Application of Janus Kinase Inhibitors in Atopic Dermatitis: An Updated Systematic Review and Meta-Analysis of Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Literature Search
2.2. Study Selection and Eligibility
2.3. Data Extraction and Efficacy and Safety Outcomes
2.4. Risk of Bias Assessment
2.5. Data Synthesis and Statistical Analyses
3. Results
3.1. Search Results
3.2. Characteristics of Eligible Studies
3.3. Risk of Bias Assessment
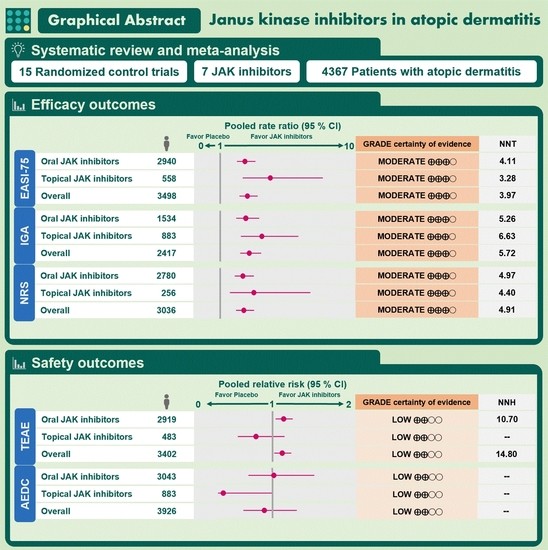
3.4. Efficacy Outcomes
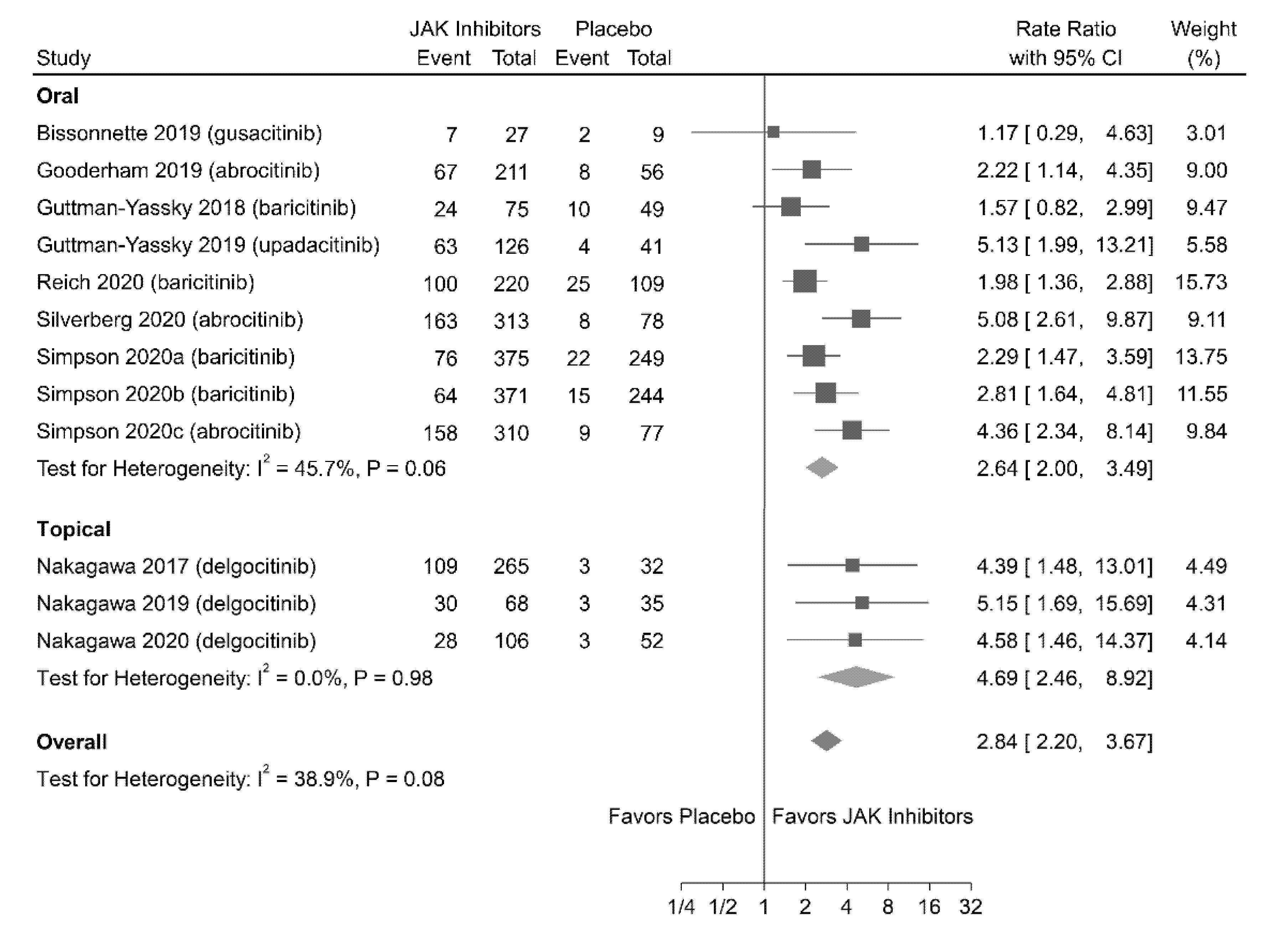
3.4.1. EASI-75 Response
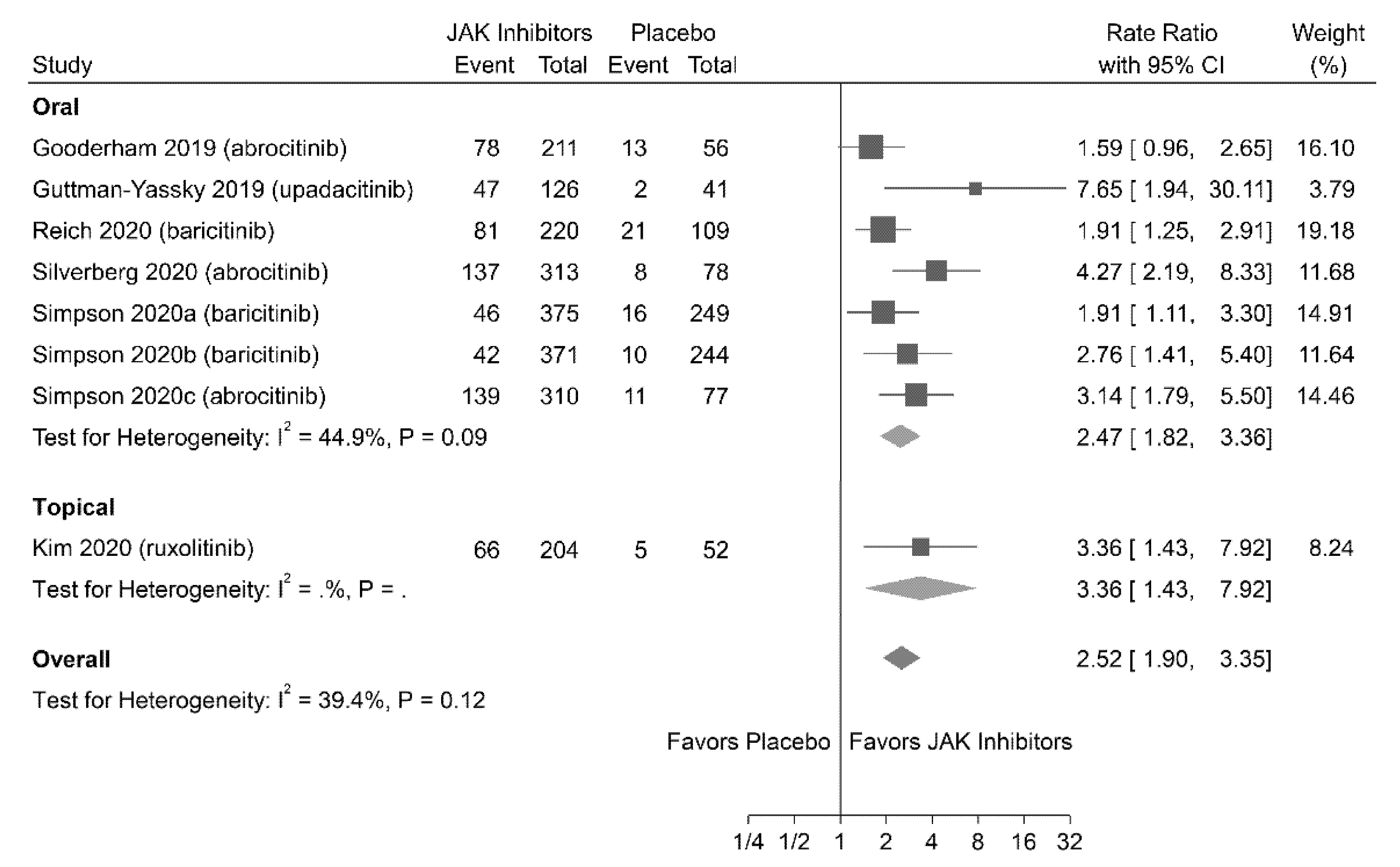
3.4.2. IGA Response
3.4.3. Pruritus-NRS Response
3.4.4. Subgroup Analyses and Meta-Regression of Efficacy Outcomes
3.5. Safety Outcomes
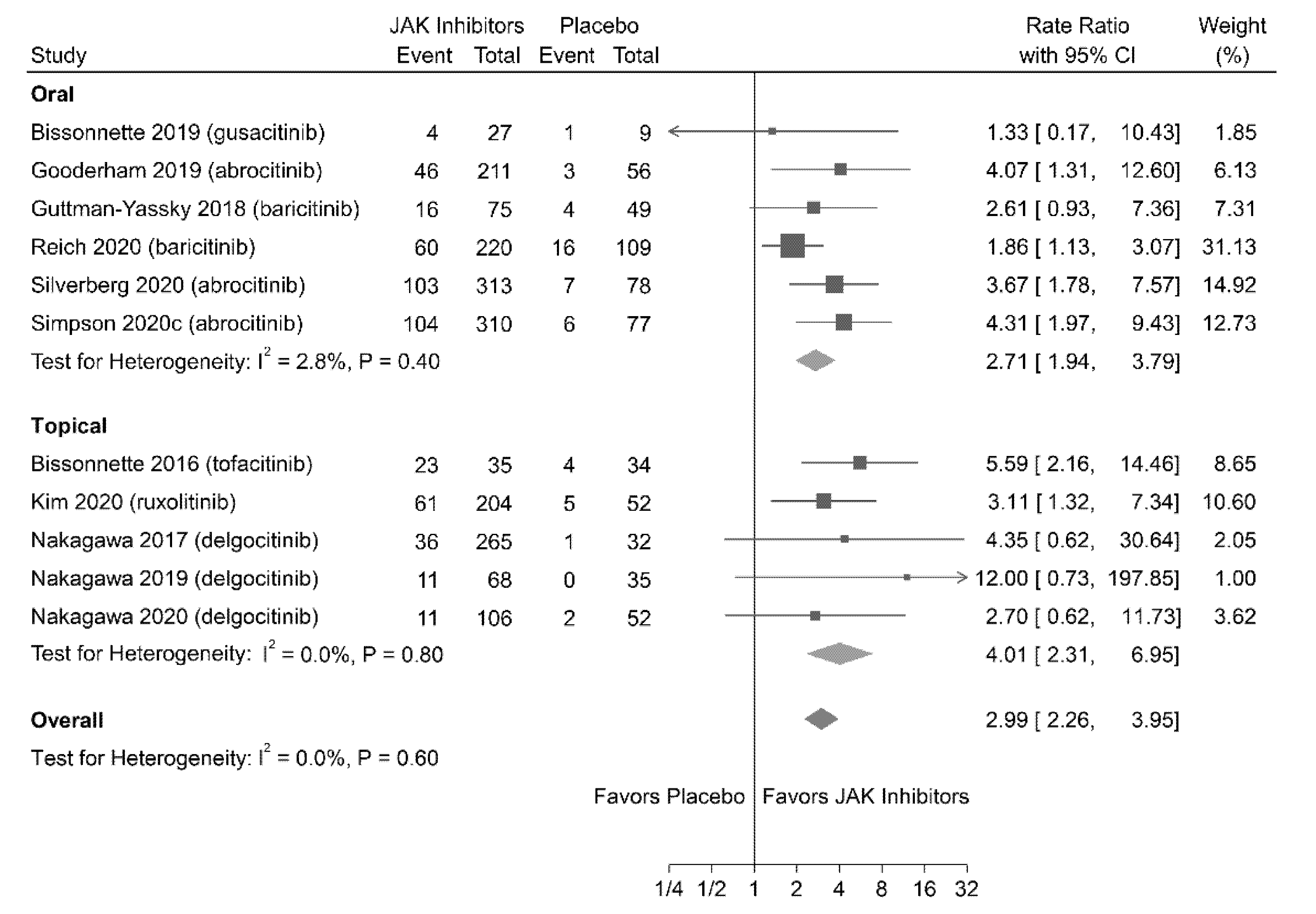
3.5.1. TEAEs
3.5.2. AEs Leading to Drug Discontinuation
3.5.3. Sensitivity Analyses
3.6. Publication Bias
3.7. GRADE Approach for CoE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silverberg, J.I.; Hanifin, J.M. Adult eczema prevalence and associations with asthma and other health and demographic factors: A US population-based study. J. Allergy Clin. Immunol. 2013, 132, 1132–1138. [Google Scholar] [CrossRef]
- Bylund, S.; von Kobyletzki, L.B.; Svalstedt, M.; Svensson, Å. Prevalence and incidence of atopic dermatitis: A systematic review. Acta Derm. Venereol. 2020, 100, adv00160. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Kaufman, B.P.; Guttman-Yassky, E.; Alexis, A.F. Atopic dermatitis in diverse racial and ethnic groups-Variations in epidemiology, genetics, clinical presentation and treatment. Exp. Dermatol. 2018, 27, 340–357. [Google Scholar] [CrossRef]
- Nettis, E.; Ortoncelli, M.; Pellacani, G.; Foti, C.; Di Leo, E.; Patruno, C.; Rongioletti, F.; Argenziano, G.; Ferrucci, S.M.; Macchia, L.; et al. A multicenter study on the prevalence of clinical patterns and clinical phenotypes in adult atopic dermatitis. J. Investig. Allergol. Clin. Immunol. 2020, 30, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Laughter, M.R.; Maymone, M.B.; Mashayekhi, S.; Arents, B.W.; Karimkhani, C.; Langan, S.M.; Dellavalle, R.P.; Flohr, C. The global burden of atopic dermatitis: Lessons from the global burden of disease study 1990–2017. Br. J. Dermatol. 