Dynamic Collaborations for the Development of Immune Checkpoint Blockade Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Data Sources
2.2. Variables and Data Sources
- An acquisition is when one company purchases all or most of another company’s shares to gain control of that company.
- An alliance is an agreement between two or more companies regarding a pharmaceutical product, technology, service, etc.
- A financing involves a company raising money publicly or privately through the sale of equity, debt, or royalty monetization.
2.3. Statistical Analysis
3. Results
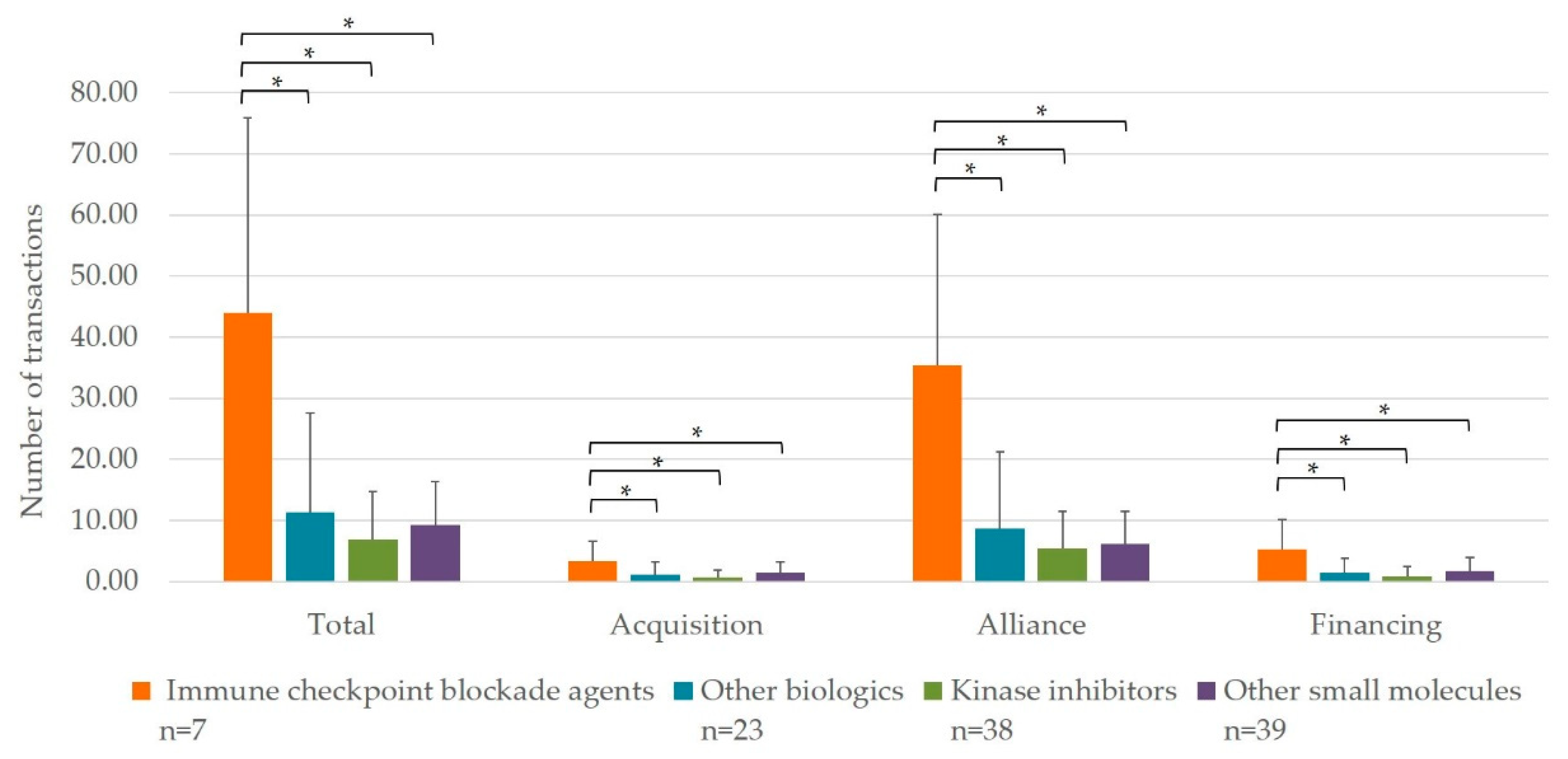
3.1. Interorganizational Transaction per Mechanism of Action
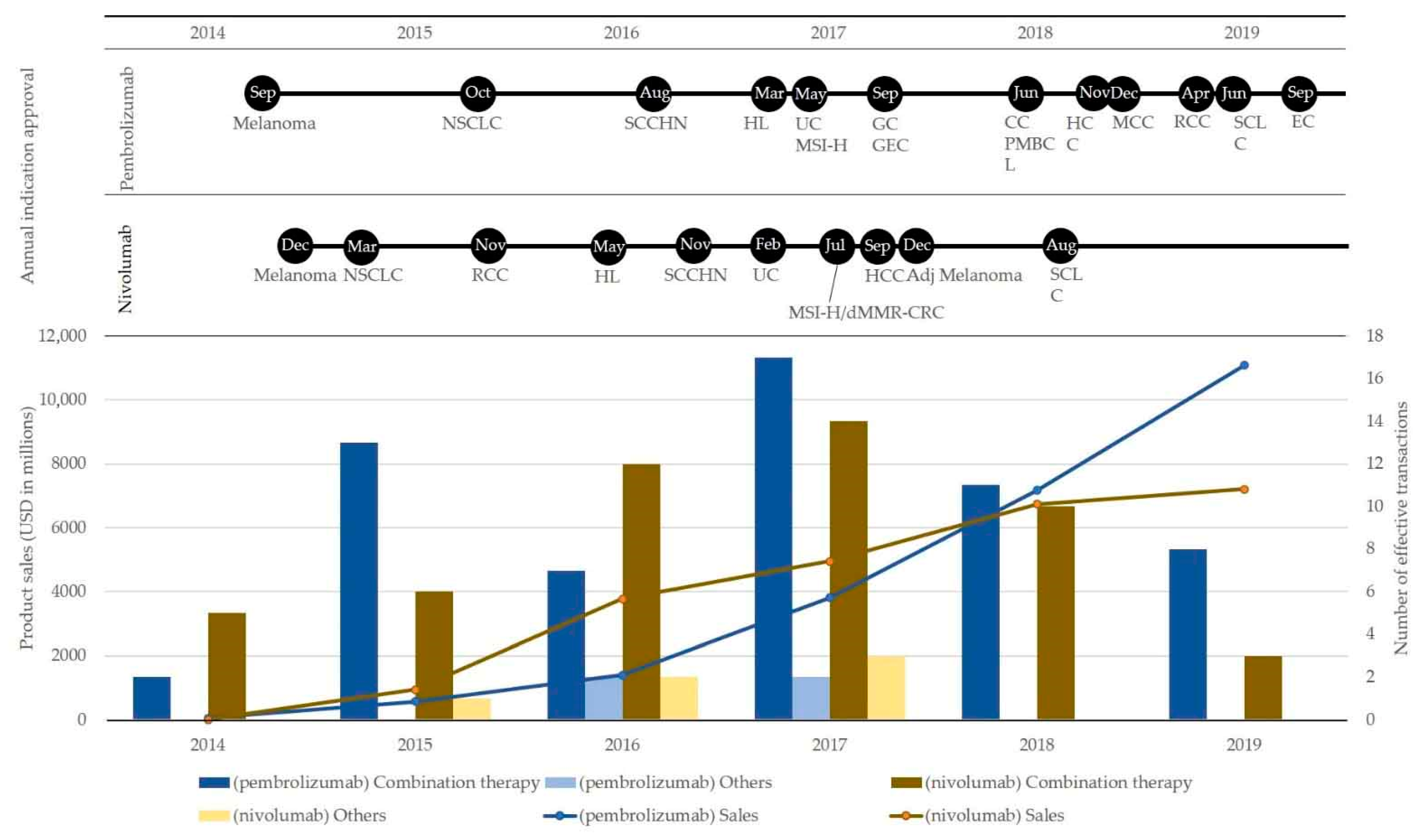
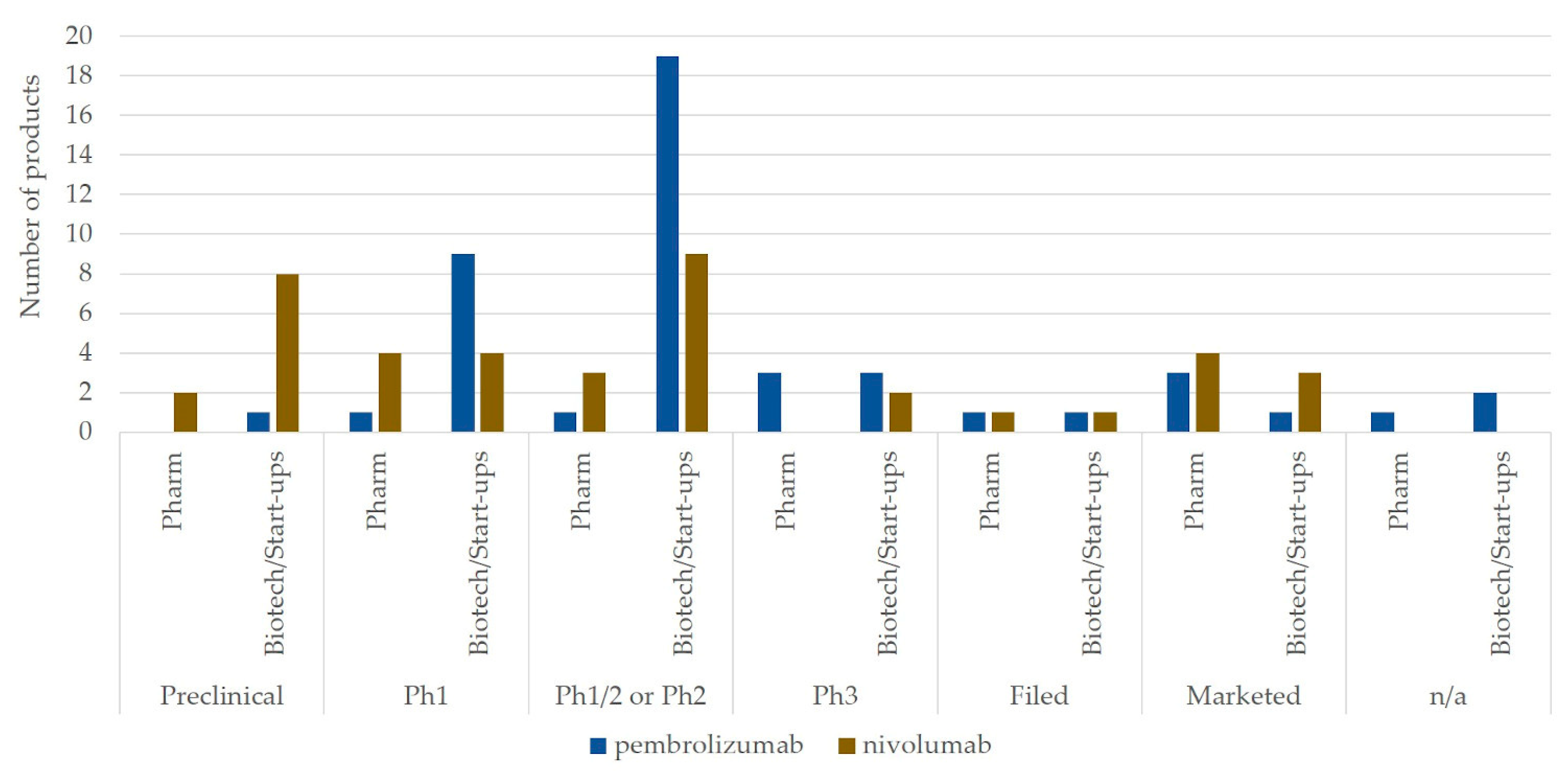
3.2. Market Landscape and Interorganizational Collaborations for Combination Therapy Development of Top Two Immune Checkpoint Blockades: Pembrolizumab and Nivolumab
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kola, I.; Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004, 3, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.P.; Van Brantner, V. Market watch: Estimating the cost of new drug development: Is it really $802 million? Health Aff. 2006, 25, 420–428. [Google Scholar] [CrossRef] [PubMed]
- DiMasi, J.A.; Hansen, R.W.; Grabowski, H.G. The price of innovation: New estimates of drug development costs. J. Health Econ. 2003, 22, 151–185. [Google Scholar] [CrossRef]
- Ciani, O.; Jommi, C. The role of health technology assessment bodies in shaping drug development. Drug Des. Devel. Ther. 2014, 8, 2273–2281. [Google Scholar] [CrossRef]
