Prognostic Factors in Primary Biliary Cholangitis: A Retrospective Study of Joint Slovak and Croatian Cohort of 249 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirschfield, G.M.; Beuers, U.; Corpechot, C.; Invernizzi, P.; Jones, D.; Marzioni, M.; Schramm, C. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef]
- Boonstra, K.; Beuers, U.; Ponsioen, C.Y. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: A systematic review. J. Hepatol. 2012, 56, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Drazilova, S.; Babinska, I.; Gazda, J.; Halanova, M.; Janicko, M.; Kucinsky, B.; Safcak, D.; Martinkova, D.; Tarbajova, L.; Cekanova, A.; et al. Epidemiology and clinical course of primary biliary cholangitis in Eastern Slovakia. Int. J. Public Health 2020, 65, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Madir, A.; Božin, T.; Mikolašević, I.; Milić, S.; Štimac, D.; Mijić, M.; Kanižaj, T.F.; Biloglav, Z.; Lucijanić, M.; Lucijanić, I.; et al. Epidemiological and clinical features of primary biliary cholangitis in two Croatian regions: A retrospective study. Croat. Med. J. 2019, 60, 494–502. [Google Scholar] [CrossRef]
- Lu, M.; Li, J.; Haller, I.V.; Romanelli, R.J.; VanWormer, J.J.; Rodriguez, C.V.; Raebel, M.A.; Boscarino, J.A.; Schmidt, M.A.; Daida, Y.G.; et al. Factors associated with prevalence and treatment of primary biliary cholangitis in United States health systems. Clin. Gastroenterol. Hepatol. 2018, 16, 1333–1341.e1336. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D.; Bowlus, C.L.; Boyer, J.; Levy, C.; Mayo, M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2019, 69, 394–419. [Google Scholar] [CrossRef] [PubMed]
- Corpechot, C.; Chazouillères, O.; Rousseau, A.; Le Gruyer, A.; Habersetzer, F.; Mathurin, P.; Goria, O.; Potier, P.; Minello, A.; Silvain, C.; et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N. Engl. J. Med. 2018, 378, 2171–2181. [Google Scholar] [CrossRef]
- Nevens, F.; Andreone, P.; Mazzella, G.; Strasser, S.I.; Bowlus, C.; Invernizzi, P.; Drenth, J.P.H.; Pockros, P.J.; Regula, J.; Beuers, U.; et al. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N. Engl. J. Med. 2016, 375, 631–643. [Google Scholar] [CrossRef]
- Lin, Z.H.; Xin, Y.N.; Dong, Q.J.; Wang, Q.; Jiang, X.J.; Zhan, S.H.; Sun, Y.; Xuan, S.Y. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology 2011, 53, 726–736. [Google Scholar] [CrossRef]
- Angulo, P.; Lindor, K.D.; Therneau, T.M.; Jorgensen, R.A.; Malinchoc, M.; Kamath, P.S.; Dickson, E.R. Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ursodeoxycholic acid. Liver 1999, 19, 115–121. [Google Scholar] [CrossRef]
- Corpechot, C.; Abenavoli, L.; Rabahi, N.; Chrétien, Y.; Andréani, T.; Johanet, C.; Chazouillères, O.; Poupon, R. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology 2008, 48, 871–877. [Google Scholar] [CrossRef]