2021, 184, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, L.F.; Tom, W.L.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis, Section 2: Management and treatment of atopic dermatitis with topical therapies. J. Am. Acad. Dermatol. 2014, 71, 116–132. [Google Scholar] [CrossRef]
- Ring, J.; Alomar, A.; Bieber, T.; Deleuran, M.; Fink-Wagner, A.; Gelmetti, C.; Gieler, U.; Lipozencic, J.; Luger, T.; Oranje, A.P.; et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Katayama, I.; Aihara, M.; Ohya, Y.; Saeki, H.; Shimojo, N.; Shoji, S.; Taniguchi, M.; Yamada, H. Japanese guidelines for atopic dermatitis 2017. Allergol. Int. 2017, 66, 230–247. [Google Scholar] [CrossRef]
- Worm, M.; Francuzik, W.; Kraft, M.; Alexiou, A. Modern therapies in atopic dermatitis: Biologics and small molecule drugs. J. Dtsch. Dermatol. Ges. 2020, 18, 1085–1092. [Google Scholar] [CrossRef]
- Dattola, A.; Bennardo, L.; Silvestri, M.; Nisticò, S.P. What′s new in the treatment of atopic dermatitis? Dermatol. Ther. 2019, 32, e12787. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Fukada, T.; Nishida, K.; Nakayama, M.; Matsuda, M.; Miura, I.; Dainichi, T.; Fukuda, S.; Kabashima, K.; Nakaoka, S.; et al. Hyperactivation of JAK1 tyrosine kinase induces stepwise, progressive pruritic dermatitis. J. Clin. Invest. 2016, 126, 2064–2076. [Google Scholar] [CrossRef] [PubMed]
- Amano, W.; Nakajima, S.; Kunugi, H.; Numata, Y.; Kitoh, A.; Egawa, G.; Dainichi, T.; Honda, T.; Otsuka, A.; Kimoto, Y.; et al. The Janus kinase inhibitor JTE-052 improves skin barrier function through suppressing signal transducer and activator of transcription 3 signaling. J. Allergy Clin. Immunol. 2015, 136, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Fridman, J.S.; Scherle, P.A.; Collins, R.; Burn, T.; Neilan, C.L.; Hertel, D.; Contel, N.; Haley, P.; Thomas, B.; Shi, J.; et al. Preclinical evaluation of local JAK1 and JAK2 inhibition in cutaneous inflammation. J. Invest. Dermatol. 2011, 131, 1838–1844. [Google Scholar] [CrossRef]
