Significant Association of Polymorphisms in the TCF7L2 Gene with a Higher Risk of Type 2 Diabetes in a Moroccan Population
Abstract
1. Background
2. Materials and Methods
2.1. Study Subject
2.2. Sample Collection and Biochemical Assay
2.3. DNA Isolation
2.4. Genotyping
2.5. Statistical Analysis
3. Results
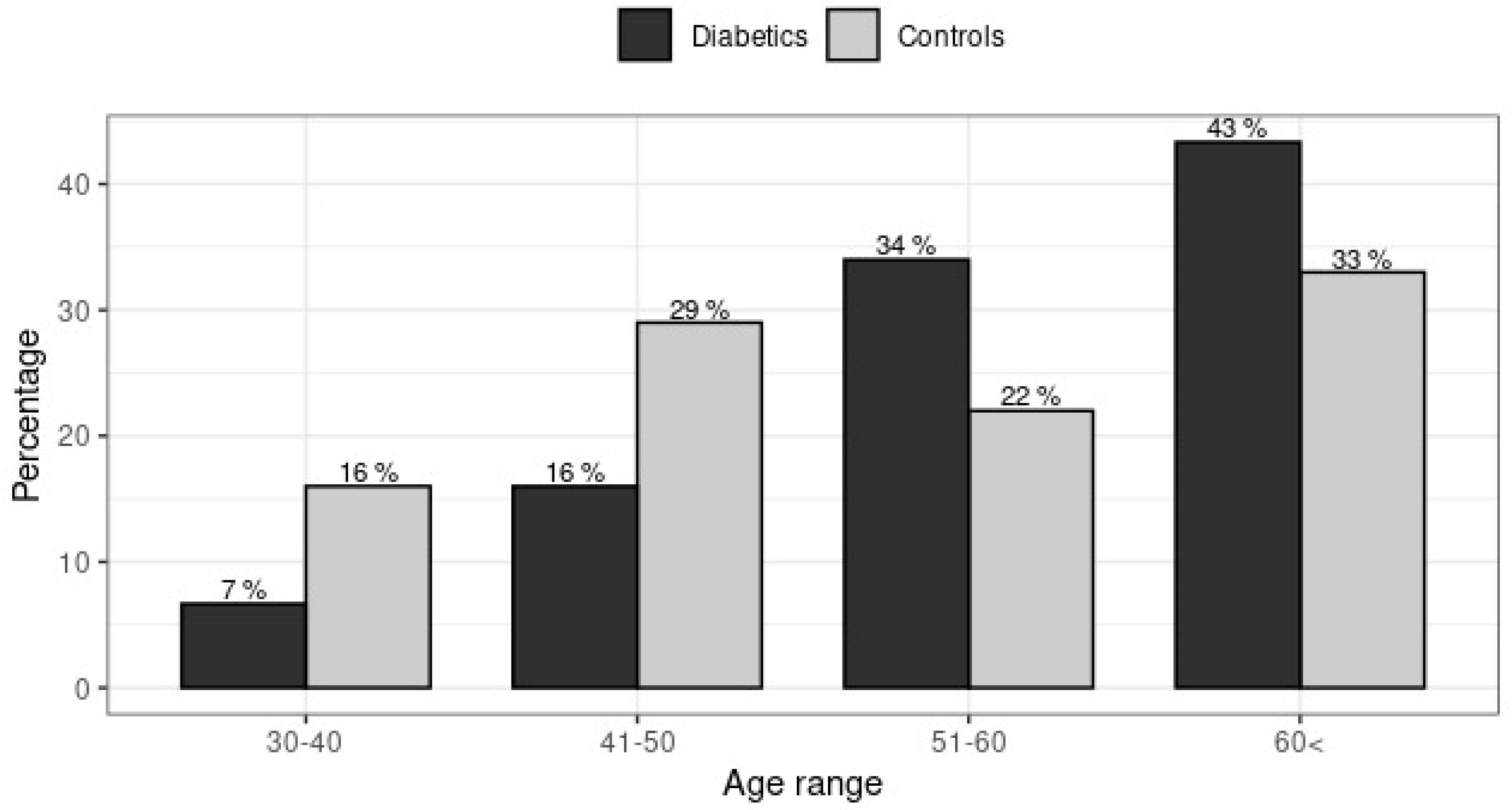
3.1. Genotypes and Alleles Frequencies
3.2. Comparison between TCF7L2 rs7903146 and rs12255372 According to Anthropometric and Laboratory Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| T2DM | type 2 diabetes mellitus |
| TCF7L2 | transcription factor 7 like 2 |
| SNP | single nucleotide polymorphism |
| HT | hypertension |
| BMI | body mass index |
| HbA1c | glycated hemoglobin |
References
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Malecki, M.T. Genetics of type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2005, 68, S10–S21. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Bhatti, G.K.; Kaur, S.; Bhadada, S.K.; Singh, S.; Bhatti, J.S. Transcription factor 7-like 2 gene, rs12255372 (G/T) variant and susceptibility to type 2 diabetes mellitus in North Indians. Gene Rep. 2020, 19, 100595. [Google Scholar] [CrossRef]
- Chetoui, A.; Kaoutar, K.; Elmoussaoui, S.; Boutahar, K.; El Kardoudi, A.; Chigr, F.; Najimi, M. Prevalence and determinants of poor glycaemic control: A cross-sectional study among Moroccan type 2 diabetes patients. Int. Health 2020, ihz107. [Google Scholar] [CrossRef] [PubMed]
- Duval, A.; Busson-Leconiat, M.; Berger, R.; Hamelin, R. Assignment of the TCF-4 gene (TCF7L2) to human chromosome band 10q25.3. Cytogenet. Cell Genet. 2000, 88, 264–265. [Google Scholar] [CrossRef]
- Huang, Z.-Q.; Liao, Y.-Q.; Huang, R.-Z.; Chen, J.-P.; Sun, H.-L. Possible role of TCF7L2 in the pathogenesis of type 2 diabetes mellitus. Biotechnol. Biotechnol. Equip. 2018, 32, 830–834. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Yang, X.-X.; Geng, H.-Y.; Gong, G. Type 2 diabetes mellitus and TCF7L2 gene rs12255372 G/T polymorphism: A meta-analysis involving 7990 subjects. Int. J. Diabetes Dev. Ctries. 2017, 38, 55–61. [Google Scholar] [CrossRef]
- Basile, K.J.; Guy, V.C.; Schwartz, S.; Grant, S.F.A. Overlap of Genetic Susceptibility to Type 1 Diabetes, Type 2 Diabetes, and Latent Autoimmune Diabetes in Adults. Curr. Diabetes Rep. 2014, 14, 1–7. [Google Scholar] [CrossRef]
