Effect of Tocilizumab in Reducing the Mortality Rate in COVID-19 Patients: A Systematic Review with Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection, Data Extraction and Quality Assessment
2.3. Statistical Analysis
3. Results
3.1. Search Results
3.2. Study Characteristics
3.3. Quality Assessment
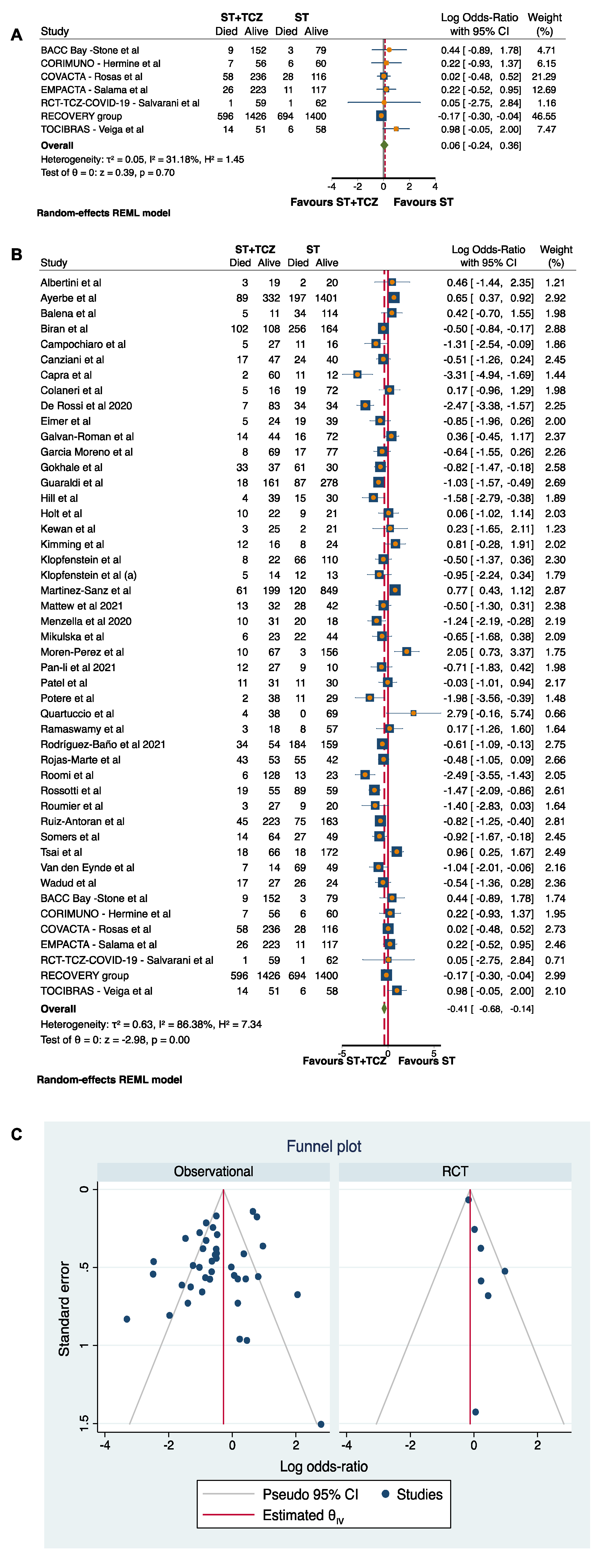
3.4. Meta-Analysis of the TCZ Therapy on Mortality
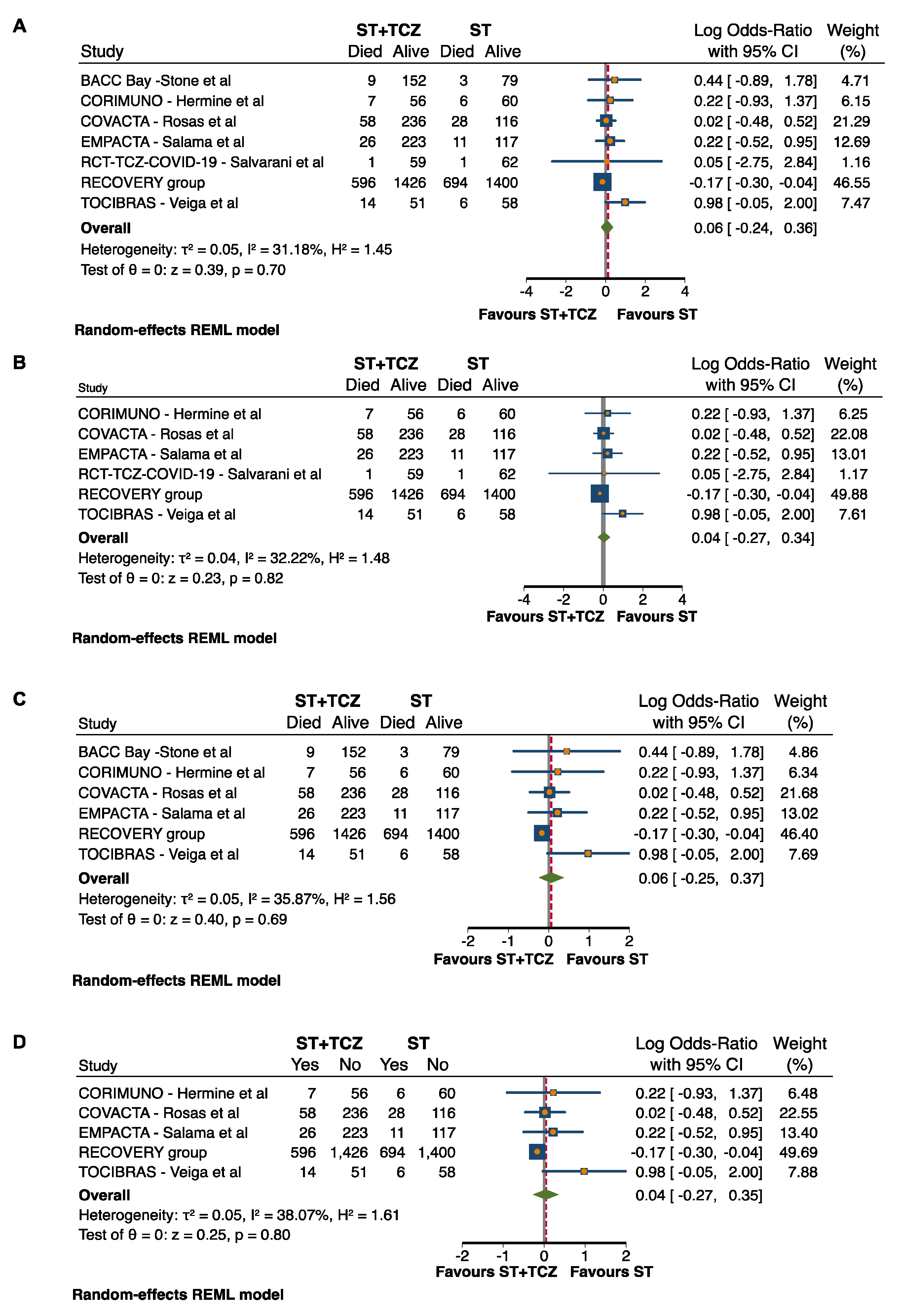
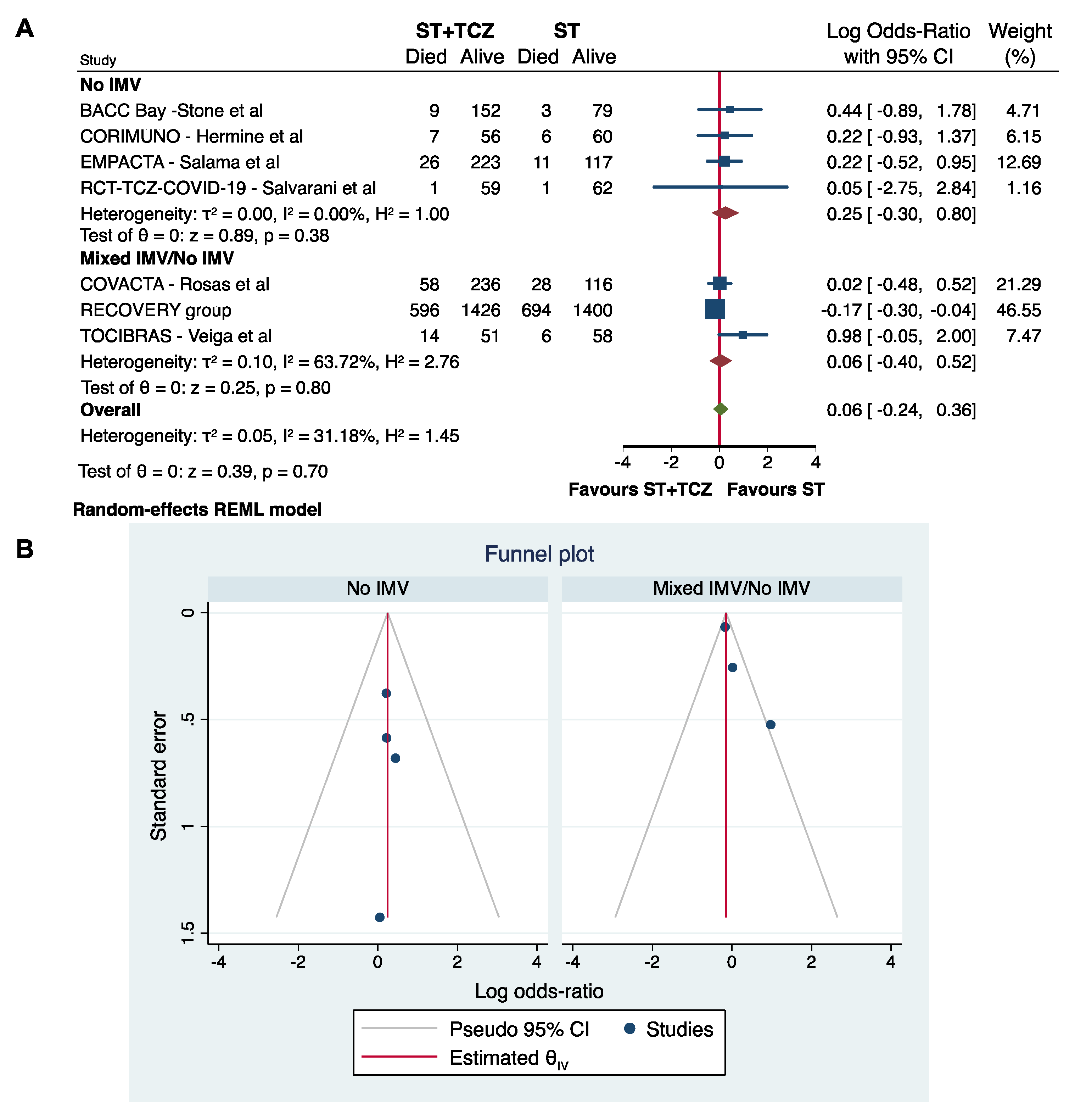
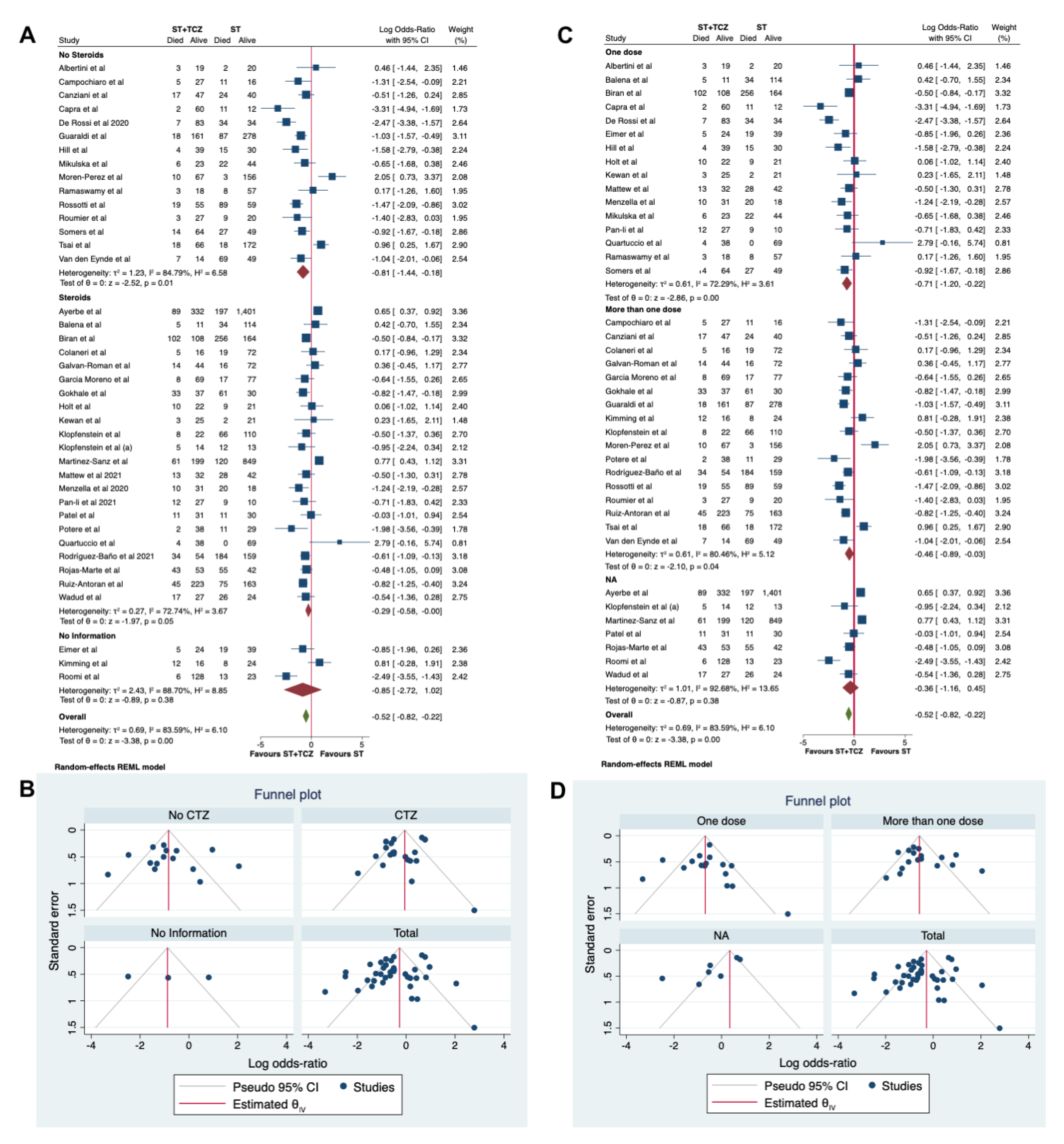
3.5. Sensitivity and Subgroup Analyses in RCTs
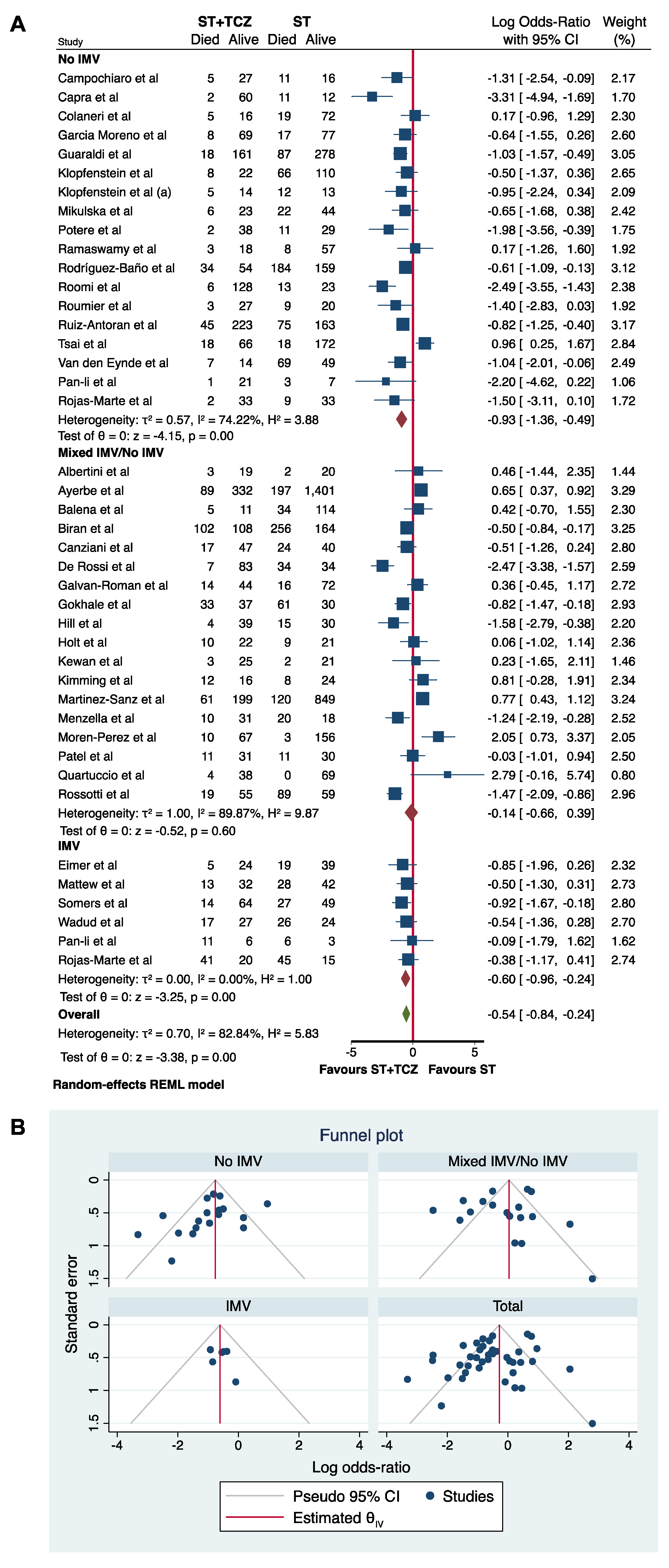
3.6. Sensitivity and Subgroup Analyses in the Observational Studies
3.7. Meta-Regression Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stasi, C.; Fallani, S.; Voller, F.; Silvestri, C. Treatment for COVID-19: An overview. Eur. J. Pharmacol. 2020, 889, 173644. [Google Scholar] [CrossRef]
- Pagliano, P.; Scarpati, G.; Sellitto, C.; Conti, V.; Spera, A.M.; Ascione, T.; Piazza, O.; Filippelli, A. Experimental Pharmacotherapy for COVID-19: The Latest Advances. J. Exp. Pharmacol. 2021, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.H.; Wong, J.Y.H.; Tang, E.H.M.; Au, C.H.; Wai, A.K.C. Clinical presentations, laboratory and radiological findings, and treatments for 11,028 COVID-19 patients: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 19765. [Google Scholar] [CrossRef] [PubMed]
