The Lausanne Infant Crying Stress Paradigm: Validation of an Early Postpartum Stress Paradigm with Women at Low vs. High Risk of Childbirth-Related Posttraumatic Stress Disorder
Abstract
1. Introduction
1.1. Childbirth-Related Posttraumatic Stress Disorder
1.2. Psychophysiological Stress Reactivity and Posttraumatic Stress Disorder
1.3. The Current Study
2. Materials and Methods
2.1. Design and Study Population
2.2. Procedure and Measures
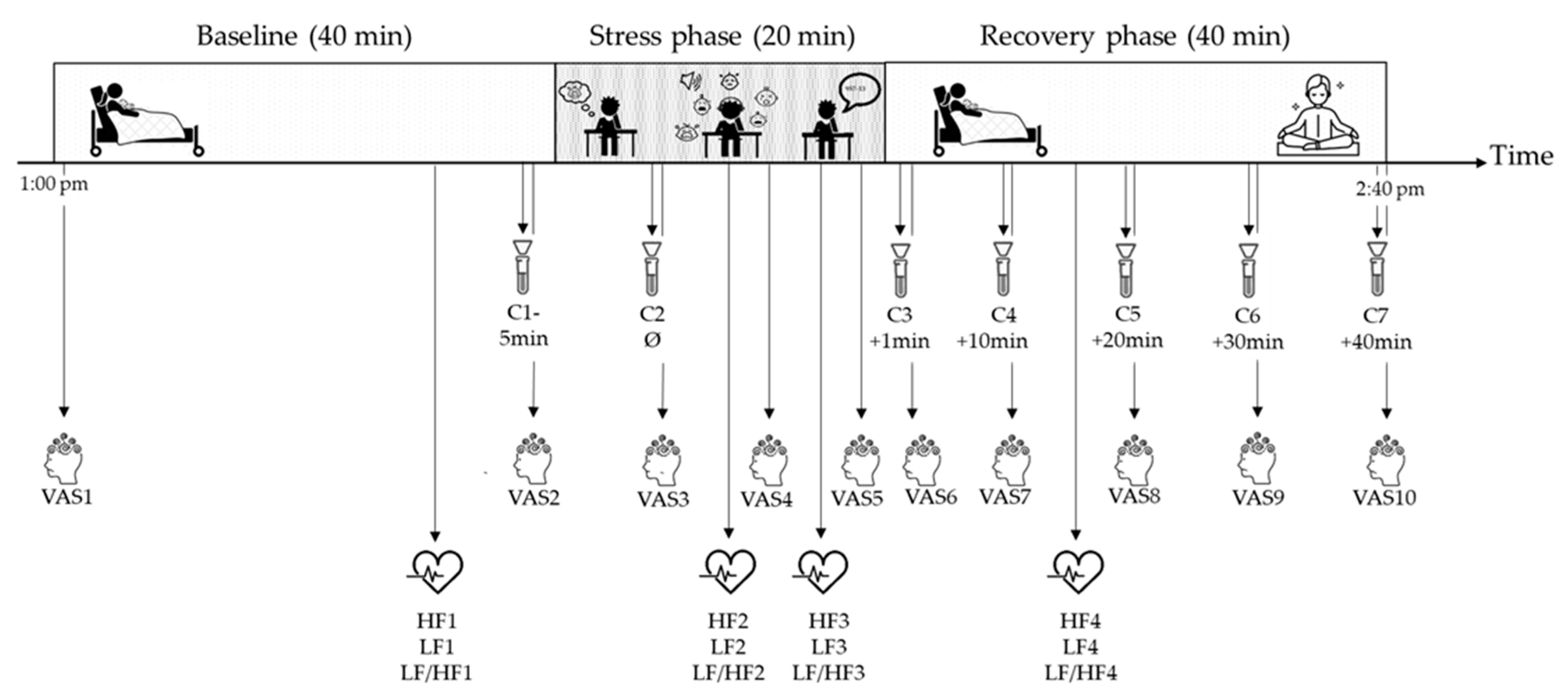
2.2.1. The Lausanne Infant Crying Paradigm Procedure
2.2.2. Psychophysiological Stress Responses
2.2.3. Psychosocial and Medical Information
2.3. Evaluation of the Stress Phase of the Lausanne Infant Crying Stress Paradigm
2.4. Sample Size Calculation
2.5. Data Analysis
3. Results
3.1. Characteristics of the Sample and of the Lausanne Infant Crying Stress Paradigm
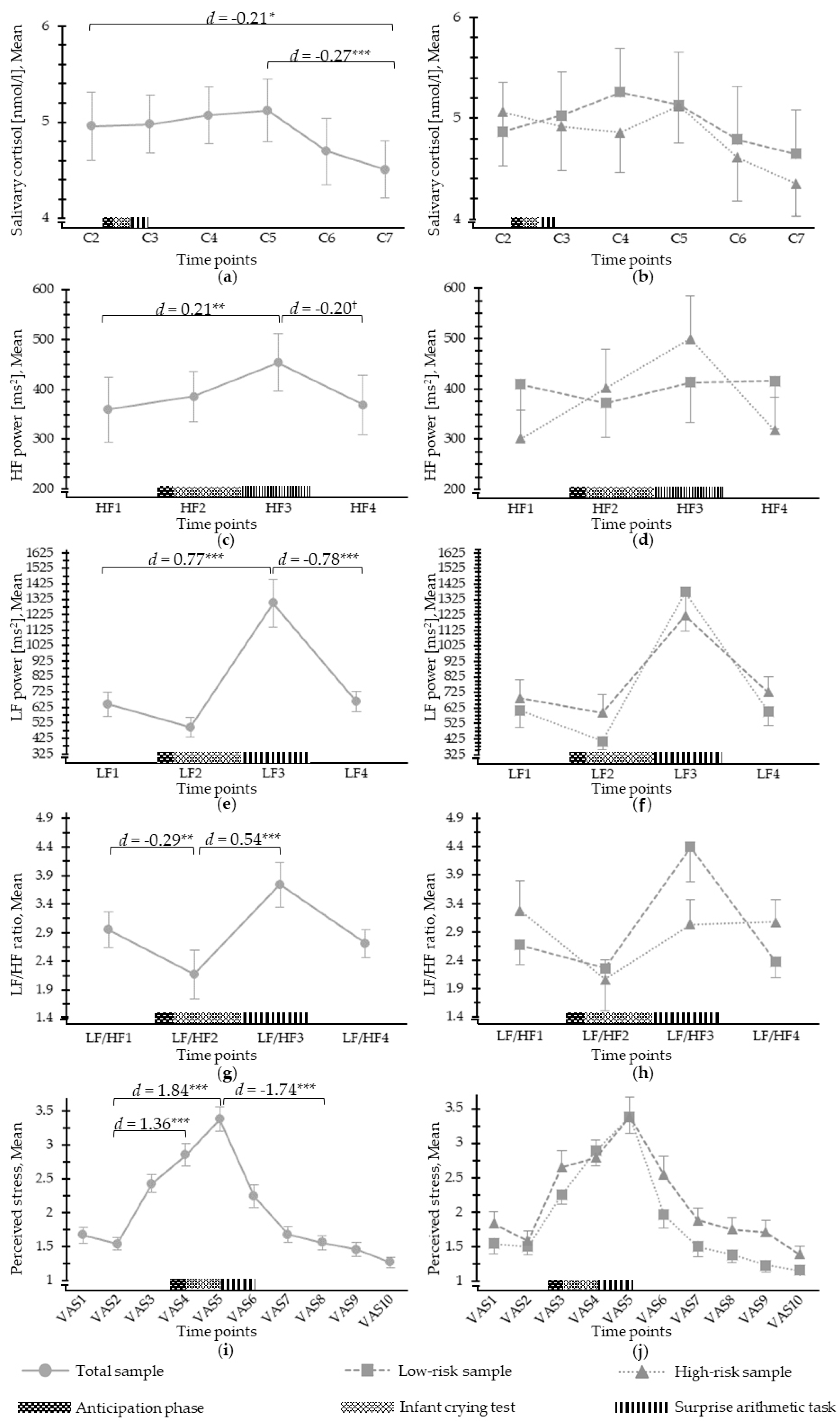
3.2. Salivary Cortisol Response to Psychosocial Stress
3.3. ANS Response to Psychosocial Stress
3.4. Perceived Stress in Response to Psychosocial Stress
4. Discussion
4.1. Strengths and Limitations
4.2. Future Research Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Olde, E.; van der Hart, O.; Kleber, R.; van Son, M. Posttraumatic stress following childbirth: A review. Clin. Psychol. Rev. 2006, 26, 1–16. [Google Scholar] [CrossRef]
- King, L.; McKenzie-McHarg, K.; Horsch, A. Testing a cognitive model to predict posttraumatic stress disorder following childbirth. BMC Pregnancy Childbirth 2017, 17, 32. [Google Scholar] [CrossRef]
- Ehlert, U.; Gaab, J.; Heinrichs, M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: The role of the hypothalamus–pituitary–adrenal axis. Biol. Psychol. 2001, 57, 141–152. [Google Scholar] [CrossRef]
- Nagpal, M.; Gleichauf, K.; Ginsberg, J. Meta-analysis of heart rate variability as a psychophysiological indicator of posttraumatic stress disorder. Trauma Treat. 2013, 3. [Google Scholar] [CrossRef]
- Soet, J.E.; Brack, G.A.; DiIorio, C. Prevalence and predictors of women’s experience of psychological trauma during childbirth. Birth 2003, 30, 36–46. [Google Scholar] [CrossRef]
- Ayers, S. Delivery as a traumatic event: Prevalence, risk factors, and treatment for postnatal posttraumatic stress disorder. Clin. Obstet. Gynecol. 2004, 47, 552–567. [Google Scholar] [CrossRef]
- Slade, P. Towards a conceptual framework for understanding post-traumatic stress symptoms following childbirth and implications for further research. J. Psychosom. Obstet. Gynecol. 2006, 27, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Horsch, A.; Garthus-Niegel, S. Posttraumatic stress disorder following childbirth. In Childbirth, Vulnerability and Law: Exploring Issues of Violence and Control, 1st ed.; Herring, C.P.J., Ed.; Routledge: London, UK, 2019; pp. 49–66. [Google Scholar]