- Florez, J.C.; Jablonski, K.A.; Bayley, N.; Pollin, T.I.; De Bakker, P.I.; Shuldiner, A.R.; Knowler, W.C.; Nathan, D.M.; Altshuler, D. TCF7L2Polymorphisms and Progression to Diabetes in the Diabetes Prevention Program. N. Engl. J. Med. 2006, 355, 241–250. [Google Scholar] [CrossRef]
- El Achhab, Y.; Berraho, M.; Benslimane, A.; Nejjari, C.; Frogue, P.; Chikri, M. Épidémiologie génétique et diabète de type 2 au Maroc. Revue d’Épidémiologie Santé Publique 2009, 57, S23. [Google Scholar] [CrossRef]
- Assmann, T.S.; Duarte, G.C.K.; Rheinheimer, J.; Cruz, L.A.; Canani, L.H.; Crispim, D. The TCF7L2 rs7903146 (C/T) polymorphism is associated with risk to type 2 diabetes mellitus in Southern-Brazil. Arq. Bras. Endocrinol. Metabol. 2014, 58, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.F.; Thorleifsson, G.; Reynisdottir, I.; Benediktsson, R.; Manolescu, A.; Sainz, J.; Helgason, A.; Stefansson, H.; Emilsson, V.; Helgadottir, A.; et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 2006, 38, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, H.; Han, X.; Ren, Q.; Wang, F.; Zhang, X.; Sun, X.; Zhou, X.; Ji, L. Meta-analysis of the association between SNPs in TCF7L2 and type 2 diabetes in East Asian population. Diabetes Res. Clin. Pract. 2009, 85, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Cauchi, S.; Meyre, D.; Dina, C.; Choquet, H.; Samson, C.; Gallina, S.; Balkau, B.; Charpentier, G.; Pattou, F.; Stetsyuk, V.; et al. Transcription Factor TCF7L2 Genetic Study in the French Population: Expression in Human -Cells and Adipose Tissue and Strong Association with Type 2 Diabetes. Diabetes 2006, 55, 2903–2908. [Google Scholar] [CrossRef]
- Van Vliet-Ostaptchouk, J.V.; Shiri-Sverdlov, R.; Zhernakova, A.; Strengman, E.; Van Haeften, T.W.; Hofker, M.H.; Wijmenga, C. Association of variants of transcription factor 7-like 2 (TCF7L2) with susceptibility to type 2 diabetes in the Dutch Breda cohort. Diabetologia 2006, 50, 59–62. [Google Scholar] [CrossRef]
- Groves, C.J.; Zeggini, E.; Minton, J.; Frayling, T.M.; Weedon, M.; Rayner, N.W.; Hitman, G.A.; Walker, M.; Wiltshire, S.; Hattersley, A.T.; et al. Association Analysis of 6,736 U.K. Subjects Provides Replication and ConfirmsTCF7L2as a Type 2 Diabetes Susceptibility Gene with a Substantial Effect on Individual Risk. Diabetes 2006, 55, 2640–2644. [Google Scholar] [CrossRef]