- Du, R.H.; Liang, L.R.; Yang, C.Q.; Wang, W.; Cao, T.Z.; Li, M.; Guo, G.Y.; Du, J.; Zheng, C.L.; Zhu, Q.; et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, S.L.; Fazio-Eynullayeva, E.; Lane, D.A.; Underhill, P.; Lip, G.Y.H. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. 2020, 17, e1003321. [Google Scholar] [CrossRef]
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. 2020, 14, 303–310. [Google Scholar] [CrossRef]
- Matthay, M.A.; Leligdowicz, A.; Liu, K.D. Biological Mechanisms of COVID-19 Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 202, 1489–1491. [Google Scholar] [CrossRef] [PubMed]
- Torres Acosta, M.A.; Singer, B.D. Pathogenesis of COVID-19-induced ARDS: Implications for an ageing population. Eur. Respir. J. 2020, 56, 2002049. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef] [PubMed]
- Jose, R.J.; Manuel, A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Nishimoto, N.; Yoshizaki, K.; Miyasaka, N.; Yamamoto, K.; Kawai, S.; Takeuchi, T.; Hashimoto, J.; Azuma, J.; Kishimoto, T. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: A multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004, 50, 1761–1769. [Google Scholar] [CrossRef]
- Santomasso, B.; Bachier, C.; Westin, J.; Rezvani, K.; Shpall, E.J. The Other Side of CAR T-Cell Therapy: Cytokine Release Syndrome, Neurologic Toxicity, and Financial Burden. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 433–444. [Google Scholar] [CrossRef]
- Parr, J.B. Time to Reassess Tocilizumab’s Role in COVID-19 Pneumonia. JAMA Intern. Med. 2021, 181, 12–15. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, B.G.A.; Shea, D.; O’Connell, J.; Peterson, V.; Welch, M.; Losos, P.T. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. Otawwa Hosp. Res. Inst. 2014. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 December 2020). [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Montori, V.; Akl, E.A.; Djulbegovic, B.; Falck-Ytter, Y. GRADE guidelines: 4. Rating the quality of evidence–Study limitations (risk of bias). J Clin Epidemiol. 2011, 64, 407–415. [Google Scholar] [CrossRef]
- Bornstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley and Sons: Chichester-West Sussex, UK, 2009; Volume 16, pp. 107–125. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 [updated September 2020]. The Cochrane Collaboration. 2020. Available online: www.training.cochrane.org/handbook (accessed on 8 February 2021).
