Circulating Tumor Cell Persistence Associates with Long-Term Clinical Outcome to a Therapeutic Cancer Vaccine in Prostate Cancer
Abstract
:1. Introduction
2. Materials and Method
2.1. Patients
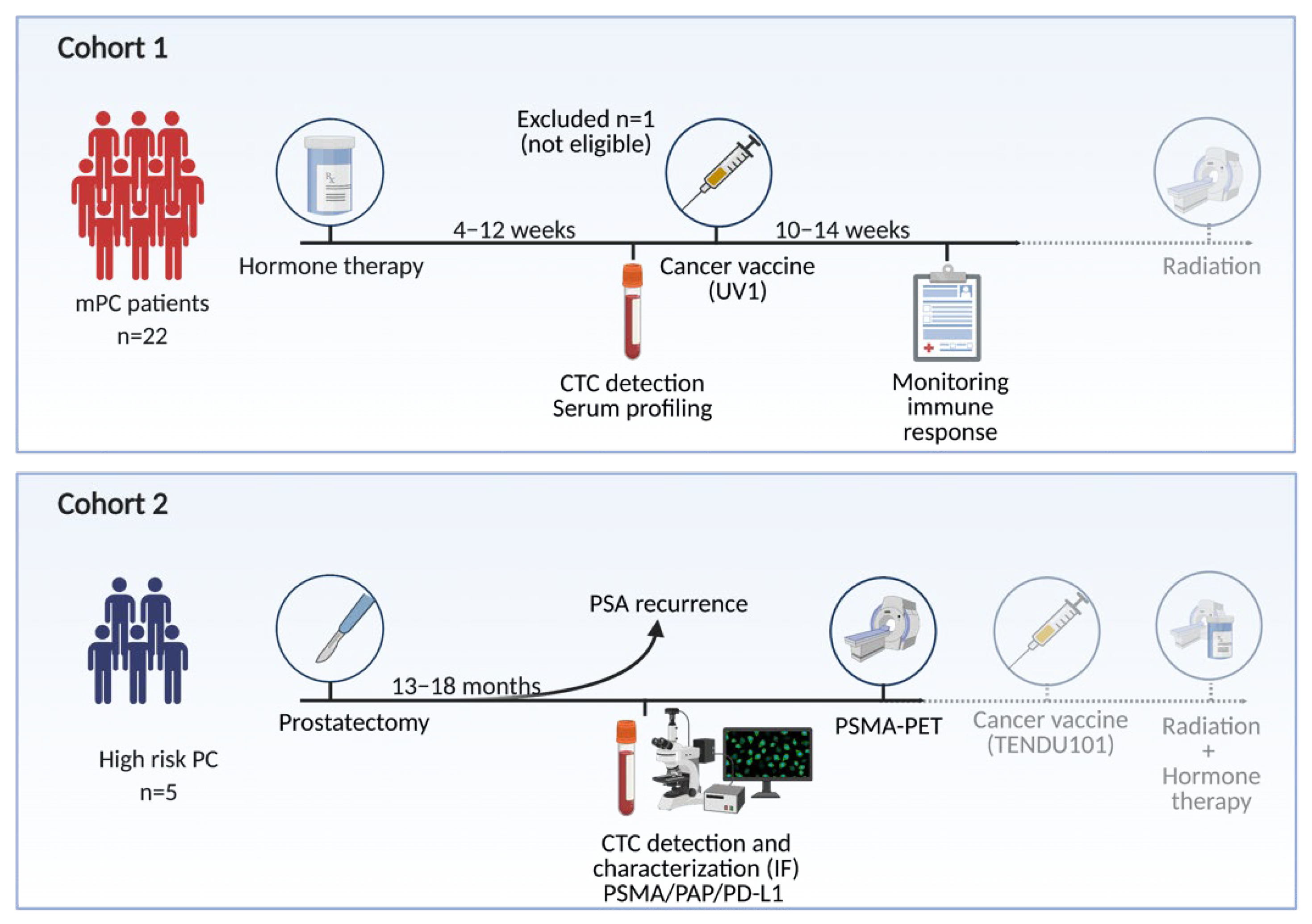
2.1.1. Cohort 1 (mPC)
2.1.2. Cohort 2 (bPC)
2.2. Laboratory Analyses
2.2.1. Capture of CTCs
2.2.2. Immunocytochemical Analysis and CTC Enumeration in Cohort 1
2.2.3. Immunofluorescence Staining for PSMA, PD-L1, and PAP in Cohort 2
2.2.4. Detection of UV1® Vaccine-Specific T-Cell Response in Cohort 1
2.2.5. Targeted Serum Profiling in Cohort 1
2.3. Statistical Analysis
3. Results
3.1. CTC Presence Predicts Long-Term Survival Benefit of a Therapeutic Cancer Vaccine
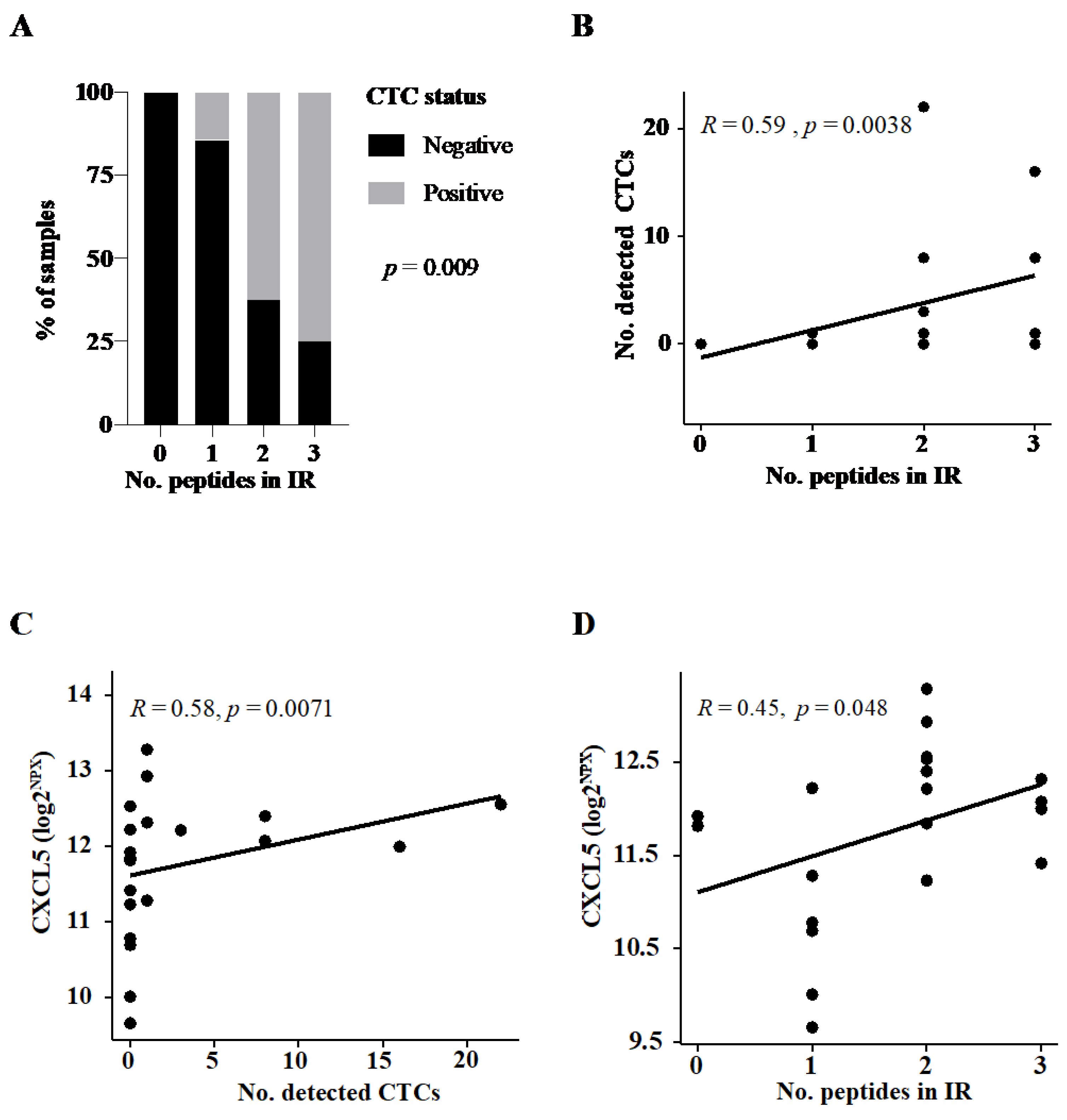
3.1.1. CTC Detection Predicts Broad Immune Response to Therapeutic Cancer Vaccine
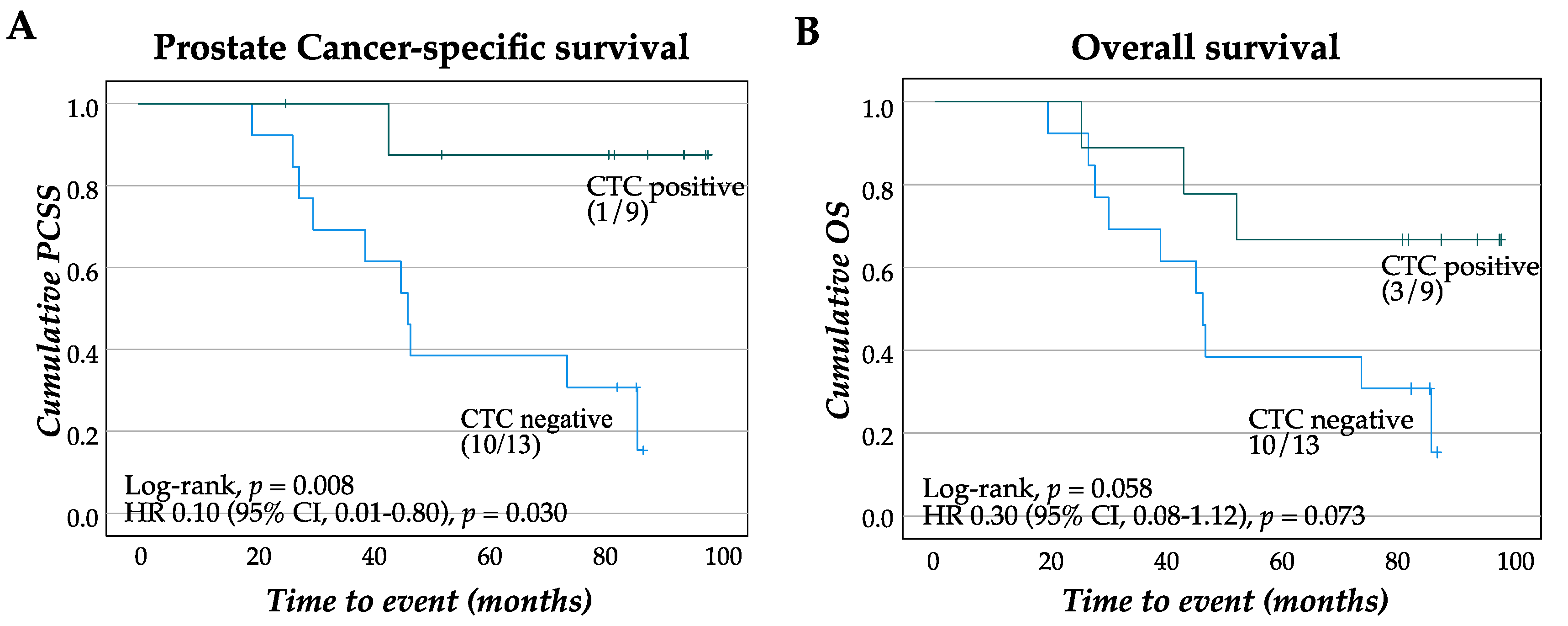
3.1.2. Patients with CTC Show Survival Benefit of Therapeutic Cancer Vaccination
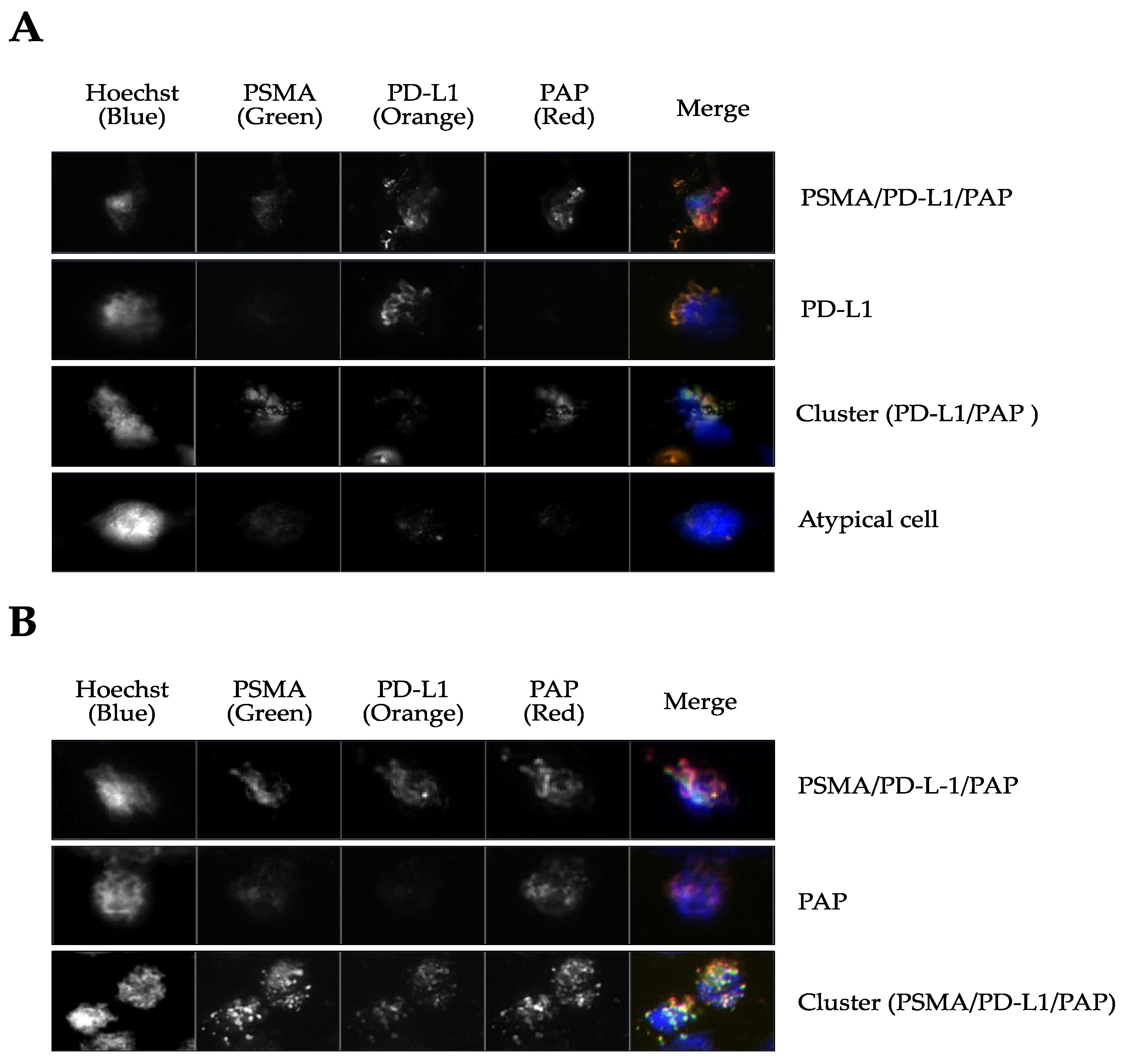
3.2. CTCs are Present at Biochemical Relapse and Express PSMA, PD-L1, and PAP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Broeck, T.V.D.; Bergh, R.C.V.D.; Briers, E.; Cornford, P.; Cumberbatch, M.; Tilki, D.; De Santis, M.; Fanti, S.; Fossati, N.; Gillessen, S.; et al. Biochemical Recurrence in Prostate Cancer: The European Association of Urology Prostate Cancer Guidelines Panel Recommendations. Eur. Urol. Focus 2020, 6, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Cancer Registry of Norway. Cancer in Norway 2019—Cancer Incidence, Mortality, Survival and Prevalence in Norway; Cancer Registry of Norway: Oslo, Norway, 2020. [Google Scholar]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.; Joniau, S.