- Zaers, S.; Waschke, M.; Ehlert, U. Depressive symptoms and symptoms of post-traumatic stress disorder in women after childbirth. J. Psychosom. Obstet. Gynecol. 2008, 29, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Ayers, S.; Bond, R.; Bertullies, S.; Wijma, K. The aetiology of post-traumatic stress following childbirth: A meta-analysis and theoretical framework. Psychol. Med. 2016, 46, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Grekin, R.; O’Hara, M.W. Prevalence and risk factors of postpartum posttraumatic stress disorder: A meta-analysis. Clin. Psychol. Rev. 2014, 34, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Dikmen-Yildiz, P.; Ayers, S.; Phillips, L. The prevalence of posttraumatic stress disorder in pregnancy and after birth: A systematic review and meta-analysis. J. Affect. Disord. 2017, 208, 634–647. [Google Scholar] [CrossRef]
- Ayers, S.; Harris, R.; Sawyer, A.; Parfitt, Y.; Ford, E. Posttraumatic stress disorder after childbirth: Analysis of symptom presentation and sampling. J. Affect. Disord. 2009, 119, 200–204. [Google Scholar] [CrossRef]
- Soderquist, J.; Wijma, K.; Wijma, B. Traumatic Stress after Childbirth: The Role of Obstetric Variables. J. Psychosom. Obstet. Gynecol. 2002, 23, 31–39. [Google Scholar] [CrossRef] [PubMed]
- van Heumen, M.A.; Hollander, M.H.; van Pampus, M.G.; van Dillen, J.; Stramrood, C.A.I. Psychosocial Predictors of Postpartum Posttraumatic Stress Disorder in Women With a Traumatic Childbirth Experience. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.B.; Melvaer, L.B.; Videbech, P.; Lamont, R.F.; Joergensen, J.S. Risk factors for developing post-traumatic stress disorder following childbirth: A systematic review. Acta Obstet. Gynecol. Scand. 2012, 91, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Sandoz, V.; Deforges, C.; Stuijfzand, S.; Epiney, M.; Vial, Y.; Sekarski, N.; Messerli-Bürgy, N.; Ehlert, U.; Bickle-Graz, M.; Morisod Harari, M.; et al. Improving mental health and physiological stress responses in mothers following traumatic childbirth and in their infants: Study protocol for the Swiss TrAumatic biRth Trial (START). BMJ Open 2019, 9, e032469. [Google Scholar] [CrossRef]
- Horesh, D.; Garthus-Niegel, S.; Horsch, A. Childbirth-related PTSD: Is it a unique post-traumatic disorder? J. Reprod. Infant Psychol. 2021, 39, 221–224. [Google Scholar] [CrossRef]
- Garthus-Niegel, S.; Horsch, A.; Handtke, E.; von Soest, T.; Ayers, S.; Weidner, K.; Eberhard-Gran, M. The impact of postpartum posttraumatic stress and depression symptoms on couples’ relationship satisfaction: A population-based prospective study. Front. Psychol. 2018, 9, 1728. [Google Scholar] [CrossRef]
- Delicate, A.; Ayers, S.; Easter, A.; McMullen, S. The impact of childbirth-related post-traumatic stress on a couple’s relationship: A systematic review and meta-synthesis. J. Reprod. Infant Psychol. 2018, 36, 102–115. [Google Scholar] [CrossRef]
- Cook, N.; Ayers, S.; Horsch, A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: A systematic review. J. Affect. Disord. 2018, 225, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Garthus-Niegel, S.; Horsch, A.; von Soest, T.; Haga, S.M.; Drozd, F.; Ayers, S.; Eberhard-Gran, M. Posttraumatic stress symptoms following childbirth: Associations with prenatal attachment in subsequent pregnancies. Arch. Women’s Ment. Health 2019, 23, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Garthus-Niegel, S.; Ayers, S.; Martini, J.; Von Soest, T.; Eberhard-Gran, M. The impact of postpartum post-traumatic stress