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albertini, L.; Soletchnik, M.; Razurel, A.; Cohen, J.; Bidegain, F.; Fauvelle, F.; Safrano, G.; Piquet, J.; Maurer, C.; Goldgran-Toledano, D. Observational study on off-label use of tocilizumab in patients with severe COVID-19. Eur. J. Hosp. Pharm. 2021, 28, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Ayerbe, L.; Risco, C.; Ayis, S. The association between treatment with heparin and survival in patients with Covid-19. J. Thromb. Thrombolysis 2020, 50, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Balena, F.; Bavaro, D.F.; Fabrizio, C.; Bottalico, I.F.; Calamo, A.; Santoro, C.R.; Brindicci, G.; Bruno, G.; Mastroianni, A.; Greco, S.; et al. Tocilizumab and corticosteroids for COVID-19 treatment in elderly patients. JGG 2020, 68, 197–203. [Google Scholar] [CrossRef]
- Biran, N.; Ip, A.; Ahn, J.; Go, R.C.; Wang, S.; Mathura, S.; Sinclaire, B.A.; Bednarz, U.; Marafelias, M.; Hansen, E.; et al. Tocilizumab among patients with COVID-19 in the intensive care unit: A multicentre observational study. Lancet Rheumatol. 2020, 2, e603–e612. [Google Scholar] [CrossRef]
- Campochiaro, C.; Della-Torre, E.; Cavalli, G.; De Luca, G.; Ripa, M.; Boffini, N.; Tomelleri, A.; Baldissera, E.; Rovere-Querini, P.; Ruggeri, A.; et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: A single-centre retrospective cohort study. Eur. J. Intern. Med. 2020, 76, 43–49. [Google Scholar] [CrossRef]
- Canziani, L.M.; Trovati, S.; Brunetta, E.; Testa, A.; De Santis, M.; Bombardieri, E.; Guidelli, G.; Albano, G.; Folci, M.; Squadroni, M.; et al. Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: A retrospective case-control survival analysis of 128 patients. J. Autoimmun. 2020, 114, 102511. [Google Scholar] [CrossRef]
- Capra, R.; De Rossi, N.; Mattioli, F.; Romanelli, G.; Scarpazza, C.; Sormani, M.P.; Cossi, S. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur. J. Intern. Med. 2020, 76, 31–35. [Google Scholar] [CrossRef]
- Colaneri, M.; Bogliolo, L.; Valsecchi, P.; Sacchi, P.; Zuccaro, V.; Brandolino, F.; Montecucco, C.; Mojoli, F.; Giusti, E.M.; Bruno, R.; et al. Tocilizumab for Treatment of Severe COVID-19 Patients: Preliminary Results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms 2020, 8, 695. [Google Scholar] [CrossRef]
- De Rossi, N.; Scarpazza, C.; Filippini, C.; Cordioli, C.; Rasia, S.; Mancinelli, C.R.; Rizzoni, D.; Romanelli, G.; Cossi, S.; Vettoretto, N.; et al. Early use of low dose tocilizumab in patients with COVID-19: A retrospective cohort study with a complete follow-up. EClinicalMedicine 2020, 25, 100459. [Google Scholar] [CrossRef]
- Eimer, J.; Vesterbacka, J.; Svensson, A.; Stojanovic, B.; Wagrell, C.; Sönnerborg, A.; Nowak, P. Tocilizumab shortens time on mechanical ventilation and length of hospital stay in patients with severe COVID-19: A retrospective cohort study. J. Intern. Med. 2021, 289, 434–436. [Google Scholar] [CrossRef]
- Galvan-Roman, J.M.; Rodriguez-Garcia, S.C.; Roy-Vallejo, E.; Marcos-Jimonez, A.; Sanchez-Alonso, S.; Fernandez-Diaz, C.; Alcaraz-Serna, A.; Mateu-Albero, T.; Rodriguez-Cortes, P.; Sanchez-Cerrillo, I.; et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: An observational study. J. Allergy Clin. Immunol 2021, 147, 72–80.e8. [Google Scholar] [CrossRef]
- Moreno-García, E.; Rico, V.; Albiach, L.; Agüero, D.; Ambrosioni, J.; Bodro, M.; Cardozo, C.; Chumbita, M.; de la Mora, L.; García-Pouton, N.; et al. Tocilizumab is associated with reduced risk of ICU admission and mortality in patients with SARS-CoV-2 infection. medRxiv 2020. [Google Scholar] [CrossRef]
- Gokhale, Y.; Mehta, R.; Karnik, N.; Kulkarni, U.; Gokhale, S. Tocilizumab improves survival in patients with persistent hypoxia in severe COVID-19 pneumonia. EClinicalMedicine 2020, 24, 100467. [Google Scholar] [CrossRef]
- Guaraldi, G.; Meschiari, M.; Cozzi-Lepri, A.; Milic, J.; Tonelli, R.; Menozzi, M.; Franceschini, E.; Cuomo, G.; Orlando, G.; Borghi, V.; et al. Tocilizumab in patients with severe COVID-19: A retrospective cohort study. Lancet Rheumatol. 2020, 2, e474–e484. [Google Scholar] [CrossRef]
- Hill, J.A.; Menon, M.P.; Dhanireddy, S.; Wurfel, M.M.; Green, M.; Jain, R.; Chan, J.D.; Huang, J.; Bethune, D.; Turtle, C.; et al. Tocilizumab in hospitalized patients with COVID-19: Clinical outcomes, inflammatory marker kinetics, and safety. J. Med. Virol. 2021, 93, 2270–2280. [Google Scholar] [CrossRef]
- Holt, G.E.; Batra, M.; Murthi, M.; Kambali, S.; Santos, K.; Bastidas, M.V.P.; Asif, H.; Haddadi, S.; Arias, S.; Mirsaeidi, M. Lack of tocilizumab effect on mortality in COVID19 patients. Sci. Rep. 2020, 10, 17100. [Google Scholar] [CrossRef] [PubMed]
- Kewan, T.; Covut, F.; Al-Jaghbeer, M.J.; Rose, L.; Gopalakrishna, K.V.; Akbik, B. Tocilizumab for treatment of patients with severe COVID-19: A retrospective cohort study. EClinicalMedicine 2020, 24, 100418. [Google Scholar] [CrossRef] [PubMed]
- Kimmig, L.M.; Wu, D.; Gold, M.; Pettit, N.N.; Pitrak, D.; Mueller, J.; Husain, A.N.; Mutlu, E.A.; Mutlu, G.M. IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated with Increased Secondary Infections. Front. Med. 2020, 7, 583897. [Google Scholar] [CrossRef]