- Marzi, C.; Huth, C.; Kolz, M.; Grallert, H.; Meisinger, C.; Wichmann, H.-E.; Rathmann, W.; Herder, C.; Illig, T. Variants of the Transcription Factor 7-Like 2 Gene (TCF7L2) are Strongly Associated with Type 2 Diabetes but not with the Metabolic Syndrome in the MONICA/KORA Surveys. Horm. Metab. Res. 2007, 39, 46–52. [Google Scholar] [CrossRef]
- Saxena, R.; Gianniny, L.; Burtt, N.P.; Lyssenko, V.; Giuducci, C.; Sjögren, M.; Florez, J.C.; Almgren, P.; Isomaa, B.; Orho-Melander, M.; et al. Common Single Nucleotide Polymorphisms in TCF7L2 Are Reproducibly Associated with Type 2 Diabetes and Reduce the Insulin Response to Glucose in Nondiabetic Individuals. Diabetes 2006, 55, 2890–2895. [Google Scholar] [CrossRef]
- Zhang, C.; Qi, L.; Hunter, D.J.; Meigs, J.B.; Manson, J.E.; Van Dam, R.M.; Hu, F.B. Variant of Transcription Factor 7-Like 2 (TCF7L2) Gene and the Risk of Type 2 Diabetes in Large Cohorts of U.S. Women and Men. Diabetes 2006, 55, 2645–2648. [Google Scholar] [CrossRef]
- Horikoshi, M.; Hara, K.; Ito, C.; Nagai, R.; Froguel, P.; Kadowaki, T. A genetic variation of the transcription factor 7-like 2 gene is associated with risk of type 2 diabetes in the Japanese population. Diabetologia 2007, 50, 747–751. [Google Scholar] [CrossRef]
- Humphries, S.E.; Gable, D.; Cooper, J.A.; Ireland, H.; Stephens, J.W.; Hurel, S.J.; Li, K.W.; Palmen, J.; Miller, M.A.; Cappuccio, F.P.; et al. Common variants in the TCF7L2 gene and predisposition to type 2 diabetes in UK European Whites, Indian Asians and Afro-Caribbean men and women. J. Mol. Med. 2006, 84, 1005–1014. [Google Scholar] [CrossRef]
- Damcott, C.M.; Pollin, T.I.; Reinhart, L.J.; Ott, S.H.; Shen, H.; Silver, K.D.; Mitchell, B.D.; Shuldiner, A.R. Polymorphisms in the Transcription Factor 7-Like 2 (TCF7L2) Gene Are Associated with Type 2 Diabetes in the Amish: Replication and evidence for a role in both insulin secretion and insulin resistance. Diabetes 2006, 55, 2654–2659. [Google Scholar] [CrossRef] [PubMed]
- Yutong, Y.; Lin, Y.; Zhang, Y.; Yang, J.; Zhang, Y.; Liu, H.; Zhang, B. Association between TCF7L2gene polymorphisms and susceptibility to Type 2 Diabetes Mellitus: A large Human Genome Epidemiology (HuGE) review and meta-analysis. BMC Med. Genet. 2009, 10, 15. [Google Scholar] [CrossRef]