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.; Raghavan, D. Circulating Tumor Cells Predict Survival Benefit from Treatment in Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danila, D.C.; Heller, G.; Gignac, G.A.; Gonzalez-Espinoza, R.; Anand, A.; Tanaka, E.; Lilja, H.; Schwartz, L.; Larson, S.; Fleisher, M.; et al. Circulating Tumor Cell Number and Prognosis in Progressive Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2007, 13, 7053–7058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuske, A.; Gorges, T.M.; Tennstedt, P.; Tiebel, A.-K.; Pompe, R.S.; Preißer, F.; Prues, S.; Mazel, M.; Markou, A.; Lianidou, E.; et al. Improved detection of circulating tumor cells in non-metastatic high-risk prostate cancer patients. Sci. Rep. 2016, 6, 39736. [Google Scholar] [CrossRef] [Green Version]
- Lilleby, W.; Gaudernack, G.; Brunsvig, P.F.; Vlatkovic, L.; Schulz, M.; Mills, K.; Hole, K.H.; Inderberg, E.M. Phase I/IIa clinical trial of a novel hTERT peptide vaccine in men with metastatic hormone-naive prostate cancer. Cancer Immunol. Immunother. 2017, 66, 891–901. [Google Scholar] [CrossRef]
- Scher, H.I.; Lu, D.; Schreiber, N.A.; Louw, J.; Graf, R.P.; Vargas, H.A.; Johnson, A.; Jendrisak, A.; Bambury, R.; Danila, D.; et al. Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker With Outcomes and Survival in Castration-Resistant Prostate Cancer. JAMA Oncol. 2016, 2, 1441–1449. [Google Scholar] [CrossRef]