- Jin, W.; Huang, W.; Chen, L.; Jin, M.; Wang, Q.; Gao, Z.; Jin, Z. Topical application of JAK1/JAK2 inhibitor momelotinib exhibits significant anti-inflammatory responses in DNCB-induced atopic dermatitis model mice. Int. J. Mol. Sci. 2018, 19, 3973. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 17, 78. [Google Scholar] [CrossRef]
- Honstein, T.; Werfel, T. The show must go on: An update on clinical experiences and clinical studies on novel pharmaceutical developments for the treatment of atopic dermatitis. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 386–394. [Google Scholar] [CrossRef]
- Gadina, M.; Le, M.T.; Schwartz, D.M.; Silvennoinen, O.; Nakayamada, S.; Yamaoka, K.; O’Shea, J.J. Janus kinases to jakinibs: From basic insights to clinical practice. Rheumatology 2019, 58, i4–i16. [Google Scholar] [CrossRef]
- Bieber, T.; Thyssen, J.P.; Reich, K.; Simpson, E.L.; Katoh, N.; Torrelo, A.; De Bruin-Weller, M.; Thaci, D.; Bissonnette, R.; Gooderham, M.; et al. Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. J. Eur. Acad. Dermatol. Venereol. 2020, 35, 476–485. [Google Scholar] [CrossRef]
- Arora, C.J.; Khattak, F.A.; Yousafzai, M.T.; Ibitoye, B.M.; Shumack, S. The effectiveness of Janus kinase inhibitors in treating atopic dermatitis: A systematic review and meta-analysis. Dermatol. Ther. 2020, 33, e13685. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. Available online: www.training.cochrane.org/handbook (accessed on 25 January 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Sarri, G.; Patorno, E.; Yuan, H.; Guo, J.J.; Bennett, D.; Wen, X.; Zullo, A.R.; Largent, J.; Panaccio, M.; Gokhale, M.; et al. Framework for the synthesis of non-randomised studies and randomised controlled trials: A guidance on conducting a systematic review and meta-analysis for healthcare decision making. BMJ Evid. Based Med. 2020, 9. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Chau, M.; Green, S.E.; Forbes, A. Methods to select results to include in meta-analyses deserve more consideration in systematic reviews. J. Clin. Epidemiol. 