- Peng, S.; Zhu, Y.; Lü, B.; Xu, F.; Li, X.; Lai, M. TCF7L2 gene polymorphisms and type 2 diabetes risk: A comprehensive and updated meta-analysis involving 121 174 subjects. Mutagenesis 2012, 28, 25–37. [Google Scholar] [CrossRef]
- Verma, S.; Srivastava, N.; Banerjee, M. PS 04-25 association of tcf7l2 and pparγ gene variants with type 2 diabetes mellitus in north indian population. J. Hypertens. 2016, 34, e139–e140. [Google Scholar] [CrossRef]
- Guewo-Fokeng, M.; Sobngwi, E.; Atogho-Tiedeu, B.; Donfack, O.S.; Noubiap, J.J.N.; Ngwa, E.N.; Mato-Mofo, E.P.; Fosso, P.P.; Djahmeni, E.; Djokam-Dadjeu, R.; et al. Contribution of the TCF7L2 rs7903146 (C/T) gene polymorphism to the susceptibility to type 2 diabetes mellitus in Cameroon. J. Diabetes Metab. Disord. 2015, 14, 1–5. [Google Scholar] [CrossRef][Green Version]
- Barros, C.; Araújo-Neto, A.; Lopes, T.; Barros, M.; Motta, F.; Canalle, R.; Nunes, L.; Rey, J.; Burbano, R.; Lima-Barros, M.; et al. Association of the rs7903146 and rs12255372 polymorphisms in the TCF7L2 gene with type 2 diabetes in a population from northeastern Brazil. Genet. Mol. Res. 2014, 13, 7889–7898. [Google Scholar] [CrossRef] [PubMed]
- Turki, A.; Al-Zaben, G.S.; Mtiraoui, N.; Marmmuoch, H.; Mahjoub, T.; Almawi, W.Y. Transcription factor-7-like 2 gene variants are strongly associated with type 2 diabetes in Tunisian Arab subjects. Gene 2013, 513, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Alsmadi, O.; Al-Rubeaan, K.; Mohamed, G.; Alkayal, F.; Al-Saud, H.; Abu Al-Saud, N.; Al-Daghri, N.; Mohammad, S.; Meyer, B.F. Weak or no association of TCF7L2 variants with Type 2 diabetes risk in an Arab population. BMC Med. Genet. 2008, 9, 72. [Google Scholar] [CrossRef]
- Saadi, H.; Nagelkerke, N.; Carruthers, S.G.; Benedict, S.; Abdulkhalek, S.; Reed, R.; Lukic, M.; Nicholls, M.G. Association of TCF7L2 polymorphism with diabetes mellitus, metabolic syndrome, and markers of beta cell function and insulin resistance in a population-based sample of Emirati subjects. Diabetes Res. Clin. Pract. 2008, 80, 392–398. [Google Scholar] [CrossRef]