- Zhang, T.; Agarwal, A.; Almquist, R.G.; Runyambo, D.; Park, S.; Bronson, E.; Boominathan, R.; Rao, C.; Anand, M.; Oyekunle, T.; et al. Expression of immune checkpoints on circulating tumor cells in men with metastatic prostate cancer. Biomark. Res. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Millner, L.M.; Linder, M.W.; Valdes, R. Circulating Tumor Cells: A Review of Present Methods and the Need to Identify Heterogeneous Phenotypes. Ann. Clin. Lab. Sci. 2013, 43, 295–304. [Google Scholar]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef] [Green Version]
- Imrich, S.; Hachmeister, M.; Gires, O. EpCAM and its potential role in tumor-initiating cells. Cell Adhes. Migr. 2012, 6, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Munz, M.; Baeuerle, P.A.; Gires, O. The Emerging Role of EpCAM in Cancer and Stem Cell Signaling. Cancer Res. 2009, 69, 5627–5629. [Google Scholar] [CrossRef] [Green Version]
- Scher, H.I.; Heller, G.; Molina, A.; Attard, G.; Danila, D.C.; Jia, X.; Peng, W.; Sandhu, S.; Olmos, D.; Riisnaes, R.; et al. Circulating Tumor Cell Biomarker Panel as an Individual-Level Surrogate for Survival in Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2015, 33, 1348–1355. [Google Scholar] [CrossRef]
- Goldkorn, A.; Ely, B.; Tangen, C.M.; Tai, Y.-C.; Xu, T.; Li, H.; Twardowski, P.; Van Veldhuizen, P.J.; Agarwal, N.; Carducci, M.A.; et al. Circulating tumor cell telomerase activity as a prognostic marker for overall survival in SWOG 0421: A phase III metastatic castration resistant prostate cancer trial. Int. J. Cancer 2014, 136, 