- Klopfenstein, T.; Zayet, S.; Lohse, A.; Balblanc, J.C.; Badie, J.; Royer, P.Y.; Toko, L.; Mezher, C.; Kadiane-Oussou, N.J.; Bossert, M.; et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med. Mal. Infect. 2020, 50, 397–400. [Google Scholar] [CrossRef]
- Klopfenstein, T.; Zayet, S.; Lohse, A.; Selles, P.; Zahra, H.; Kadiane-Oussou, N.J.; Toko, L.; Royer, P.Y.; Balblanc, J.C.; Gendrin, V.; et al. Impact of tocilizumab on mortality and/or invasive mechanical ventilation requirement in a cohort of 206 COVID-19 patients. Int. J. Infect. Dis. 2020, 99, 491–495. [Google Scholar] [CrossRef]
- Martínez-Sanz, J.; Muriel, A.; Ron, R.; Herrera, S.; Pérez-Molina, J.A.; Moreno, S.; Serrano-Villar, S. Effects of tocilizumab on mortality in hospitalized patients with COVID-19: A multicentre cohort study. Clin. Microbiol Infect. 2021, 27, 238–243. [Google Scholar] [CrossRef]
- Fisher, M.J.; Marcos Raymundo, L.A.; Monteforte, M.; Taub, E.M.; Go, R. Tocilizumab in the treatment of critical COVID-19 pneumonia: A retrospective cohort study of mechanically ventilated patients. Int. J. Infect. Dis. 2021, 103, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Menzella, F.; Fontana, M.; Salvarani, C.; Massari, M.; Ruggiero, P.; Scelfo, C.; Barbieri, C.; Castagnetti, C.; Catellani, C.; Gibellini, G.; et al. Efficacy of tocilizumab in patients with COVID-19 ARDS undergoing noninvasive ventilation. Crit. Care 2020, 24, 589. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Nicolini, L.A.; Signori, A.; Di Biagio, A.; Sepulcri, C.; Russo, C.; Dettori, S.; Berruti, M.; Sor-mani, M.P.; Giacobbe, D.R.; et al. Tocilizumab and steroid treatment in patients with COVID-19 pneumonia. PLoS ONE 2020, 15, e0237831. [Google Scholar] [CrossRef]
- Moreno-Pérez, O.; Andres, M.; Leon-Ramirez, J.-M.; Sánchez-Payá, J.; Rodríguez, J.C.; Sánchez, R.; García-Sevila, R.; Boix, V.; Gil, J.; Merino, E. Experience with tocilizumab in severe COVID-19 pneumonia after 80 days of follow-up: A retrospective cohort study. J. Autoimmun. 2020, 114, 102523. [Google Scholar] [CrossRef]
- Li, P.; Lu, Z.; Li, Q.; Wang, Z.; Guo, Y.; Cai, C.; Wang, S.; Liu, P.; Su, X.; Huang, Y.; et al. Administration Timing and Efficacy of Tocilizumab in Patients With COVID-19 and Elevated IL-6. Front. Mol. Biosci. 2021, 8, 651662. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Gooley, T.A.; Bailey, N.; Bailey, M.; Hegerova, L.; Batchelder, A.; Holdread, H.; Dunleavy, V.; Downey, T.; Frisvold, J.; et al. Use of the IL-6R antagonist tocilizumab in hospitalized COVID-19 patients. J. Intern. Med. 2021, 289, 430–433. [Google Scholar] [CrossRef]
- Potere, N.; Di Nisio, M.; Cibelli, D.; Scurti, R.; Frattari, A.; Porreca, E.; Abbate, A.; Parruti, G. Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyperinflammation: A case-control study. Ann. Rheum. Dis. 2021, 80, 1–2. [Google Scholar] [CrossRef]
- Quartuccio, L.; Sonaglia, A.; McGonagle, D.; Fabris, M.; Peghin, M.; Pecori, D.; De Monte, A.; Bove, T.; Curcio, F.; Bassi, F.; et al. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: Results from a single Italian Centre study on tocilizumab versus standard of care. J. Clin. Virol. 2020, 129, 104444. [Google Scholar] [CrossRef]
- Ramaswamy, M.; Mannam, P.; Comer, R.; Sinclair, E.; McQuaid, D.B.; Schmidt, M.L. Off-Label Real World Experience Using Tocilizumab for Patients Hospitalized with COVID-19 Disease in a Regional Community Health System: A Case-Control Study. medRxiv 2020. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Pachón, J.; Carratala, J.; Ryan, P.; Jarrín, I.; Yllescas, M.; Arribas, J.R.; Berenguer, J.; Muñoz, E.A.; Gil Divasson, P.; et al. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: A multicentre cohort study (SAM-COVID-19). Clin. Microbiol. Infect. 2021, 27, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Marte, G.; Khalid, M.; Mukhtar, O.; Hashmi, A.T.; Waheed, M.A.; Ehrlich, S.; Aslam, A.; Siddiqui, S.; Agarwal, C.; Malyshev, Y.; et al. Outcomes in patients with severe COVID-19 disease treated with tocilizumab: A case-controlled study. QJM 2020, 113, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Roomi, S.; Ullah, W.; Ahmed, F.; Farooq, S.; Sadiq, U.; Chohan, A.; Jafar, M.; Saddique, M.; Khanal, S.; Watson, R.; et al. Efficacy of Hydroxychloroquine and Tocilizumab in Patients With COVID-19: Single-Center Retrospective Chart Review. J. Med. Internet Res. 2020, 22, e21758. [Google Scholar] [CrossRef]
- Rossotti, R.; Travi, G.; Ughi, N.; Corradin, M.; Baiguera, C.; Fumagalli, R.; Bottiroli, M.; Mondino, M.; Merli, M.; Bellone, A.; et al. Safety and efficacy of anti-il6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: A comparative analysis. J. Infect. 2020, 81, e11–e17. [Google Scholar] [CrossRef] [PubMed]