- Corpechot, C.; Chazouillères, O.; Poupon, R. Early primary biliary cirrhosis: Biochemical response to treatment and prediction of long-term outcome. J. Hepatol. 2011, 55, 1361–1367. [Google Scholar] [CrossRef]
- Kuiper, E.M.; Hansen, B.E.; de Vries, R.A.; den Ouden–Muller, J.W.; Van Ditzhuijsen, T.J.; Haagsma, E.B.; Houben, M.H.; Witteman, B.J.; van Erpecum, K.J.; van Buuren, H.R. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology 2009, 136, 1281–1287. [Google Scholar] [CrossRef]
- Kumagi, T.; Guindi, M.; Fischer, S.E.; Arenovich, T.; Abdalian, R.; Coltescu, C.; Heathcote, J.E.; Hirschfield, G.M. Baseline Ductopenia and Treatment Response Predict Long-Term Histological Progression in Primary Biliary Cirrhosis. Am. J. Gastroenterol. 2010, 105, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Parés, A.; Caballería, L.; Rodés, J. Excellent Long-Term Survival in Patients With Primary Biliary Cirrhosis and Biochemical Response to Ursodeoxycholic Acid. Gastroenterology 2006, 130, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Shi, T.Y.; Shi, X.H.; Wang, L.; Yang, Y.J.; Liu, B.; Gao, L.X.; Shuai, Z.W.; Kong, F.; Chen, H.; et al. Early biochemical response to ursodeoxycholic acid and long-term prognosis of primary biliary cirrhosis: Results of a 14-year cohort study. Hepatology 2013, 58, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Mells, G.F.; Pells, G.; Dawwas, M.F.; Newton, J.L.; Heneghan, M.A.; Neuberger, J.M.; Day, D.B.; Ducker, S.J.; Sandford, R.N.; et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology 2013, 144, 560–569.e567. [Google Scholar] [CrossRef]
- Raszeja-Wyszomirska, J.; Wunsch, E.; Krawczyk, M.; Rigopoulou, E.I.; Kostrzewa, K.; Norman, G.L.; Bogdanos, D.P.; Milkiewicz, P. Assessment of health related quality of life in polish patients with primary biliary cirrhosis. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Quarneti, C.; Muratori, P.; Lalanne, C.; Fabbri, A.; Menichella, R.; Granito, A.; Masi, C.; Lenzi, M.; Cassani, F.; Pappas, G.; et al. Fatigue and pruritus at onset identify a more aggressive subset of primary biliary cirrhosis. Liver Int. 2015, 35, 636–641. [Google Scholar] [CrossRef]
- Carbone, M.; Nardi, A.; Flack, S.; Carpino, G.; Varvaropoulou, N.; Gavrila, C.; Spicer, A.; Badrock, J.; Bernuzzi, F.; Cardinale, V.; et al. Pretreatment prediction of response to ursodeoxycholic acid in primary biliary cholangitis: Development and validation of the UDCA Response Score. Lancet Gastroenterol. Hepatol. 2018, 3, 626–634. [Google Scholar] [CrossRef]