- Shokouhi, S.; Delpisheh, A.; Haghani, K.; Mahdizadeh, M.; Bakhtiyari, S. Association of rs7903146, rs12255372, and rs290487 Polymorphisms in TCF7L2 Gene with Type 2 Diabetes in an Iranian Kurdish Ethnic Group. Clin. Lab. 2014, 60, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Martínez, P.P.; García-Rios, A.; Phillips, C.; Williams, C.; Gulseth, H.; Helal, O.; Blaak, E.; Kiec-Wilk, B.; Basu, S.; et al. Pleiotropic effects of TCF7L2 gene variants and its modulation in the metabolic syndrome: From the LIPGENE study. Atherosclerosis 2011, 214, 110–116. [Google Scholar] [CrossRef] [PubMed]
| Genetic Polymorphism | Assay ID | Amplified Region |
|---|---|---|
| rs7903146 | C__29347861_10 | TAGAGAGCTAAGCACTTTTTAGATA[C/T] TATATAATTTAATTGCCGTATGAGG |
| rs12255372 | C___291484_20 | TGCCCAGGAATATCCAGGCAAGAAT[G/T] ACCATATTCTGATAATTACTCAGGC |
| T2DM Group n = 150 | Control Group n = 100 | p Value * | |
|---|---|---|---|
| Gender | 52 M (34.7%)98 F (65.3%) | 36 M (36%)64 F (64%) | 0.82 |
| Age (years) | 58.32 ± 11.38 | 52.16 ± 12.78 | 0.048 |
| BMI (kg/m2) | 28.39 ± 4.55 | 25.72 ± 2.71 | <0.0001 |
| Glycemia (g/L) | 1.68 ± 0.63 | 1.0 ± 0.12 | <0.0001 |
| HbA1c (%) | 7.87 ± 1.800 | 6.20 ± 0.01 | <0.0001 |
| Cholesterol (g/L) | 1.87 ± 0.93 | 1.85 ± 0.90 | 0.61 |
| HDL-cholesterol (g/L) | 0.95 ± 0.73 | 0.91 ± 0.64 | 0.73 |
| LDL-cholesterol (g/L) | 1.33 ± 1.16 | 1.31 ± 0.95 | 0.66 |
| Triglycerides(g/L) | 1.32 ± 0.48 | 1.28 ± 0.53 | 0.49 |
| T2DM (n = 150) | Control (n = 100) | OR (CI) | p Value * | |
|---|---|---|---|---|
| rs7903146 | ||||
| CC | 50 (33.3%) | 36 (36.0%) | 1 | Reference |
| CT | 64 (42.7%) | 59 (59.0%) | 0.72 [0.65–1.30] | 0.14 |
| TT | 36 (24.0%) | 5 (5.0%) | 4.08 [1.95–11.80] | <0.0001 |
| C allele | 165 (54.8%) | 131 (65.5%) | 1 | 0.005 |
| T allele | 136 (45.2%) | 69 (34.5%) | 2.13 [1.12–7.31] | |
| rs12255372 | ||||
| GG | 42 (28.0%) | 30 (30.0%) | 1 | Reference |
| GT | 80 (53.3%) | 64 (64.0%) | 1.50 [0.91–2.62] | 0.11 |
| TT | 28 (18.7%) | 6 (6.0%) | 3.11 [1.33–7.24] | 0.004 |
| G allele | 164 (54.7%) | 124 (62.0%) | 1 | 0.01 |
| T allele | 136 (45.3%) | 76 (38.0%) | 2.01 [1.04–3.10] | |
| Variable | CC (n = 50) | TT (n = 36) | CT (n = 64) | p Value * |
|---|---|---|---|---|
| Age (year) | 58.28 ± 11.59 | 58.45 ± 10.75 | 58.27 ± 12.47 | 0.43 |
| BMI (kg/m2) | 28.26 ± 4.02 | 28.64 ± 3.75 | 27.96 ± 4.23 | 0.08 |
| HbA1c (%) | 8.01 ± 1.17 | 7.96 ± 1.70 | 7.75 ± 1.88 | 0.33 |
| Glycemia (g/L) | 1.69 ± 0.51 | 1.69 ± 0.76 | 1.66 ± 0.65 | 0.24 |
| Cholesterol (g/L) | 1.94 ± 0.99 | 1.82 ±1.00 | 1.82 ± 0.84 | 0.75 |