2015, 68, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Cadham, C.J.; Jayasekera, J.C.; Advani, S.M.; Fallon, S.J.; Stephens, J.L.; Braithwaite, D.; Jeon, J.; Cao, P.; Levy, D.T.; Meza, R.; et al. Smoking cessation interventions for potential use in the lung cancer screening setting: A systematic review and meta-analysis. Lung Cancer 2019, 135, 205–216. [Google Scholar] [CrossRef]
- Ueta, T.; Noda, Y.; Toyama, T.; Yamaguchi, T.; Amano, S. Systemic vascular safety of ranibizumab for age-related macular degeneration: Systematic review and meta-analysis of randomized trials. Ophthalmology 2014, 121, 2193–2203. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 18, 25. [Google Scholar] [CrossRef]
- Hartung, J.; Knapp, G. On tests of the overall treatment effect in meta-analysis with normally distributed responses. Stat. Med. 2001, 20, 1771–1782. [Google Scholar] [CrossRef]
- Saueressig, T.; Owen, P.J.; Zebisch, J.; Herbst, M.; Belavy, D.L. Evaluation of exercise interventions and outcomes after hip arthroplasty: A systematic review and meta-analysis. JAMA Netw. Open 2021, 4, e210254. [Google Scholar] [CrossRef]
- Mc Cord, K.A.; Ewald, H.; Agarwal, A.; Glinz, D.; Aghlmandi, S.; Ioannidis, J.P.; Hemkens, L.G. Treatment effects in randomised trials using routinely collected data for outcome assessment versus traditional trials: Meta-research study. BMJ 2021, 372, n450. [Google Scholar] [CrossRef]
- Janiaud, P.; Axfors, C.; Schmitt, A.M.; Gloy, V.; Ebrahimi, F.; Hepprich, M.; Smith, E.R.; Haber, N.A.; Khanna, N.; Moher, D.; et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: A systematic review and meta-analysis. JAMA 2021, 325, 1185–1195. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Kaino, H.; Nagata, T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J. Am. Acad. Dermatol. 2020, 82, 823–831. [Google Scholar] [CrossRef]