- Rugman, A.M.; Verbeke, A. A perspective on regional and global strategies of multinational enterprises. J. Int. Bus. Stud. 2004, 35, 3–18. [Google Scholar] [CrossRef]
- Teramae, F.; Makino, T.; Lim, Y.; Sengoku, S.; Kodama, K. International strategy for sustainable growth in multinational pharmaceutical companies. Sustainability 2020, 12, 867. [Google Scholar] [CrossRef]
- Sengoku, S.; Yoda, T.; Seki, A. Assessment of Pharmaceutical Research and Development Productivity With a Novel Net Present Value–based Project Database. Ther. Innov. Regul. Sci. 2011, 45, 175–185. [Google Scholar] [CrossRef]
- Bianchi, M.; Cavaliere, A.; Chiaroni, D.; Frattini, F.; Chiesa, V. Organisational modes for Open Innovation in the bio-pharmaceutical industry: An exploratory analysis. Technovation 2011, 31, 22–33. [Google Scholar] [CrossRef]
- Makino, T.; Sengoku, S.; Ishida, S.; Kodama, K. Trends in interorganizational transactions in personalized medicine development. Drug Discov. Today 2019, 24, 364–370. [Google Scholar] [CrossRef]
- Djurian, A.; Makino, T.; Lim, Y.; Sengoku, S.; Kodama, K. Trends of business-to-business transactions to develop innovative cancer drugs. Sustainability 2020, 12, 5535. [Google Scholar] [CrossRef]
- Wang, L.; Plump, A.; Ringel, M. Racing to define pharmaceutical R&D external innovation models. Drug Discov. Today 2015, 20, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Schuhmacher, A.; Germann, P.-G.; Trill, H.; Gassmann, O. Models for open innovation in the pharmaceutical industry. Drug Discov. Today 2013, 18, 1133–1137. [Google Scholar] [CrossRef]