1856–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Gregory, S.G.; Garcia-Blanco, M.A.; Armstrong, A.J. Using circulating tumor cells to inform on prostate cancer biology and clinical utility. Crit. Rev. Clin. Lab. Sci. 2015, 52, 191–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulop, T.; Kotb, R.; Fortin, C.F.; Pawelec, G.; De Angelis, F.; Larbi, A. Potential role of immunosenescence in cancer development. Ann. N. Y. Acad. Sci. 2010, 1197, 158–165. [Google Scholar] [CrossRef]
- Drake, C.G.; Doody, A.D.; Mihalyo, M.A.; Huang, C.-T.; Kelleher, E.; Ravi, S.; Hipkiss, E.L.; Flies, D.B.; Kennedy, E.P.; Long, M.; et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell 2005, 7, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Mercader, M.; Bodner, B.K.; Moser, M.T.; Kwon, P.S.; Park, E.S.Y.; Manecke, R.G.; Ellis, T.M.; Wojcik, E.M.; Yang, D.; Flanigan, R.C.; et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 14565–14570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebelt, K.; Babaryka, G.; Frankenberger, B.; Stief, C.G.; Eisenmenger, W.; Kirchner, T.; Schendel, D.J.; Noessner, E. Prostate cancer lesions are surrounded by FOXP3+, PD-1+ and B7-H1+ lymphocyte clusters. Eur. J. Cancer 2009, 45, 1664–1672. [Google Scholar] [CrossRef]
- Olson, B.M.; Gamat-Huber, M.; Seliski, J.; Sawicki, T.; Jeffery, J.; Ellis, L.; Drake, C.G.; Weichert, J.; McNeel, D.G. Prostate Cancer Cells Express More Androgen Receptor (AR) Following Androgen Deprivation, Improving Recognition by AR-Specific T Cells. Cancer Immunol. Res. 2017, 5, 1074–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardella, C.; Clohessy, J.G.; Alimonti, A.; Pandolfi, P.P. Pro-senescence