- Roumier, M.; Paule, R.; Groh, M.; Vallée, A.; Ackermann, F. Interleukin-6 blockade for severe COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Ruiz-Antorán, B.; the TOCICOV-study group; Sancho-López, A.; Torres, F.; Moreno-Torres, V.; de Pablo-López, I.; García-López, P.; Abad-Santos, F.; Rosso-Fernández, C.M.; Aldea-Perona, A.; et al. Combination of Tocilizumab and Steroids to Improve Mortality in Patients with Severe COVID-19 Infection: A Spanish, Multicenter, Cohort Study. Infect. Dis. Ther. 2021, 10, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Somers, E.C.; Eschenauer, G.A.; Troost, J.P.; Golob, J.L.; Gandhi, T.N.; Wang, L.; Zhou, N.; Petty, L.A.; Baang, J.H.; Dillman, N.O.; et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin. Infect. Dis. 2020, 11, ciaa954. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.; Diawara, O.; Nahass, R.G.; Brunetti, L. Impact of tocilizumab administration on mortality in severe COVID-19. Sci. Rep. 2020, 10, 19131. [Google Scholar] [CrossRef]
- Van den Eynde, E.; Gasch, O.; Oliva, J.C.; Prieto, E.; Calzado, S.; Gomila, A.; Machado, M.L.; Falgueras, L.; Ortonobes, S.; Morón, A.; et al. Corticosteroids and tocilizumab reduce in-hospital mortality in severe COVID-19 pneumonia: A retrospective study in a Spanish hospital. Infect. Dis. 2021, 53, 291–302. [Google Scholar] [CrossRef]
- Wadud, N.; Ahmed, N.; Shergill, M.; Khan, M.; Krishna, M.; Gilani, A.; El Zarif, S.; Galaydick, J.; Linga, K.; Kooragayalu, S.; et al. Improved survival outcome in patients with SARS-COV-2 (COVID-19) ARDS with tocilizumab administration. Chest 2020, 158, A696–A697. [Google Scholar] [CrossRef]
- Stone, J.H.; Frigault, M.J.; Serling-Boyd, N.J.; Fernandes, A.D.; Harvey, L.; Foulkes, A.S.; Horick, N.K.; Healy, B.C.; Shah, R.; Bensaci, A.M.; et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N. Engl. J. Med. 2020, 383, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Hermine, O.; Mariette, X.; Tharaux, P.L.; Resche-Rigon, M.; Porcher, R.; Ravaud, P.; CORIMUNO-19 Collaborative Group. Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 32–40. [Google Scholar] [CrossRef]
- Rosas, I.O.; Bräu, N.; Waters, M.; Go, R.C.; Hunter, B.D.; Bhagani, S.; Skiest, D.; Aziz, M.S.; Cooper, N.; Douglas, I.S.; et al. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N. Engl. J. Med. 2021, 384, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L.; et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N. Engl. J. Med. 2021, 384, 20–30. [Google Scholar] [CrossRef]
- Salvarani, C.; Dolci, G.; Massari, M.; Merlo, D.F.; Cavuto, S.; Savoldi, L.; Bruzzi, P.; Boni, F.; Braglia, L.; Turrà, C.; et al. Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.W.; Pessoa-Amorim, G.; Peto, L.; Brightling, C.E.; Sarkar, R.; Jeebun, K.T.V.J.; Ashish, A.; Tully, R.; Chadwick, D.; Sharafat, M. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): Preliminary results of a randomised, controlled, open-label, platform trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Farias, D.L.C.; Prats, J.; Cavalcanti, A.B.; Rosa, R.G.; Machado, F.R.; Berwanger, O.; Azevedo, L.C.P.; Lopes, R.D.; Avezum, Á.; Kawano-Dourado, L.; et al. Rationale and design of the "Tocilizumab in patients with moderate to severe COVID-19: An open-label multicentre randomized controlled" trial (TOCIBRAS). Rev. Bras. Ter. Intensiva 2020, 32, 337–347. [Google Scholar] [CrossRef]
- Berardicurti, O.; Ruscitti, P.; Ursini, F.; D’Andrea, S.; Ciaffi, J.; Meliconi, R.; Iagnocco, A.; Cipriani, P.; Giacomelli, R. Mortality in tocilizumab-treated patients with COVID-19: A systematic review and meta-analysis. Clin. Exp. Rheumatol. 2020, 38, 1247–1254. [Google Scholar] [PubMed]
- Talaie, H.; Hosseini, S.M.; Nazari, M.; Fakhri, Y.; Mousavizadeh, A.; Vatanpour, H.; Firoozfar, A. Is there any potential management against COVID-19? A systematic review and meta-analysis. DARU 2020, 28, 765–777. [Google Scholar] [CrossRef]
- Misra, S.; Nath, M.; Hadda, V.; Vibha, D. Efficacy of various treatment modalities for nCOV-2019: A systematic review and meta-analysis. Eur. J. Clin. Invest. 2020, 50, e13383. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Haghbin, H.; Abu Sitta, E.; Nawras, Y.; Fatima, R.; Sharma, S.; Lee-Smith, W.; Duggan, J.; Kammeyer, J.A.; Hanarahan, J.; et al. Efficacy of tocilizumab in COVID-19: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 1620–1630. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Fatemi, B.; Karimi Majd, Z.; Minaei, H.; Peikanpour, M.; Anjidani, N.; Taheri, A.; Dastan, F.; Mosaed, R. Efficacy and safety of Tocilizumab in severe and critical COVID-19: A Systematic Review and Meta-Analysis. Expert Rev. Clin. Immunol. 2021, 17, 499–511. [Google Scholar] [CrossRef]
- Tleyjeh, I.M.; Kashour, Z.; Damlaj, M.; Riaz, M.; Tlayjeh, H.; Altannir, M.; Altannir, Y.; Al-Tannir, M.; Tleyjeh, R.; Hassett, L.; et al. Efficacy and safety of tocilizumab in COVID-19 patients: A living systematic review and meta-analysis. Clin Microbiol. Infect. 2021, 27, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-X.; Hu, F.; Wei, J.; Yuan, L.-T.; Wen, T.-M.; Gale, R.P.; Liang, Y. Systematic review and meta-analysis of tocilizumab in persons with coronavirus disease-2019 (COVID-19). Leukemia 2021, 35, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Wang, W.; Hayek, S.S.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Association Between Early Treatment with Tocilizumab and Mortality Among Critically Ill Patients with COVID-19. JAMA Intern. Med. 2021, 181, 41–51. [Google Scholar] [CrossRef]
- Johns Hopkins Center for Systems Science and Engineering. Mortality Analyses. Available online: https://coronavirus.jhu.edu/data/mortality (accessed on 8 February 2021).