- Yoo, J.-J.; Cho, E.J.; Lee, B.; Kim, S.G.; Kim, Y.S.; Lee, Y.B.; Lee, J.-H.; Yu, S.J.; Kim, Y.J.; Yoon, J.-H. Prognostic value of biochemical response models for primary biliary cholangitis and the additional role of the neutrophil-to-lymphocyte ratio. Gut Liver 2018, 12, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Gulamhusein, A.F.; Hansen, B.E. Beyond Biochemical Responses, Use of Histologic Staging to Predict Outcomes of Patients With Primary Biliary Cholangitis. Clin. Gastroenterol. Hepatol. 2020, 18, 1033–1035. [Google Scholar] [CrossRef] [PubMed]
- Corpechot, C.; El Naggar, A.; Poujol-Robert, A.; Ziol, M.; Wendum, D.; Chazouillères, O.; de Lédinghen, V.; Dhumeaux, D.; Marcellin, P.; Beaugrand, M.; et al. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology 2006, 43, 1118–1124. [Google Scholar] [CrossRef]
- Perez, C.F.M.; Gulamhusein, A.; Carbone, M.; Trivedi, P.; van der Meer, A.; Corpechot, C.; Battezzati, P.; Lammers, W.; Floreani, A.; Pares, A.; et al. Raising awareness and messaging risk in patients with primary biliary cholangitis: The rapid Global PBC Screening Test. Red 2019, 70, e404. [Google Scholar] [CrossRef]
- Osman, K.T.; Maselli, D.B.; Idilman, I.S.; Rowan, D.J.; Viehman, J.K.; Harmsen, W.S.; Harnois, D.M.; Carey, E.J.; Gossard, A.A.; LaRusso, N.F.; et al. Liver Stiffness Measured by Either Magnetic Resonance or Transient Elastography Is Associated With Liver Fibrosis and Is an Independent Predictor of Outcomes Among Patients With Primary Biliary Cholangitis. J. Clin. Gastroenterol. 2020, 55, 449–457. [Google Scholar] [CrossRef]
- Delgado, J.-S.; Vodonos, A.; Delgado, B.; Jotkowitz, A.; Rosenthal, A.; Fich, A.; Novack, V. Primary biliary cirrhosis in Southern Israel: A 20 year follow up study. Eur. J. Intern. Med. 2012, 23, e193–e198. [Google Scholar] [CrossRef]
- Guo, G.Y.; Shi, Y.Q.; Wang, L.; Ren, X.; Han, Z.Y.; Guo, C.C.; Cui, L.N.; Wang, J.B.; Zhu, J.; Wang, N.; et al. Serum vitamin D level is associated with disease severity and response to ursodeoxycholic acid in primary biliary cirrhosis. Aliment. Pharmacol. Ther. 2015, 42, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Sharp, S.J.; Flack, S.; Paximadas, D.; Spiess, K.; Adgey, C.; Griffiths, L.; Lim, R.; Trembling, P.; Williamson, K.; et al. The UK-PBC risk scores: Derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology 2016, 63, 930–950. [Google Scholar] [CrossRef] [PubMed]
- Lammers, W.J.; Hirschfield, G.M.; Corpechot, C.; Nevens, F.; Lindor, K.D.; Janssen, H.L.; Floreani, A.; Ponsioen, C.Y.; Mayo, M.J.; Invernizzi, P.; et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology 2015, 149, 1804–1812.e1804. [Google Scholar] [CrossRef]
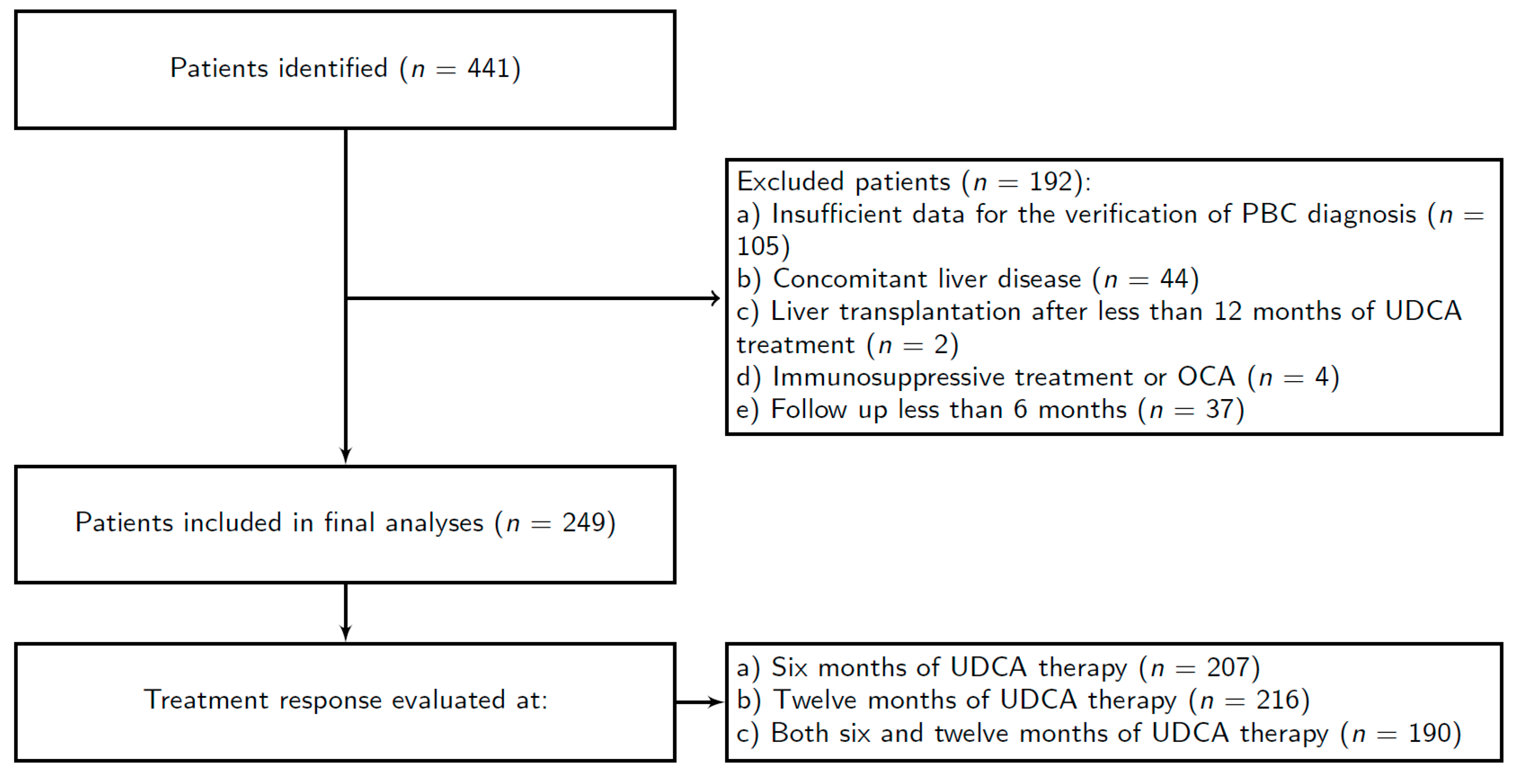
| Patients Included | n (%) | 249 (100%) | |
|---|---|---|---|
| Patients evaluated for the treatment response to UDCA | after 6 months after 12 months after both 6 and 12 months | n (%) | 207 (83.1%) 216 (86.8%) 190 (76.3%) |