- Makino, T.; Lim, Y.; Kodama, K. Strategic R&D transactions in personalized drug development. Drug Discov. Today 2018, 23, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T.; Chu, E. A History of Cancer Chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef]
- Chabner, B.A.; Roberts, T.G. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Sawyers, C. Targeted cancer therapy. Nature 2004, 432, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. Biology-driven cancer drug development: Back to the future. BMC Biol. 2010, 8, 1–12. [Google Scholar] [CrossRef][Green Version]
- Al-Lazikani, B.; Banerji, U.; Workman, P. Combinatorial drug therapy for cancer in the post-genomic era. Nat. Biotechnol. 2012, 30, 679–692. [Google Scholar] [CrossRef]
- Finn, O.J. Immuno-oncology: Understanding the function and dysfunction of the immune system in cancer. Ann. Oncol. 2012, 23, 8–11. [Google Scholar] [CrossRef]
- Hoos, A. Development of immuno-oncology drugs-from CTLA4 to PD1 to the next generations. Nat. Rev. Drug Discov. 2016, 15, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed on 5 December 2020).
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Han, X. Anti-PD-1/PD-L1 therapy of human cancer: Past, present, and future Find the latest version: Anti– PD-1/PD-L1 therapy of human cancer: Past, present, and future. J. Clin. Investig. 