- Reich, K.; Kabashima, K.; Peris, K.; Silverberg, J.I.; Eichenfield, L.F.; Bieber, T.; Kaszuba, A.; Kolodsick, J.; Yang, F.E.; Gamalo, M.; et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2020, 156, 1333–1343. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Simpson, E.L.; Thyssen, J.P.; Gooderham, M.; Chan, G.; Feeney, C.; Biswas, P.; Valdez, H.; DiBonaventura, M.; Nduaka, C.; et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2020, 156, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Lacour, J.P.; Spelman, L.; Galimberti, R.; Eichenfield, L.; Bissonnette, R.; King, B.; Thyssen, J.; Silverberg, J.; Bieber, T.; et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: Results from two randomized monotherapy phase III trials. Br. J. Dermatol. 2020, 183, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Sinclair, R.; Forman, S.; Wollenberg, A.; Aschoff, R.; Cork, M.; Bieber, T.; Thyssen, J.P.; Yosipovitch, G.; Flohr, C.; et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 2020, 396, 255–266. [Google Scholar] [CrossRef]
- Bissonnette, R.; Papp, K.A.; Poulin, Y.; Gooderham, M.; Gooderham, M.; Raman, M.; Mallbris, L.; Wang, C.; Purohit, V.; Mamolo, C.; et al. Topical tofacitinib for atopic dermatitis: A phase IIa randomized trial. Br. J. Dermatol. 2016, 175, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Gooderham, M.J.; Forman, S.B.; Bissonnette, R.; Beebe, J.S.; Zhang, W.; Banfield, C.; Zhu, L.; Papacharalambous, J.; Vincent, M.S.; Peeva, E. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: A phase 2 randomized clinical trial. JAMA Dermatol. 2019, 155, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Silverberg, J.I.; Nemoto, O.; Forman, S.B.; Wilke, A.; Prescilla, R.; de la Peña, A.; Nunes, F.P.; Janes, J.; Gamalo, M.; et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: A phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J. Am. Acad. Dermatol. 2019, 80, 913–921.e9. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Thaçi, D.; Pangan, A.L.; Hong, H.C.; Papp, K.A.; Reich, K.; Beck, L.A.; Mohamed, M.F.; Othman, A.A.; Anderson, J.K.; et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2020, 145, 877–884. [Google Scholar] [CrossRef]