disorder symptoms on child development: A population-based, 2-year follow-up study. Psychol. Med. 2017, 47, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Garthus-Niegel, S.; Horsch, A.; Ayers, S.; Junge-Hoffmeister, J.; Weidner, K.; Eberhard-Gran, M. The influence of postpartum PTSD on breastfeeding: A longitudinal population-based study. Birth 2018, 45, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Stuijfzand, S.; Garthus-Niegel, S.; Horsch, A. Parental birth-related PTSD symptoms and bonding in the early postpartum period: A prospective population-based cohort study. Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef]
- Bastos, M.H.; Furuta, M.; Small, R.; McKenzie-McHarg, K.; Bick, D. Debriefing interventions for the prevention of psychological trauma in women following childbirth. Cochrane Database Syst. Rev. 2015, CD007194. [Google Scholar] [CrossRef] [PubMed]
- Horsch, A. Post traumatic stress disorder following childbirth and pregnancy loss. In Clinical Psychology in Practice; Beinard, H., Kennedy, P.S.L., Eds.; Blackwell, BPS: Oxford, UK, 2009; pp. 274–287. [Google Scholar]
- Del Giudice, M.; Ellis, B.J.; Shirtcliff, E.A. The Adaptive Calibration Model of stress responsivity. Neurosci. Biobehav. Rev. 2011, 35, 1562–1592. [Google Scholar] [CrossRef] [PubMed]
- Benfield, R.D.; Newton, E.R.; Tanner, C.J.; Heitkemper, M.M. Cortisol as a biomarker of stress in term human labor: Physiological and methodological issues. Biol. Res. Nurs. 2014, 16, 64–71. [Google Scholar] [CrossRef]
- Falk-Smith, N.L.; Madrigal, L. Maternal stress, anxiety, and fear of childbirth among planned homebirth mothers: Results from a mixed methods study. In Proceedings of the 88th Annual Meeting of the American Association of Physical Anthropologists, Cleveland, OH, USA, 27 March 2019; pp. 71–72. [Google Scholar]
- Miller, N.; Asali, A.A.; Agassi-Zaitler, M.; Neumark, E.; Eisenberg, M.M.; Hadi, E.; Elbaz, M.; Pasternak, Y.; Fishman, A.; Biron-Shental, T. Physiological and psychological stress responses to labor and delivery as expressed by salivary cortisol: A prospective study. Am. J. Obstet. Gynecol. 2019, 221, 351.e351–e357. [Google Scholar] [CrossRef]
- Meulenberg, P.M.; Hofman, J.A. Differences between concentrations of salivary cortisol and cortisone and of free cortisol and cortisone in plasma during pregnancy and postpartum. Clin. Chem. 1990, 36, 70–75. [Google Scholar] [CrossRef]
- Heinrichs, M.; Neumann, I.; Ehlert, U. Lactation and stress: Protective effects of breast-feeding in humans. Stress 2002, 5, 195–203. [Google Scholar] [CrossRef]
- Pitman, R.K.; Rasmusson, A.M.; Koenen, K.C.; Shin, L.M.; Orr, S.P.; Gilbertson, M.W.; Milad, M.R.; Liberzon, I. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012, 13, 769–787. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.E.; Chen, E.; Zhou, E.S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 2007, 133, 25–45. [Google Scholar] [CrossRef]