therapy for cancer treatment. Nat. Rev. Cancer 2011, 11, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Toso, A.; DI Mitri, D.; Alimonti, A. Enhancing chemotherapy efficacy by reprogramming the senescence-associated secretory phenotype of prostate tumors: A way to reactivate the antitumor immunity. Oncoimmunology 2015, 4, e994380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhou, J.; Zhang, J.; Li, S.; Wang, H.; Du, J. Cancer-associated fibroblasts promote PD-L1 expression in mice cancer cells via secreting CXCL5. Int. J. Cancer 2019, 145, 1946–1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roca, H.; Jones, J.D.; Purica, M.C.; Weidner, S.; Koh, A.J.; Kuo, R.; Wilkinson, J.E.; Wang, Y.; Daignault-Newton, S.; Pienta, K.J.; et al. Apoptosis-induced CXCL5 accelerates inflammation and growth of prostate tumor metastases in bone. J. Clin. Investig. 2017, 128, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhao, D.; Spring, D.J.; Depinho, R.A. Genetics and biology of prostate cancer. Genes Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rejniak, K.A. Circulating Tumor Cells: When a Solid Tumor Meets a Fluid Microenvironment. Adv. Exp. Med. Biol. 2016, 936, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Tauber, G.; Langsenlehner, T.; Schmölzer, L.M.; Pötscher, M.; Riethdorf, S.; Kuske, A.; Leitinger, G.; Kashofer, K.; Czyż, Z.T.; et al. In Vivo Detection of Circulating Tumor Cells in High-Risk Non-Metastatic Prostate Cancer Patients Undergoing Radiotherapy. Cancers 2019, 11, 933. [Google Scholar] [CrossRef] [Green Version]
