrossRef]
- Yoshimura, B.; Yada, Y.; So, R.; Takaki, M.; Yamada, N. The Critical Treatment Window of Clozapine in Treatment-Resistant Schizophrenia: Secondary Analysis of an Observational Study. Psychiatry Res. 2017, 250, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Aboraya, A.; Leucht, S.; Nasrallah, H.A.; Samara, M.; Haro, J.M.; Elshazly, A.; Zangeneh, M. A Novel Approach to Measuring Response and Remission in Schizophrenia in Clinical Trials. Schizophr. Res. 2017, 190, 123–128. [Google Scholar] [CrossRef]
- Malla, A.; Norman, R.; Scholten, D.; Manchanda, R.; McLean, T. A Community Intervention for Early Identification of First Episode Psychosis. Soc. Psychiatry Psychiatr. 2005, 40, 337–344. [Google Scholar] [CrossRef]
- Cannon-Spoor, H.E.; Potkin, S.G.; Wyatt, R.J. Measurement of Premorbid Adjustment in Chronic Schizophrenia. Schizophr. Bull. 1982, 8, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Malla, A.; Norman, R.; Schmitz, N.; Manchanda, R.; Béchard-Evans, L.; Takhar, J.; Haricharan, R. Predictors of Rate and Time to Remission in First-Episode Psychosis: A Two-Year Outcome Study. Psychol. Med. 2006, 36, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.G.; Geisler, S.; Chakos, M.; Goldman, R. Predictors of Treatment Response from a First Episode of Schizophrenia or Schizoaffective Disorder. Am. J. Psychiatry 1999, 156, 544–549. [Google Scholar]
- Emsley, R.; Chiliza, B.; Asmal, L.; Harvey, B.H. The Nature of Relapse in Schizophrenia. BMC Psychiatry 2013, 13, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siskind, D.; McCartney, L.; Goldschlager, R.; Kisely, S. Clozapine v. First- and Second-Generation Antipsychotics in Treatment-Refractory Schizophrenia: Systematic Review and Meta-Analysis. Br. J. Psychiatry 2016, 209, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Siskind, D.; Siskind, V.; Kisely, S. Clozapine Response Rates among People with Treatment-Resistant Schizophrenia: Data from a Systematic Review and Meta-Analysis. Can. J. Psychiatry 2017, 62, 772–777. [Google Scholar] [CrossRef]
- Cassidy, C.; Rabinovitch, M.; Schmitz, N.; Joober, R.; Malla, A. A Comparison Study of Multiple Measures of Adherence to Antipsychotic Medication in First-Episode Psychosis. J. Clin. Psychopharmacol. 2010, 30, 64–67. [Google Scholar] [CrossRef]
- Correll, C.U.; Kishimoto, T.; Nielsen, J.; Kane, J.M. Quantifying Clinical Relevance in the Treatment of Schizophrenia. Clin. Ther. 2011, 33, B16–B39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | Sample (n = 92) |