- England-Mason, G.; Kimber, M.; Khoury, J.; Atkinson, L.; MacMillan, H.; Gonzalez, A. Difficulties with emotion regulation moderate the association between childhood history of maltreatment and cortisol reactivity to psychosocial challenge in postpartum women. Horm. Behav. 2017, 95, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Pierce, M.E.; Pritchard, L.M. Lower stress-reactive cortisol in female veterans associated with military status but not PTSD. Stress 2016, 19, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, B.M.; Schmahl, C.G.; Vermetten, E.; Van Dyck, R.; Bremner, J.D. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology 2003, 28, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.I.; Moser, D.A.; Manini, A.; Suardi, F.; Sancho-Rossignol, A.; Torrisi, R.; Rossier, M.F.; Ansermet, F.; Dayer, A.G.; Rusconi-Serpa, S.; et al. Effects of interpersonal violence-related post-traumatic stress disorder (PTSD) on mother and child diurnal cortisol rhythm and cortisol reactivity to a laboratory stressor involving separation. Horm. Behav. 2017, 90, 15–24. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5. [Google Scholar] [CrossRef]
- Klinkenberg, A.V.; Nater, U.M.; Nierop, A.; Bratsikas, A.; Zimmermann, R.; Ehlert, U. Heart rate variability changes in pregnant and non-pregnant women during standardized psychosocial stress 1. Acta Obstet. Gynecol. Scand. 2009, 88, 77–82. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Finan, P.H.; Zautra, A.J.; Wershba, R. The dynamics of emotions in adaptation to stress. In The Handbook of Stress Science: Biology, Psychology, and Health; Contrada, R.J., Baum, A., Eds.; Springer Publishing Company: New York, NY, USA, 2011; pp. 209–220. [Google Scholar] [CrossRef]
- Kudielka, B.M.; Hellhammer, D.H.; Kirschbaum, C. Ten years of research with the Trier Social Stress Test—revisited. In Social Neuroscience: Integrating Biological and Psychological Explanations of Social Behavior; Winkielman, E.H.-J.P., Ed.; The Guilford Press: New York, NY, USA, 2007; pp. 56–83. [Google Scholar] [CrossRef]
- Dickerson, S.S.; Kemeny, M.E. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004, 130, 355–391. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Pirke, K.-M.; Hellhammer, D.H. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Ayers, S.; Crawley, R.; Webb, R.; Button, S.; Thornton, A.; the HABiT collaborative group. What are women stressed about after birth? Birth 2019, 46, 678–685. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Meyer, T.; Albrecht, J.; Bornschein, G.; Sachsse, U.; Herrmann-Lingen, C. Posttraumatic Stress Disorder (PTSD) patients exhibit a blunted parasympathetic response to an emotional stressor. Appl. Psychophysiol. Biofeedback 2016, 41, 395–404. [Google Scholar] [CrossRef]
- Bouchet, H.; Plat, A.; Levréro, F.; Reby, D.; Patural, H.; Mathevon, N. Baby cry recognition is independent of motherhood but improved by experience and exposure. Proc. R. Soc. B Biol. Sci. 2020, 287, 20192499. [Google Scholar] [CrossRef]
- Bozovic, D.; Racic, M.; Ivkovic, N. Salivary cortisol levels as a biological marker of stress reaction. Med. Arch. 2013, 67, 374–377. [Google Scholar] [CrossRef]
- Hellhammer, D.H.; Wust, S.; Kudielka, B.M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009, 34, 163–171. [Google Scholar] [CrossRef]