- Klein, C.A. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer 2009, 9, 302–312. [Google Scholar] [CrossRef]
- Benko, G.; Spajić, B.; Krušlin, B.; Tomas, D. Impact of the EpCAM expression on biochemical recurrence-free survival in clinically localized prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 468–474. [Google Scholar] [CrossRef]
- Cieślikowski, W.A.; Budna-Tukan, J.; Świerczewska, M.; Ida, A.; Hrab, M.; Jankowiak, A.; Mazel, M.; Nowicki, M.; Milecki, P.; Pantel, K.; et al. Circulating Tumor Cells as a Marker of Disseminated Disease in Patients with Newly Diagnosed High-Risk Prostate Cancer. Cancers 2020, 12, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikhbahaei, S.; Afshar-Oromieh, A.; Eiber, M.; Solnes, L.B.; Javadi, M.S.; Ross, A.E.; Pienta, K.J.; Allaf, M.E.; Haberkorn, U.; Pomper, M.G.; et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2117–2136. [Google Scholar] [CrossRef] [PubMed]
- Sheikhbahaei, S.; Werner, R.A.; Solnes, L.B.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A.; Rowe, S.P. Prostate-Specific Membrane Antigen (PSMA)-Targeted PET Imaging of Prostate Cancer: An Update on Important Pitfalls. Semin. Nucl. Med. 2019, 49, 255–270. [Google Scholar] [CrossRef] [PubMed]
| Cohort 1 (mPC) | Cohort 2 (bPC) | ||||
|---|---|---|---|---|---|
| CTC Status | CTC Status | ||||
| Positive | Negative | p= | Positive | Negative | |
| n (%) | 9 (40.9) | 13 (59.1) | <0.001 | 5 (100) | 0 (0.00) |
| Time FU (month, median [IQR]) | 77.6 [52.6, 82.5] | 46.6 [30.2, 76.9] | |||