| Gender | Men women | n (%) | 15 (6%) 234 (94%) |
| Women ≤ 40 years | n (%) | 15 (6.4% of female patients, 62% of all patients) | |
| Age | Years | median (IQR) | 56.00 (13) |
| Significant liver fibrosis (APRI > 0.7) | n (%) | 79 (31.7%) | |
| Follow up | Years | median (IQR) | 5 (6) |
| Liver cirrhosis decompensations | n (%) | 20 (8%) | |
|
| ||
| UDCA dosage | mg per day | median (IQR) | 1000 (500) |
| 207 Patients | Non-Responders 66 (31.9%) | Responders 141 (68.1%) | P | ||
|---|---|---|---|---|---|
| Age at diagnosis | years | median (IQR) | 56.00 (14.75) | 54.00 (14.00) | 0.77 |
| Gender | Female patients male patients | number (%) | 63 (30.4) 3 (1.5) | 130 (62.8) 11 (5.3) | 0.57 |
| Total bilirubin | ×ULN | median (IQR) | 0.59 (0.89) | 0.52 (0.21) | 0.03 |
| Albumin | g/L | median (IQR) | 42.00 (5.32) | 43.00 (3.16) | 0.005 |
| Platelets | ×109/L | median (IQR) | 231.50 (108.50) | 245.00 (97.00) | 0.13 |
| Glycemia | mmol/l | median (IQR) | 5.41 (1.05) | 5.39 (0.94) | 0.20 |
| ALT | ×ULN | median (IQR) | 1.91 (1.94) | 1.20 (0.82) | 0.0001 |
| AST | ×ULN | median (IQR) | 1.68 (1.91) | 1.10 (0.63) | 0.00002 |
| ALP | ×ULN | median (IQR) | 2.84 (3.10) | 1.43 (0.75) | <0.00001 |
| AST/ALT | median (IQR) | 0.99 (0.43) | 0.96 (0.42) | 0.810 | |
| ALT/ALP | median (IQR) | 0.46 (0.46) | 0.72 (0.52) | 0.00004 | |
| TC | mmol/L | median (IQR) | 6.18 (2.20) | 5.96 (1.37) | 0.03 |
| APRI | median (IQR) | 0.74 (0.82) | 0.43 (0.33) | 0.00003 |
| OR | 95% CI | p Value | |
|---|---|---|---|
| Total bilirubin (×ULN) | 0.3388 | 0.1671–0.6077 | 0.001 |
| Albumin (g/L) | 1.1612 | 1.0706–1.2688 | 0.0005 |
| Platelets (×109/L) | 1.0036 | 0.9993–1.0080 | 0.10 |
| Glycemia (mmol/L) | 0.8717 | 0.7298–1.0187 | 0.10 |
| ALT (×ULN) | 0.5306 | 0.3830–0.7080 | 0.00005 |
| AST (×ULN) | 0.4065 | 0.2690–0.5834 | <0.00001 |
| ALP (×ULN) | 0.3440 | 0.2356–0.4723 | <0.00001 |
| AST/ALT | 0.8810 | 0.3916–2.0264 | 0.76 |
| ALT/ALP | 2.4596 | 1.2095–5.5472 | 0.02 |
| TC (mmol/L) | 0.7730 | 0.6242–0.9271 | 0.01 |
| APRI | 0.3375 | 0.1833–0.5774 | 0.0002 |
| 216 Patients | Non-Responders 50 (23.2%) | Responders 166 (76.9%) | p Value | ||
|---|---|---|---|---|---|
| Age at diagnosis | years | median (IQR) | 55.50 (11) | 56.00 (12) | 0.30 |
| Gender | female patients male patients | number (%) | 49 (22.7) 1 (0.5) | 153 (70.8) 13 (6) | 0.25 |
| Total bilirubin | ×ULN | median (IQR) | 0.65 (0.91) | 0.52 (0.23) | 0.07 |
| Albumin | g/L | median (IQR) | 41.99 (6.35) | 43.00 (3.04) | 0.001 |
| Platelets | ×109/L | median (IQR) | 236.00 (124.25) | 245.00 (93.50) | 0.06 |
| Glycemia | mmol/L | median (IQR) | 5.24 (0.78) | 5.40 (1.10) | 0.80 |
| ALT | ×ULN | median (IQR) | 1.72 (1.60) | 1.17 (0.90) | 0.001 |