- Messerli-Bürgy, N.; Kakebeeke, T.H.; Arhab, A.; Stülb, K.; Zysset, A.E.; Leeger-Aschmann, C.S.; Schmutz, E.A.; Fares, F.; Meyer, A.H.; Munsch, S.; et al. The Swiss Preschoolers’ health study (SPLASHY): Objectives and design of a prospective multi-site cohort study assessing psychological and physiological health in young children. BMC Pediatrics 2016, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Schobinger, E.; Stuijfzand, S.; Horsch, A. Acute and post-traumatic stress disorder symptoms in mothers and fathers following childbirth: A prospective cohort study. Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Guedeney, N.; Fermanian, J. Validation study of the French version of the Edinburgh Postnatal Depression Scale (EPDS): New results about use and psychometric properties. Eur. Psychiatry 1998, 13, 83–89. [Google Scholar] [CrossRef]
- Horsch, A.; Tolsa, J.-F.; Gilbert, L.; Jan Du Chêne, L.; Müller-Nix, C.; Bickle-Graz, M. Improving maternal mental health following preterm birth using an expressive writing intervention: A randomized controlled trial. Child Psychiatry Human Dev. 2016, 47, 780–791. [Google Scholar] [CrossRef]
- Sandoz, V.; Bickle-Graz, M.; Camos, V.; Horsch, A. Maternal postpartum depression symptoms are negatively associated with emotion regulation of children born very preterm. Acta Paediatr. 2019, 108, 969–970. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Bocéréan, C.; Dupret, E. A validation study of the Hospital Anxiety and Depression Scale (HADS) in a large sample of French employees. BMC Psychiatry 2014, 14. [Google Scholar] [CrossRef]
- Horsch, A.; Vial, Y.; Favrod, C.; Morisod Harari, M.; Blackwell, S.E.; Watson, P.; Iyadurai, L.; Bonsall, M.B.; Holmes, E.A. Reducing intrusive traumatic memories after emergency caesarean section: A proof-of-principle randomized controlled study. Behav. Res. Ther. 2017, 94, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.W. A review of psychoendocrine research on the pituitary-adrenal cortical system. Psychosom. Med. 1968, 30, 576–607. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Van Buuren, S.; Groothuis-Oudshoorn, K. MICE: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–68. [Google Scholar] [CrossRef]
- Heinrichs, M.; Baumgartner, T.; Kirschbaum, C.; Ehlert, U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry 2003, 54, 1389–1398. [Google Scholar] [CrossRef]
- Wright, K.P.; Drake, A.L.; Frey, D.J.; Fleshner, M.; Desouza, C.A.; Gronfier, C.; Czeisler, C.A. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav. Immun. 2015, 47, 24–34. [Google Scholar] [CrossRef]
- Almanza-Sepulveda, M.L.; Fleming, A.S.; Jonas, W. Mothering revisited: A role for cortisol? Horm. Behav. 2020, 121, 104679. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.A.; Lawrence, R.M. Physiology of lactation. In Breastfeeding, 7th ed.; Lawrence, R.A., Lawrence, R.M., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 62–97. [Google Scholar]
- Kaye, J.; Soothill, P.; Hunt, M.; Lightman, S. Responses to the 35% CO challenge in postpartum women. Clin. Endocrinol. 2004, 61, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.E.; Wouk, K.; Grewen, K.; Gottfredson, N.C.; Meltzer-Brody, S.; Propper, C.; Mills-Koonce, R.; Pearson, B.; Whitley, J.; Stuebe, A.M. Associations of postpartum depression symptoms and infant feeding with Hypothalamic Pituitary Adrenal (HPA) axis reactivity. Am. J. Obstet. Gynecol. 2020, 222, S114. [Google Scholar] [CrossRef]