| Age (yr, median [IQR]) | 66.9 [59.2, 75.4] | 66.8 [63.9, 73.9] | 0.85 | 65.8 [57.4, 74.7] | |
| T stage (%) | 0.72 * | ||||
| cT2 | 1 (11.1) | 1 (7.7) | |||
| cT3 | 6 (66.7) | 7 (53.8) | |||
| cT4 | 2 (22.2) | 5 (38.5) | |||
| pT2 | 2 (40.0) | ||||
| pT3 | 3 (60.0) | ||||
| Gleason grade group (%) | 0.61 ** | ||||
| 2 + 3 | 0 (0.00) | 0 (0.00) | 3 (60.0) | ||
| 4 | 3 (33.3) | 2 (14.3) | 1 (20.0) | ||
| 5 | 6 (66.7) | 11 (84.6) | 1 (20.0) | ||
| PSA values (ng/mL, median [IQR]) | |||||
| PSA at diagnosis | 26.0 [12.0, 72.0] | 33.0 [12.0, 58.0] | 0.95 | ||
| PSA after ADT | 1.10 [0.40, 7.60] | 3.00 [0.60, 9.20] | 0.66 | ||
| PSA at relapse | 0.26 [0.20,0.54] | ||||
| Time ADT/DC01 (mo., median [IQR]) | 3.42 [1.74, 3.72] | 2.30 [1.64, 4.77] | 0.92 | ||
| Time RP/DC01 (mo., median [IQR]) | 13.2 [7.02, 14.5] | ||||
| No. reactive peptides in IR (%) | 0.009 * | ||||
| 0 | 0 (0.0) | 3 (23.1) | |||
| 1 | 1 (11.1) | 6 (46.2) | |||
| 2 | 5 (55.6) | 3 (23.1) | |||
| 3 | 3 (33.3) | 1 (7.7) | |||
| p= * | ||||||
|---|---|---|---|---|---|---|
| Patient ID | 1 | 3 | 5 | 2 | 4 | |
| Gleason Grade Group | 2 | 3 | 3 | 4 | 5 | |
| PSMA-PET status | + | - | + | - | + | |
| Total no. CTC | 31 | 19 | 18 | 27 | 29 | |
| PSMA, n (%) | 0 (0.0) | 0 (0.0) | 5 (27.8) | 0 (0.0) | 0 (0.0) | 0.96 |
| PD-L1, n (%) | 20 (64.5) | 3 (15.8) | 3 (16.7) | 0 (0.0) | 0 (0.0) | <0.001 |
| PAP, n (%) | 0 (0.0) | 6 (31.6) | 4 (22.2) | 3 (11.1) | 2 (6.9) | 0.92 |
| PSMA/PD-L1, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| PSMA/PAP, n (%) | 6 (19.4) | 3 (15.8) | 6 (33.3) | 0 (0.0) | 0 (0.0) | 0.005 |