| HDL-cholesterol (g/L) | 1.35 ± 1.15 | 1.42 ± 0.66 | 1.10 ± 0.63 | 0.12 |
| LDL-cholesterol (g/L) | 1.24 ± 0.89 | 1.11 ± 0.68 | 1.53 ± 2.53 | 0.48 |
| Triglyceride (g/L) | 1.32 ± 0.40 | 1.26 ± 0.65 | 1.35 ± 0.43 | 0.70 |
| Variable | GG (n = 42) | TT (n = 28) | GT (n = 80) | p Value* |
|---|---|---|---|---|
| Age (year) | 58.37 ± 10.37 | 58.36 ± 11.03 | 58.25 ± 12.13 | 0.53 |
| BMI (kg/m2) | 28.33 ± 4.31 | 28.53 ± 4.43 | 28.01 ± 4.84 | 0.10 |
| HbA1c (%) | 7.97 ± 1.26 | 7.88 ± 1.23 | 7.82 ± 1.74 | 0.68 |
| Glycemia (g/L) | 1.67 ± 0.67 | 1.70 ± 0.83 | 1.69 ± 0.71 | 0.48 |
| Cholesterol (g/L) | 1.95 ± 0.97 | 1.85 ± 0.96 | 1.82 ± 0.90 | 0.74 |
| HDL-cholesterol (g/L) | 1.15 ± 0.75 | 1.46 ± 0.63 | 1.25 ± 0.96 | 0.33 |
| LDL-cholesterol (g/L) | 1.22 ± 0.72 | 1.27 ± 1.04 | 1.44 ± 2.28 | 0.83 |
| Triglyceride (g/L) | 1.35 ± 0.43 | 1.39 ± 0.55 | 1.28 ± 0.48 | 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhourch, S.; Arrouchi, H.; Mekkaoui, N.; Allou, Y.; Ghrifi, F.; Allam, L.; Elhafidi, N.; Belyamani, L.; Ibrahimi, A.; Elomri, N.; et al. Significant Association of Polymorphisms in the TCF7L2 Gene with a Higher Risk of Type 2 Diabetes in a Moroccan Population. J. Pers. Med. 2021, 11, 461. https://doi.org/10.3390/jpm11060461
Elhourch S, Arrouchi H, Mekkaoui N, Allou Y, Ghrifi F, Allam L, Elhafidi N, Belyamani L, Ibrahimi A, Elomri N, et al. Significant Association of Polymorphisms in the TCF7L2 Gene with a Higher Risk of Type 2 Diabetes in a Moroccan Population. Journal of Personalized Medicine. 2021; 11(6):461. https://doi.org/10.3390/jpm11060461
Chicago/Turabian StyleElhourch, Sarah, Housna Arrouchi, Nour Mekkaoui, Younes Allou, Fatima Ghrifi, Loubna Allam, Naima Elhafidi, Lahcen Belyamani, Azeddine Ibrahimi, Naoual Elomri, and et al. 2021. "Significant Association of Polymorphisms in the TCF7L2 Gene with a Higher Risk of Type 2 Diabetes in a Moroccan Population" Journal of Personalized Medicine 11, no. 6: 461. https://doi.org/10.3390/jpm11060461
APA StyleElhourch, S., Arrouchi, H., Mekkaoui, N., Allou, Y., Ghrifi, F., Allam, L., Elhafidi, N., Belyamani, L., Ibrahimi, A., Elomri, N., & Eljaoudi, R. (2021). Significant Association of Polymorphisms in the TCF7L2 Gene with a Higher Risk of Type 2 Diabetes in a Moroccan Population. Journal of Personalized Medicine, 11(6), 461. https://doi.org/10.3390/jpm11060461