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- Leaf, D.E.; Gupta, S.; Wang, W. Tocilizumab in Covid-19. N. Engl. J. Med. 2021, 384, 86–87. [Google Scholar] [CrossRef]


| Condition | Definition |
|---|---|
| Population | COVID-19 patients admitted in hospital |
| Intervention | Patients treated with Tocilizumab plus Standard Therapy |
| Comparator | Patients treated with Standard Therapy alone |
| Outcome | Mortality |
| Source | Country | Primary Outcome | Total Pts (n) | Mean Age | Sex, (Male %) | Oxygen Support | TCZ Administer Route and Dosage | Time from Symptoms Onset to TCZ Start (days) | Time from Hospital Admission to TCZ Start (days) | Death (%) (p Value) |
|---|---|---|---|---|---|---|---|---|---|---|
| Albertini [26] | Europe | Efficacy of TCZ on respiratory clinical conditions | 44 TCZ: 22 ST: 22 | 65 | 70.45 | No-IMV/IMV | 8 mg/kg | 10 | NA | TCZ: 13.6 ST: 9 (p = NA) |
| Ayerbe [27] | Europe | Mortality with Heparin therapy | 2019 TCZ: 421 ST: 1598 | 67.57 | 61.268 | No-IMV/IMV | NA | NA | NA | TCZ: 21.14 ST: 12.33 (p = NA) |
| Balena [28] | Europe | Crude mortality and AdE | 164 TCZ:16 ST:148 | 77.5 | 48 | No-IMV/IMV | 8 mg/kg i.v. | NA | NA | TCZ: 31 ST:23 (p = 0.074) |
| Biran [29] | America | Mortality in patients requiring ICU | 630 TCZ:210 ST:420 | 65 | 58.413 | No-IMV/IMV | 8 mg/kg i.v. | 7 | NA | TCZ: 49 ST: 61 (p = 0.0040) |
| Campochiaro [30] | Europe | Clinical improvement and overall survival | 59 TCZ:32 ST:27 | 62 | 86.154 | No- IMV | 400 mg i.v., repeated after 24 h (n = 9) | 11 | NA | TCZ: 16 ST: 33 (p = 0.15) |
| Canziani [31] | Europe | Mortality | 128 TCZ:64 ST:64 | 63.5 | 73 | No-IMV/IMV | 8 mg/kg i.v., repeated after 24 h (n = 61) | 13 | NA | TCZ: 27 ST: 38 (p = 0.185) |
| Capra [32] | Europe | Mortality | 85 TCZ:62 ST:23 | 66.5 | 75 | No- IMV | 400 mg i.v. 324 mg s.c. | NA | 4 | TCZ: 3.22 ST: 47.8 (p = 0.004) |
| Colaneri [33] | Europe | ICU admission and 7-day mortality rate | 112 TCZ:21 ST:91 | 63.03 | 73.215 | No- IMV | 8 mg/kg i.v. and repeated after 12 h | NA | NA | TCZ: 24 ST: 21 (p = 0.84) |
| De Rossi [34] | Europe | Survival rate | 158 TCZ:90 ST:68 | 66.95 | 71.52 | No- IMV/IMV | 400 mg i.v. or 324 mg s.c. | NA | 9 | TCZ: 7.7 ST: 50 (p < 0.001) |
| Eimer [35] | Europe | 30-day death after admission to ICU | 87 TCZ:29 ST:58 | 56.5 | 84 | IMV | 8 mg/kg i.v. | 11 | NA | TCZ: 17.2 ST: 32.8 (p = 0.20) |
| Galvan-Romàn [36] | Europe | Need for IMV, mortality | 146 TCZ: 58 ST: 88 | 63 | 66 | No-IMV/IMV | 8 mg/kg (max 800 mg), repeated after 12 h | NA | NA | TCZ: 24 ST: 18 (p = NA) |
| Garcia [37] | Europe | ICU admission and/or death | 171 TCZ: 77 ST: 94 | 61 | 65.498 | No- IMV | 400 mg/24 h iv (Pts ≤ 75 kg) 600 mg/24 h iv (Pts > 75 kg) with the possibility to repeat the dose every 12 h up to 3 doses. | NA | 6.5 | TCZ: 10.3 ST: 18 (p = 0.156) |
| Gokhale [38] | Asia | Overall survival | 161 TCZ: 70 ST: 91 | 53.5 | 62.11 | No-IMV/IMV | 400 mg/die i.v. and repeated after 24 h (n = 9) | NA | 12 | TCZ: 47 ST: 67 (p = 0.011) |
| Guaraldi [39] | Europe | IMV requirement and/or death | 544 TCZ: 179 ST: 365 | 67 | 66 | No- IMV | 8 mg/kg i.v., repeated after 12 h, or 324 mg s.c. | NA | 7 | TCZ: 7 ST: 20 (p < 0.001) |
| Hill [40] | America | Clinical improvement | 88 TCZ: 43 ST: 45 | NA | 69 | No-IMV/IMV | 400 mg i.v. | NA | 2 | TCZ: 21 ST: 33 (p = 0.26) |
| Holt [41] | America | Survival time and mortality | 62 TCZ: 32 ST: 30 | 68.5 | 70.97 | No-IMV/IMV | 400 mg i.v. | NA | 2 | TCZ: 31.25 ST: 30 (p = 0.36) |
| Kewan [42] | North America | Median LOS, ICU LOS, duration of IMV, mortality. | 51 TCZ: 28 ST: 23 | 66 | 61 | No-IMV/IMV | 400 mg i.v. | NA | 2 | TCZ: 11 ST: 9 (p > 0.99) |
| Kimmig [43] | North America | Infection and clinical outcomes (discharged, died) | 60 TCZ:28 ST: 32 | 63.15 | 55.856 | No-IMV/IMV | 400 mg i.v. with possible redosing | NA | NA | TCZ: 35.2 ST: 19.3 (p = 0.020) |
| Klopfenstein [44] | Europe | IMV requirement and/or death | 206 TCZ: 30 ST;176 | 73.75 | 60.7 | No- IMV | 8 mg/kg i.v. (1 or 2 doses) | 12 | NA | TCZ: 26.7 ST: 37.5 (p = 0.253) |
| Klopfenstein [45] | Europe | ICU admission and/or death | 44 TCZ: 19 ST: 25 | 74.95 | NA | No- IMV | NA | 13 | NA | TCZ: 25 ST: 48 (p = 0.066) |
| Martinez-Sanz [46] | Europe | Time to death | 1229 TCZ: 260 ST: 969 | 65.5 | 72.246 | No-IMV/IMV | NA | NA | 4 | TCZ: 23 ST: 12 (p < 0.001) |
| Matthew [47] | America | Overall mortality 30 days from the date of intubation | 115 TCZ: 45 ST: 70 | 58.4 | 69.566 | IMV | 400 mg i.v. | NA | 2.5 | TCZ: 29 ST: 40 (p = 0.23) |
| Menzella [48] | Europe | In-hospital mortality rate | 79 TCZ: 41 ST: 38 | 66.5 | 70.89 | No-IMV/IMV | 8 mg/kg i.v (max 800 mg) or 162 mg s.c | NA | NA | TCZ: 24 ST: 53 (p = 0.01) |
| Mikulska [49] | Europe | Failure-free survival and overall survival | 95 TCZ: 29 ST: 66 | 69 | 67.35 | No- IMV | 8 mg/kg i.v. 162 mg s.c. | NA | 7 | TCZ: 14.2 ST: 28.1 (p = NA) |
| Moreno-Pérez [50] | Europe | Death, LOS | 236 TCZ: 77 ST: 159 | 59.5 | 59.746 | No-IMV/IMV | 600 mg i.v., with second or third dose (400 mg i.v.) | 10 | NA | TCZ: 12.9 ST: 1.9 (p = 0.002) |
| Pan-Li [51] | Asia | Improvement and death | 58 TCZ: 39 ST: 19 | 73.9 | 63.8 | No- IMV/IMV | 4–8 mg/kg (max dose of 800 mg) | NA | NA | TCZ: 30.8 ST: 47.3 (p = NA) |
| Patel [52] | America | Clinical outcomes and survival | 83 TCZ: 42 ST: 41 | 67.5 | 50.603 | No-IMV/IMV | NA | NA | 4 | TCZ: 21.4 ST: 26.8 in severe Pts TCZ: 14.2 ST: 28.6 (p = NA) |