| PD-L1/PAP, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| PSMA/PD-L1/PAP, n (%) | 5 (16.1) | 7 (36.8) | 0 (0.0) | 24 (88.9) | 27 (93.1) | 0.005 |
| Clusters (no.) | 4 (12.3) | 3 (15.8) | 4 (22.2) | 1 (3.7) | 0 (0.0) | <0.0001 |
| Nuclei+ cells (no. PSMA/PD-L1/PAP) † | 4 | 5 | 8 | 11 | 0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guldvik, I.J.; Ekseth, L.; Kishan, A.U.; Stensvold, A.; Inderberg, E.M.; Lilleby, W. Circulating Tumor Cell Persistence Associates with Long-Term Clinical Outcome to a Therapeutic Cancer Vaccine in Prostate Cancer. J. Pers. Med. 2021, 11, 605. https://doi.org/10.3390/jpm11070605
Guldvik IJ, Ekseth L, Kishan AU, Stensvold A, Inderberg EM, Lilleby W. Circulating Tumor Cell Persistence Associates with Long-Term Clinical Outcome to a Therapeutic Cancer Vaccine in Prostate Cancer. Journal of Personalized Medicine. 2021; 11(7):605. https://doi.org/10.3390/jpm11070605
Chicago/Turabian StyleGuldvik, Ingrid Jenny, Lina Ekseth, Amar U. Kishan, Andreas Stensvold, Else Marit Inderberg, and Wolfgang Lilleby. 2021. "Circulating Tumor Cell Persistence Associates with Long-Term Clinical Outcome to a Therapeutic Cancer Vaccine in Prostate Cancer" Journal of Personalized Medicine 11, no. 7: 605. https://doi.org/10.3390/jpm11070605
APA StyleGuldvik, I. J., Ekseth, L., Kishan, A. U., Stensvold, A., Inderberg, E. M., & Lilleby, W. (2021). Circulating Tumor Cell Persistence Associates with Long-Term Clinical Outcome to a Therapeutic Cancer Vaccine in Prostate Cancer. Journal of Personalized Medicine, 11(7), 605. https://doi.org/10.3390/jpm11070605