| AST | ×ULN | median (IQR) | 1.72 (1.69) | 1.06 (0.63) | 0.0001 |
| ALP | ×ULN | median (IQR) | 2.93 (2.73) | 1.51 (1.01) | <0.00001 |
| AST/ALT | median (IQR) | 0.97 (0.55) | 0.96 (0.40) | 0.58 | |
| ALT/ALP | median (IQR) | 0.49 (0.37) | 0.70 (0.49) | 0.0007 | |
| TC | mmol/L | median (IQR) | 6.07 (2.08) | 5.98 (1.29) | 0.56 |
| APRI | median (IQR) | 0.72 (0.97) | 0.45 (0.36) | 0.0001 |
| OR | 95% CI | p Value | |
|---|---|---|---|
| Total bilirubin (×ULN) | 0.2777 | 0.1288–0.5228 | 0.0004 |
| Albumin (g/L) | 1.2359 | 1.1257–1.3714 | 0.00002 |
| Platelets (×109/L) | 1.0056 | 1.0011–1.0103 | 0.02 |
| Glycemia (mmol/L) | 0.9494 | 0.7970–1.1575 | 0.57 |
| ALT (×ULN) | 0.5968 | 0.4354–0.7963 | 0.0007 |
| AST (×ULN) | 0.4161 | 0.2736–0.6076 | 0.00002 |
| ALP (×ULN) | 0.4676 | 0.3487–0.6048 | <0.00001 |
| AST/ALT | 0.6137 | 0.2544–1.5115 | 0.28 |
| ALT/ALP | 2.1896 | 1.0093–5.4160 | 0.07 |
| TC (mmol/L) | 0.9604 | 0.7707–1.2082 | 0.72 |
| APRI | 0.2838 | 0.1433–0.5141 | 0.0001 |
| Treatment Response | OR | 95% CI | p Value |
|---|---|---|---|
| after 6 months of UDCA therapy | 12.1156 | 3.7192–54.4826 | 0.0002 |
| after 12 months of UDCA therapy | 21.6000 | 6.6319–97.3840 | <0.00001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazda, J.; Drazilova, S.; Janicko, M.; Grgurevic, I.; Filipec Kanizaj, T.; Koller, T.; Bodorovska, B.; Bozin, T.; Mijic, M.; Rob, Z.; et al. Prognostic Factors in Primary Biliary Cholangitis: A Retrospective Study of Joint Slovak and Croatian Cohort of 249 Patients. J. Pers. Med. 2021, 11, 495. https://doi.org/10.3390/jpm11060495
Gazda J, Drazilova S, Janicko M, Grgurevic I, Filipec Kanizaj T, Koller T, Bodorovska B, Bozin T, Mijic M, Rob Z, et al. Prognostic Factors in Primary Biliary Cholangitis: A Retrospective Study of Joint Slovak and Croatian Cohort of 249 Patients. Journal of Personalized Medicine. 2021; 11(6):495. https://doi.org/10.3390/jpm11060495
Chicago/Turabian StyleGazda, Jakub, Sylvia Drazilova, Martin Janicko, Ivica Grgurevic, Tajana Filipec Kanizaj, Tomas Koller, Beatrica Bodorovska, Tonci Bozin, Maja Mijic, Zrinka Rob, and et al. 2021. "Prognostic Factors in Primary Biliary Cholangitis: A Retrospective Study of Joint Slovak and Croatian Cohort of 249 Patients" Journal of Personalized Medicine 11, no. 6: 495. https://doi.org/10.3390/jpm11060495
APA StyleGazda, J., Drazilova, S., Janicko, M., Grgurevic, I., Filipec Kanizaj, T., Koller, T., Bodorovska, B., Bozin, T., Mijic, M., Rob, Z., Mikolasevic, I., Madir, A., Kucinsky, B., & Jarcuska, P. (2021). Prognostic Factors in Primary Biliary Cholangitis: A Retrospective Study of Joint Slovak and Croatian Cohort of 249 Patients. Journal of Personalized Medicine, 11(6), 495. https://doi.org/10.3390/jpm11060495