|---|---|
| Age | 25.72 (7.97) |
| Gender (M/F) | 73/19 |
| Age of onset in years (SD) | 24.27 (8.1) |
| DUP (mean in weeks) (SD) | 74.53 (112.5) |
| DUI (mean in weeks) (SD) | 292.3 (275.1) |
| SAPS baseline (M/SD) | 10.5 (3.58) |
| SANS baseline (M/SD) | 12.7 (5.17) |
| Substance abuse/dependence (Y/N) | 23/69 |
| Mode of onset (I/A) | 67/23 |
| Family History (Y/N) | 32/49 |
| Domain | Criteria | Probable TR (%) | AP Trials (M/SD) | Non TR (%) | AP Trials |
|---|---|---|---|---|---|
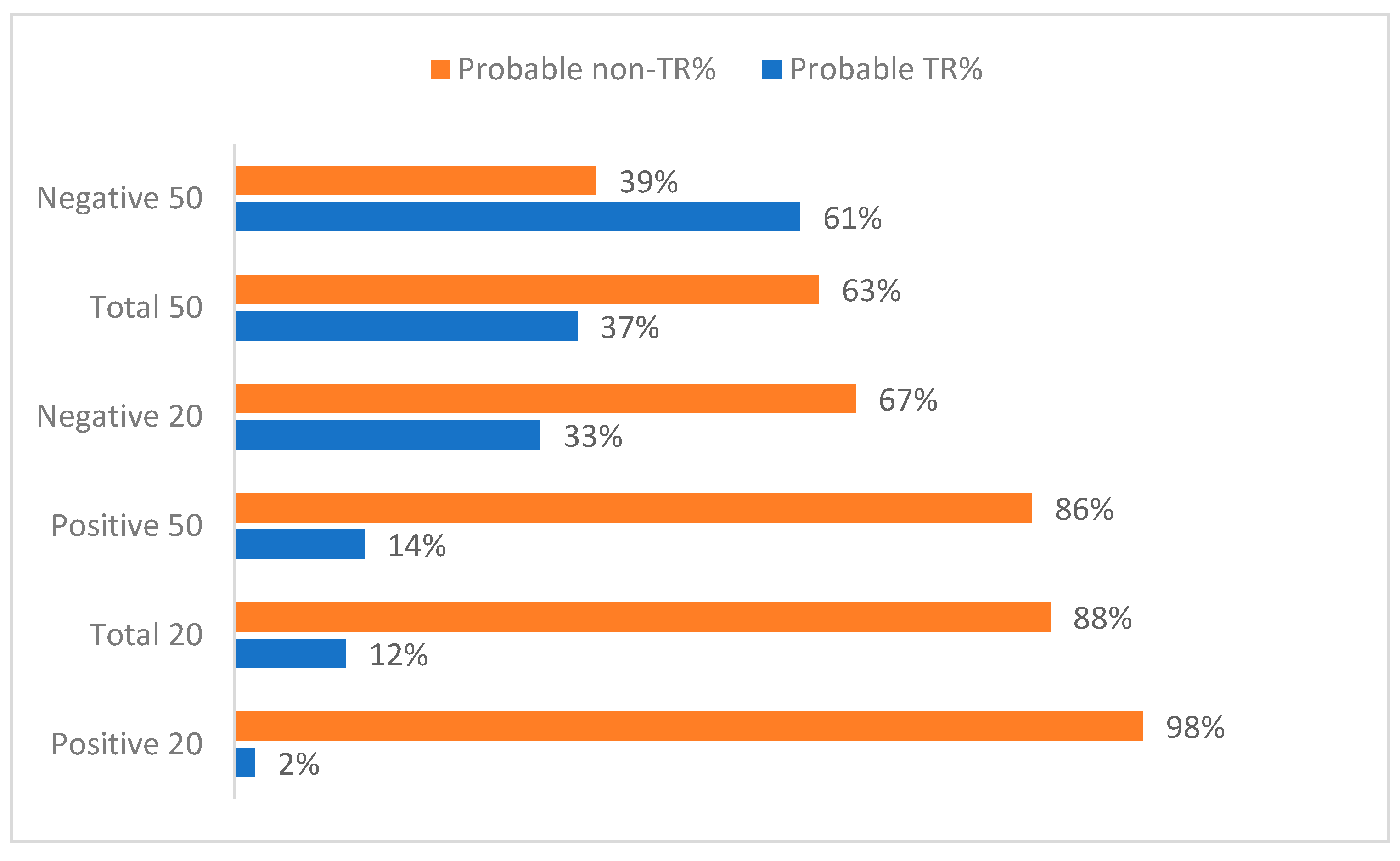
| Positive symptoms | <20% <50% | 2 (2.2) 13 (14) | 2.00/1.41 1.38/0.65 | 91 (97.8) 80 (86) | 1.30/0.50 1.30/0.05 |
| Negative symptoms | <20% <50% | 30 (33) 56 (60.8) | 1.23/0.50 1.30/0.54 | 62 (67) 36 (39.13) | 1.34/0.54 1.31/0.52 |
| Total symptoms | <20% <50% | 11 (12) 34 (36.96) | 1.36/0.67 1.32/0.59 | 81 (88) 58 (63.04) | 1.29/0.51 1.29/0.49 |
| Domain | Threshold | Ability to Select a 33% Subgroup by 6 Months of FES | Ability to Predict Low GAF over 5 Years | Ability to Predict Clozapine Use by 5 Years |
|---|---|---|---|---|
| Positive Symptoms | <20% | NO | - | - |
| <50% | NO | - | YES * | |
| Negative Symptoms | <20% | YES | - | - |
| <50% | NO | - | - | |
| Total symptoms | <20% | NO | - | - |
| <50% | YES | YES | NO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dempster, K.; Li, A.; Sabesan, P.; Norman, R.; Palaniyappan, L. Treatment Resistance: A Time-Based Approach for Early Identification in First Episode Psychosis. J. Pers. Med. 2021, 11, 711. https://doi.org/10.3390/jpm11080711
Dempster K, Li A, Sabesan P, Norman R, Palaniyappan L. Treatment Resistance: A Time-Based Approach for Early Identification in First Episode Psychosis. Journal of Personalized Medicine. 2021; 11(8):711. https://doi.org/10.3390/jpm11080711
Chicago/Turabian StyleDempster, Kara, Annie Li, Priyadharshini Sabesan, Ross Norman, and Lena Palaniyappan. 2021. "Treatment Resistance: A Time-Based Approach for Early Identification in First Episode Psychosis" Journal of Personalized Medicine 11, no. 8: 711. https://doi.org/10.3390/jpm11080711
APA StyleDempster, K., Li, A., Sabesan, P., Norman, R., & Palaniyappan, L. (2021). Treatment Resistance: A Time-Based Approach for Early Identification in First Episode Psychosis. Journal of Personalized Medicine, 11(8), 711. https://doi.org/10.3390/jpm11080711