2015, 125, 3384–3391. [Google Scholar] [CrossRef]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Tseng, S.Y.; Otsuji, M.; Gorski, K.; Huang, X.; Slansky, J.E.; Pai, S.I.; Shalabi, A.; Shin, T.; Pardoll, D.M.; Tsuchiya, H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 2001, 193, 839–845. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed Death-1 Ligand 1 Interacts Specifically with the B7-1 Costimulatory Molecule to Inhibit T Cell Responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef]
- Park, J.J.; Omiya, R.; Matsumura, Y.; Sakoda, Y.; Kuramasu, A.; Augustine, M.M.; Yao, S.; Tsushima, F.; Narazaki, H.; Anand, S.; et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 2010, 116, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yu, S.; Zhu, B.; Bedoret, D.; Bu, X.; Duke-Cohan, L.M.F.; Umetsu, D.T.; Sharpe, A.H.; DeKruyff, R.H.; Freeman, G.J. RGMb is a novel binding partner for PD-l2 and its engagement with PD-l2 promotes respiratory tolerance. J. Exp. Med. 2014, 211, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef]
- Dong, H.; Zhu, G.; Tamada, K.; Flies, D.B.; Van Deursen, J.M.A.; Chen, L. B7-H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity 2004, 20, 327–336. [Google Scholar] [CrossRef]
- Chen, L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004, 4, 336–347. [Google Scholar] [CrossRef]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef]
- Lifearc, A. LifeArc: Case Study-Keytruda. Available online: https://www.lifearc.org/case-studies/keytruda-new-generation-cancer-treatment/ (accessed on 13 December 2020).