- Giles, G.E.; Mahoney, C.R.; Brunyé, T.T.; Taylor, H.A.; Kanarek, R.B. Stress effects on mood, HPA axis, and autonomic response: Comparison of three psychosocial stress paradigms. PLoS ONE 2014, 9, e113618. [Google Scholar] [CrossRef] [PubMed]
- Schechter, D.S.; Moser, D.A.; McCaw, J.E.; Myers, M.M. Autonomic functioning in mothers with interpersonal violence-related posttraumatic stress disorder in response to separation–reunion. Dev. Psychobiol. 2014, 56, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Terkelsen, A.J.; Mølgaard, H.; Hansen, J.; Andersen, O.K.; Jensen, T.S. Acute pain increases heart rate: Differential mechanisms during rest and mental stress. Auton. Neurosci. 2005, 121, 101–109. [Google Scholar] [CrossRef]
- Harrison, S.E.; Ayers, S.; Quigley, M.A.; Stein, A.; Alderdice, F. Prevalence and factors associated with postpartum posttraumatic stress in a population-based maternity survey in England. J. Affect. Disord. 2020. [Google Scholar] [CrossRef]
- Ayers, S.; Wright, D.B.; Thornton, A. Development of a measure of postpartum PTSD: The City Birth Trauma Scale. Front. Psychiatry 2018, 9, 409. [Google Scholar] [CrossRef]
- Sandoz, V.; Hingray, C.; Stuijfzand, S.; Lacroix, A.; El Hage, W.; Horsch, A. Measurement and conceptualization of maternal PTSD following childbirth: Psychometric properties of the City Birth Trauma Scale–French version (City BiTS-F). Psychol. Trauma 2021, 12, 147–155. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
| Total Sample (n = 52) | Low-Risk Group (n = 28) | High-Risk Group (n = 24) | |
|---|---|---|---|
| M SD | M SD | M SD | |
| Salivary cortisol (nmol/L) | |||
| C1 level | 5.5 (2.57) | 5.31 (2.57) | 5.72 (2.6) |
| C2 level | 4.96 (2.19) | 4.87 (2.27) | 5.06 (2.15) |
| C3 level 1 | 4.98 (2.13) | 5.03 (2.31) | 4.92 (1.96) |
| C4 level 1 | 5.07 (2.35) | 5.26 (2.79) | 4.86 (1.79) |
| C5 level 1 | 5.12 (2.47) | 5.13 (2.8) | 5.12 (2.12) |
| C6 level 1 | 4.7 (2.13) | 4.79 (2.29) | 4.61 (1.98) |
| C7 level 1 | 4.51 (2.19) | 4.65 (2.51) | 4.35 (1.82) |
| HF power (ms2) | |||
| HF1 | 359.56 (472.95) | 409.07 (593.5) | 301.79 (275.74) |
| HF2 2 | 386.04 (364.14) | 371.93 (363.87) | 401.92 (371.61) |
| HF3 23 | 454.3 (417.7) | 413.04 (421.67) | 499.48 (418.85) |
| HF4 1 | 369.2 (427.57) | 416.19 (508.43) | 318.29 (321.41) |
| LF power (ms2) | |||
| LF1 | 646.08 (572.69) | 609.82 (574.26) | 688.38 (580.22) |
| LF2 2 | 497.08 (440.61) | 410.44 (280.52) | 594.54 (560.67) |
| LF3 23 | 1299.73 (1101.02) | 1372.04 (1336.12) | 1220.52 (793.71) |
| LF41 | 663.36 (483.53) | 602.81 (491.58) | 728.96 (476.23) |
| LF/HF ratio | |||
| LF/HF1 | 2.95 (2.22) | 2.67 (1.81) | 3.27 (2.62) |
| LF/HF2 2 | 2.17 (3.09) | 2.26 (3.96) | 2.06 (1.72) |
| LF/HF3 23 | 3.74 (2.79) | 4.39 (3.18) | 3.03 (2.13) |
| LF/HF4 1 | 2.71 (1.78) | 2.38 (1.53) | 3.07 (1.98) |
| Perceived stress | |||
| VAS1 | 1.67 (0.83) | 1.54 (0.79) | 1.83 (0.87) |
| VAS2 | 1.54 (0.67) | 1.5 (0.64) | 1.58 (0.72) |
| VAS3 | 2.43 (0.96) | 2.25 (0.7) | 2.65 (1.18) |