| Potere [53] | Europe | Overall survival and survival-free of IMV | 80 TCZ: 40 ST: 40 | 55.25 | 65 | No- IMV | 324 mg s.c. (bid) | NA | 5 | TCZ: 5 ST: 27.5 (p = 0.006) |
| Quartuccio [54] | Europe | Optimal patient selection to be treated with TCZ | 111 TCZ: 42 ST: 69 | 58.3 | 69.4 | No-IMV/IMV | 8 mg/kg i.v. | 8.4 | NA | TCZ: 9.5 ST: 0 (p = NA) |
| Ramaswamy [55] | North America | Mortality | 86 TCZ: 21 ST: 65 | 63.7 | 57 | No- IMV | 400 mg i.v. 8 mg/kg i.v. | NA | NA | TCZ: 14 ST: 12 (p = 0.81) |
| Rodríguez-Bano [56] | Europe | Intubation or death | 432 TCZ: 88 ST: 343 | 67.5 | 77.702 | No- IMV | 400–600 mg i.v. with second or third dose | 10 | NA | TCZ: 2.3 ST: 11.9 (p = 0.004) |
| Rojas-Marte [57] | North America | Mortality | 193 TCZ = 96 ST = 97 | 60.4 | 71 | No-IMV/IMV | NA | NA | NA | TCZ: 52 ST: 62 (p = 0.09) excluding intubated TCZ: 6 ST: 27 (p = 0.024) |
| Roomi [58] | America | Clinical effectiveness of HCQ and TCZ | 170 TCZ: 134 ST: 36 | 61.8 | 48.83 | No- IMV | NA | NA | NA | TCZ: 4.5 ST: 36 (p = 0.44) |
| Rossotti [59] | Europe | Overall survival and hospital discharge | 222 TCZ: 74 ST: 148 | 59 | 81.532 | No-IMV/IMV | 8 mg/kg i.v. (max dose of 800 mg) with possible second dose | NA | NA | TCZ: 25.7 ST: 60.1 (p = 0.035) |
| Roumier [60] | Europe | IMV requirement and death | 59 TCZ: 30 ST: 29 | 65 | 80 | No- IMV | 8 mg/kg i.v. (renewable once) | 14 | NA | TCZ: 17.2 ST: 18.7 (p = 0.837) unadjusted TCZ: 10 ST: 31 (p = 0.41) |
| Ruiz-Antora’n [61] | Europe | Mortality | 506 TCZ: 268 ST: 238 | 68 | 64.03 | No- IMV | 600 mg (3 doses n = 22, 2 doses n = 92, 1 dose n = 154) | 11 | NA | TCZ: 16,8 ST: 31,5 (28days/ death) (p = 0.001) |
| Somers [62] | North America | Survival probability after intubation | 154 TCZ = 78 ST = 76 | 58 | 66 | IMV | 8 mg/kg i.v. (max 800 mg) | NA | 3.9 | 18% in TCZ 36% in ST (28days/ death) (p = 0.01) |
| Tsai [63] | America | Mortality | 274 TCZ: 84 ST: 190 | 63 | 61.4 | No- IMV | 400 mg i.v. (n = 53) 600 mg i.v. (n = 3) 800 mg i.v (n = 10) (second dose n = 4) | NA | NA | TCZ: 21.4 ST: 9.4 (p = NA) |
| Van den Eynde [64] | Europe | Mortality | 139 TCZ = 21 ST = 118 | 73.2 | 66.91 | No- IMV | 400 mg or 600 mg (once or twice daily) | NA | NA | TCZ: 33,3 ST: 58,4 (p < 0.001) |
| Wadud [65] | North America | LOS, days on ventilator, in-hospital and ICU, mortality. | 94 TCZ: 44 ST: 50 | 55.5 | NA | IMV | NA | NA | NA | 3TCZ: 8.64 ST: 52 (p < 0.001) |
| Source | Country | Primary Outcome | Total Pts (n) | Mean Age | Sex, (Male %) | Oxygen Support | TCZ Administration Route and Dosage | Time from Symptoms Onset to TCZ Initiation (days) | Time from Hospital Admission to TCZ Initiation (days) | Death (%) (p Value) |
|---|---|---|---|---|---|---|---|---|---|---|
| BACC Stone [66] | America | Intubation or death | 242 TCZ:161 ST:81 | 59.8 | 58.05 | No-IMV | 8 mg/kg i.v. | NA | 9 | TCZ: 5.6 ST: 4.9 (p = 0.81) |
| CORIMUNO-19 Hermine [67] | Europe | Death or respiratory support or IMV. | 130 TCZ:63 ST:66 | 63.5 | NA | No-IMV | 8 mg/kg i.v. | NA | 10 | TCZ: 11.1 ST: 9 (day 14) (p = NA) |
| COVACTA Rosas [68] | America, Europe | Clinical Status | 438 TCZ: 294 ST: 144 | 60.75 | 69.863 | No-IMV/IMV | 8 mg/kg i.v. | 12 | NA | TCZ: 19.7 ST: 19.4 (p = 0.941) |
| EMPACTA Salama [69] | America, Africa | IMV or death by day 28 | 377 TCZ:249 ST:128 | 55.9 | NA | No-IMV | 8 mg/kg i.v. | NA | 8 | TCZ: 10.4 ST: 8.6 (day 28) (p = NA) |
| RCT-TCZ-COVID-19 Salvarani [70] | Europe | Clinical worsening within 14 days since randomization | 123 TCZ:60 ST:63 | 60 | 61.1 | No-IMV | 8 mg/kg i.v., repeated after 12h | NA | 7 | TCZ 1.7 ST 1,6 (day 14) (p = NA) |
| RECOVERY Recovery Group [71] | Europe | All-cause mortality within 28 days after randomization | 4116 TCZ:2022 ST:2094 | 63.6 | 67.4 | No-IMV/ IMV | 400 mg i.v. 600 mg i.v. 800 mg i.v | 9 | NA | TCZ: 29 ST: 33 (p= 0.007) |
| TOCIBRAS Veiga [72] | America | Clinical status at 15 days | 129 TCZ:65 ST:64 | 57.4 | NA | No-IMV/ IMV | 8 mg/kg i.v. | 10 | NA | TCZ: 17 ST: 3 (p = NA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, V.; Corbi, G.; Sellitto, C.; Sabbatino, F.; Maci, C.; Bertini, N.; De Bellis, E.; Iuliano, A.; Davinelli, S.; Pagliano, P.; et al. Effect of Tocilizumab in Reducing the Mortality Rate in COVID-19 Patients: A Systematic Review with Meta-Analysis. J. Pers. Med. 2021, 11, 628. https://doi.org/10.3390/jpm11070628
Conti V, Corbi G, Sellitto C, Sabbatino F, Maci C, Bertini N, De Bellis E, Iuliano A, Davinelli S, Pagliano P, et al. Effect of Tocilizumab in Reducing the Mortality Rate in COVID-19 Patients: A Systematic Review with Meta-Analysis. Journal of Personalized Medicine. 2021; 11(7):628. https://doi.org/10.3390/jpm11070628
Chicago/Turabian StyleConti, Valeria, Graziamaria Corbi, Carmine Sellitto, Francesco Sabbatino, Chiara Maci, Nicola Bertini, Emanuela De Bellis, Antonio Iuliano, Sergio Davinelli, Pasquale Pagliano, and et al. 2021. "Effect of Tocilizumab in Reducing the Mortality Rate in COVID-19 Patients: A Systematic Review with Meta-Analysis" Journal of Personalized Medicine 11, no. 7: 628. https://doi.org/10.3390/jpm11070628
APA StyleConti, V., Corbi, G., Sellitto, C., Sabbatino, F., Maci, C., Bertini, N., De Bellis, E., Iuliano, A., Davinelli, S., Pagliano, P., & Filippelli, A. (2021). Effect of Tocilizumab in Reducing the Mortality Rate in COVID-19 Patients: A Systematic Review with Meta-Analysis. Journal of Personalized Medicine, 11(7), 628. https://doi.org/10.3390/jpm11070628