- BMS Newsroom: Bristol-Myers Squibb to Acquire Medarex. Available online: http://news.bms.com/press-release/partnering-news/bristol-myers-squibb-acquire-medarex (accessed on 13 December 2020).
- Yu, J.X.; Hodge, J.P.; Oliva, C.; Neftelinov, S.T. Trends in clinical development for PD-1 / PD-L1 inhibitors. Nat. Rev. Drug Discov. 2019, 18, 13–14. [Google Scholar]
- Tang, J.; Yu, J.X.; Hubbard-Lucey, V.M.; Neftelinov, S.T.; Hodge, J.P.; Lin, Y. The clinical trial landscape for PD1/PDl1 immune checkpoint inhibitors. Nat. Rev. Drug Discov. 2018, 17, 854–855. [Google Scholar] [CrossRef]
- Definition of Mechanism of Action. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/mechanism-of-action (accessed on 1 November 2020).
- CenterWatch. Available online: https://www.centerwatch.com/directories/1067-fda-approved-drugs (accessed on 20 July 2019).
- Biomedtracker Deal Search. Available online: https://www.biomedtracker.com/DealSearch.cfm (accessed on 26 November 2020).
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front. Pharmacol. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Zappasodi, R.; Merghoub, T.; Wolchok, J.D. Emerging Concepts for Immune Checkpoint Blockade-Based Combination Therapies. Cancer Cell 2018, 33, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Huang, Z.; Teng, F.; Xing, L.; Yu, J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat. Rev. 2015, 41, 868–876. [Google Scholar] [CrossRef]
- Schulze, U.; Ringel, M. What matters most in commercial success: First-in-class or best-in-class. Nat. Rev. Drug Discov. 2013, 12, 419–420. [Google Scholar] [CrossRef]
- Teramae, F.; Makino, T.; Sengoku, S.; Lim, Y.; Natori, T.; Kodama, K. Research on pharmaceutical product life cycle patterns for sustainable growth. Sustainability 2020, 12, 8938. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
| Average | SD | |
|---|---|---|
| Co-promotion | 3.571 | 2.225 |
| Includes Contract | 0.714 | 0.756 |
| Includes Equity | 2.143 | 2.116 |
| Includes Royalty or Profit Split Information | 9.000 | 5.916 |
| Intra-biotech Deal | 4.714 | 6.396 |
| Marketing-licensing | 0.714 | 1.496 |
| Product or Technology Swap | 0.429 | 0.535 |
| R+D and Marketing-licensing | 14.000 | 9.678 |
| Trial Collaborations | 24.429 | 18.636 |
| Reverse Licensing | 0.286 | 0.488 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djurian, A.; Makino, T.; Lim, Y.; Sengoku, S.; Kodama, K. Dynamic Collaborations for the Development of Immune Checkpoint Blockade Agents. J. Pers. Med. 2021, 11, 460. https://doi.org/10.3390/jpm11060460
Djurian A, Makino T, Lim Y, Sengoku S, Kodama K. Dynamic Collaborations for the Development of Immune Checkpoint Blockade Agents. Journal of Personalized Medicine. 2021; 11(6):460. https://doi.org/10.3390/jpm11060460
Chicago/Turabian StyleDjurian, Arisa, Tomohiro Makino, Yeongjoo Lim, Shintaro Sengoku, and Kota Kodama. 2021. "Dynamic Collaborations for the Development of Immune Checkpoint Blockade Agents" Journal of Personalized Medicine 11, no. 6: 460. https://doi.org/10.3390/jpm11060460
APA StyleDjurian, A., Makino, T., Lim, Y., Sengoku, S., & Kodama, K. (2021). Dynamic Collaborations for the Development of Immune Checkpoint Blockade Agents. Journal of Personalized Medicine, 11(6), 460. https://doi.org/10.3390/jpm11060460