| VAS4 | 2.85 (1.19) | 2.89 (1.17) | 2.79 (1.25) |
| VAS5 1 | 3.38 (1.29) | 3.38 (1.24) | 3.38 (1.38) |
| VAS6 1 | 2.24 (1.19) | 1.96 (1.04) | 2.54 (1.28) |
| VAS7 1 | 1.68 (0.87) | 1.5 (0.81) | 1.88 (0.9) |
| VAS8 1 | 1.56 (0.73) | 1.38 (0.57) | 1.75 (0.85) |
| VAS9 1 | 1.46 (0.71) | 1.23 (0.51) | 1.71 (0.81) |
| VAS10 1 | 1.27 (0.53) | 1.15 (0.46) | 1.39 (0.57) |
| Total Sample (n = 52) | Low-Risk Group (n = 28) | High-Risk Group (n = 24) | Group Difference | |
|---|---|---|---|---|
| Sociodemographic and medical characteristics | ||||
| Age (M, SD) | 31.71 (4.00) | 33.48 (4.00) | 31.83 (3.89) | W = 382.00, p = 0.276 |
| Missing data (N, %) | 1 (1.92) | 1 (3.57) | 0 | |
| Civil status | p = 0.507 | |||
| Married or in a relationship (N, %) | 36 (69.23) | 20 (71.43) | 16 (66.67) | |
| Single, separated, divorced, or widowed (N, %) | 7 (13.46) | 3 (10.71) | 4 (16.67) | |
| Other (N, %) | 2 (3.87) | 2 (7.14) | 0 | |
| Missing data (N, %) | 7 (13.46) | 3 (10.71) | 4 (16.67) | |
| Education level | p = 0.407 | |||
| Compulsory education (N, %) | 3 (5.77) | 2 (7.14) | 1 (4.17) | |
| Post-compulsory education (N, %) | 1 (3.57) | 2 | ||
| Apprenticeship (N, %) | 6 (11.54) | 2 (7.14) | 4 (16.67) | |
| University (N, %) | 30 (57.69) | 17 (60.71) | 13 (54.17) | |
| Other (N, %) | 3 (5.77) | 3 (10.71) | 0 | |
| Missing data (N, %) | 7 (13.46) | 3 (10.71) | 4 (16.67) | |
| Smoking | p = 0.242 | |||
| Yes (N, %) | 3 (5.77) | 3 (10.71) | 0 | |
| No (N, %) | 42 (80.77) | 22 (78.58) | 20 (83.33) | |
| Missing data (N, %) | 7 (13.46) | 3 (10.71) | 4 (16.67) | |
| Parity (M, SD) | 0.40 (0.72) | 0.57 (0.74) | 0.21 (0.66) | W = 436.00, p = 0.022 |
| Gravidity (M, SD) | 1.65 (1.25) | 1.89 (1.23) | 1.38 (1.24) | W = 450.00, p = 0.013 |
| Type of delivery | p < 0.001 | |||
| Vaginal birth (N, %) | 28 (53.85) | 22 (78.57) | 6 (25.00) | |
| Planned cesarean section (N, %) | 6 (11.54) | 5 (17.86) | 1 (4.17) | |
| Vacuum-assisted vaginal delivery (N, %) | 1 (1.92) | 0 | 1 (4.17) | |
| Forceps delivery (N, %) | 3 (5.77) | 0 | 3 (12.50) | |
| Emergency cesarian section (N, %) | 14 (26.92) | 1 (3.57) | 13 (54.17) | |
| Psychological characteristics | ||||
| Perceived life threat for the mother (M, SD) | 1.77 (1.63) | 1.07 (0.26) | 2.58 (2.13) | W = 211.00, p = 0.002 |
| Perceived life threat for the infant (M, SD) | 3.00 (2.22) | 1.21 (0.42) | 5.08 (1.53) | W = 6.00, p < 0.001 |
| Anxiety, HADS-A (M, SD) | 6.91 (3.65) | 6.40 (3.25) | 7.58 (4.1) | W = 198.50, p = 0.360 |
| Cronbach’s Alpha | 0.72 | 0.68 | 0.76 | |
| Missing data (N, %) | 8 (15.38) | 3 (10.71) | 5 (20.83) | |
| Depression, EPDS (M, SD) | 6.75 (5.13) | 5.24 (3.75) | 8.74 (6.06) | W = 155.00, p = 0.051 |
| Cronbach’s Alpha | 0.85 | 0.76 | 0.90 | |
| Missing data (N, %) | 7 (13.46) | 3 (10.71) | 5 (20.83) | |
| Total Sample (n = 52) | Low-Risk Group (n = 28) | High-Risk Group (n = 24) | Group Differences | |
|---|---|---|---|---|
| Time between birth and the LICSP (hh:mm) (M, SD) | 51:04 (22:02) | 46:23 (21:50) | 56:31 (21:27) | W = 236, p = 0.068 |
| LICSP start time (hh:mm p.m.) (M, SD) | 1:13 (0:20) | 1:13 (0:20) | 1:15 (0:20) | W = 309.5, p = 0.632 |
| Baseline phase duration (mm:ss) (M, SD) | 43:43 (3:28) | 42:58 (2:45) | 44:35 (4:01) | W = 256.5, p = 0.146 |
| Stress phase duration (mm:ss) (M, SD) | 21:41 (2:39) | 21:58 (3:00) | 21:23 (2:01) | W = 349, p = 643 |
| Missing data (N, %) | 1 (1.92) | 1 (3.57) | 0 | |
| Recovery phase duration (mm:ss) (M, SD) | 42:37 (4:28) | 43:10 (5:53) | 42:02 (2:02) | W = 341.5, p = 0.561 |
| Missing data (N, %) | 2 (3.85) | 2 (7.14) | 0 | |
| LICSP duration (mm:ss) (M, SD) | 107.68 (4.61) | 107.38 (4.73) | 108 (4.56) | W = 289.5, p = 0.668 |
| Missing data (N, %) | 2 (3.85) | 2 (7.14) | 0 | |
| Breastfeeding within the hour preceding the stress phase | X2(1) = 0.06, p = 0.799 | |||
| Yes (N, %) | 9 (17.31) | 4 (14.29) | 5 (20.83) | |
| No (N, %) | 43 (82.69) | 24 (85.71) | 19 (79.17) | |
| Characteristics of the stress phase | ||||
| Novelty (M, SD) | 77.80 (28.34) | 72.12 (29.91) | 83.96 (25.75) | W = 220.5, p = 0.061 |
| Missing data (N, %) | 2 (3.85) | 2 (7.14) | 0 | |
| Difficulty (M, SD) | 63.96 (22.88) | 65.31 (23.42) | 62.50 (22.70) | W = 335, p = 0.66 |
| Missing data (N, %) | 2 (3.85) | 2 (7.14) | 0 | |
| Stress (M, SD) | 50.16 (27.09) | 52.81 (24.61) | 47.29 (29.82) | W = 345, p = 0.525 |
| Missing data (N, %) | 2 (3.85) | 2 (7.14) | 0 | |
| Controllability (M, SD) | 49.12 (29.19) | 54.04 (33.32) | 43.79 (23.48) | W = 376, p = 0.212 |
| Missing data (N, %) | 2 (3.85) | 2 (7.14) | 0 | |
| Predictability (M, SD) | 32.73 (29.23) | 36.88 (27.22) | 28.00 (31.36) | W = 303, p = 0.247 |
| Missing data (N, %) | 7 (13.46) | 4 (14.29) | 3 (12.50) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoz, V.; Stuijfzand, S.; Lacroix, A.; Deforges, C.; Quillet Diop, M.; Ehlert, U.; Rubo, M.; Messerli-Bürgy, N.; Horsch, A. The Lausanne Infant Crying Stress Paradigm: Validation of an Early Postpartum Stress Paradigm with Women at Low vs. High Risk of Childbirth-Related Posttraumatic Stress Disorder. J. Pers. Med. 2021, 11, 472. https://doi.org/10.3390/jpm11060472
Sandoz V, Stuijfzand S, Lacroix A, Deforges C, Quillet Diop M, Ehlert U, Rubo M, Messerli-Bürgy N, Horsch A. The Lausanne Infant Crying Stress Paradigm: Validation of an Early Postpartum Stress Paradigm with Women at Low vs. High Risk of Childbirth-Related Posttraumatic Stress Disorder. Journal of Personalized Medicine. 2021; 11(6):472. https://doi.org/10.3390/jpm11060472
Chicago/Turabian StyleSandoz, Vania, Suzannah Stuijfzand, Alain Lacroix, Camille Deforges, Magali Quillet Diop, Ulrike Ehlert, Marius Rubo, Nadine Messerli-Bürgy, and Antje Horsch. 2021. "The Lausanne Infant Crying Stress Paradigm: Validation of an Early Postpartum Stress Paradigm with Women at Low vs. High Risk of Childbirth-Related Posttraumatic Stress Disorder" Journal of Personalized Medicine 11, no. 6: 472. https://doi.org/10.3390/jpm11060472
APA StyleSandoz, V., Stuijfzand, S., Lacroix, A., Deforges, C., Quillet Diop, M., Ehlert, U., Rubo, M., Messerli-Bürgy, N., & Horsch, A. (2021). The Lausanne Infant Crying Stress Paradigm: Validation of an Early Postpartum Stress Paradigm with Women at Low vs. High Risk of Childbirth-Related Posttraumatic Stress Disorder. Journal of Personalized Medicine, 11(6), 472. https://doi.org/10.3390/jpm11060472