- Kim, B.S.; Howell, M.D.; Sun, K.; Papp, K.; Papp, K.; Nasir, A.; Kuligowski, M.E.; INCB 18424-206 Study Investigators. Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. J. Allergy Clin. Immunol. 2020, 145, 572–582. [Google Scholar] [CrossRef]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Nagata, T. Efficacy and safety of topical JTE-052, a Janus kinase inhibitor, in Japanese adult patients with moderate-to-severe atopic dermatitis: A phase II, multicentre, randomized, vehicle-controlled clinical study. Br. J. Dermatol. 2018, 178, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Oda, M.; Kabashima, K.; Nagata, T. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J. Allergy Clin. Immunol 2019, 144, 1575–1583. [Google Scholar] [CrossRef]
- Bissonnette, R.; Maari, C.; Forman, S.; Bhatia, N.; Lee, M.; Fowler, J.; Tyring, S.; Pariser, D.; Sofen, H.; Dhawan, S.; et al. The oral Janus kinase/spleen tyrosine kinase inhibitor ASN002 demonstrates efficacy and improves associated systemic inflammation in patients with moderate-to-severe atopic dermatitis: Results from a randomized double-blind placebo-controlled study. Br. J. Dermatol. 2019, 181, 733–742. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Syn. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Montori, V.; Vist, G.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Djulbegovic, B.; Atkins, D.; Falck-Ytter, Y.; et al. GRADE guidelines: 5. Rating the quality of evidence--publication bias. J. Clin. Epidemiol. 2011, 64, 1277–1282. [Google Scholar] [CrossRef]
- Schmitt, J.; Langan, S.; Deckert, S.; Svensson, A.; von Kobyletzki, L.; Thomas, K.; Spuls, P. Assessment of clinical signs of atopic dermatitis: A systematic review and recommendation. J. Allergy Clin. Immunol. 2013, 132, 1337–1347. [Google Scholar] [CrossRef]
- Fishbein, A.B.; Silverberg, J.I.; Wilson, E.J.; Ong, P.Y. Update on atopic dermatitis: Diagnosis, severity assessment, and treatment selection. J. Allergy Clin. Immunol. Pract. 2020, 8, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Futamura, M.; Leshem, Y.A.; Thomas, K.S.; Nankervis, H.; Williams, H.C.; Simpson, E.; Information, P.E.K.F.C. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: Many options, no standards. J. Am. Acad. Dermatol. 2016, 74, 288–294. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Margolis, D.J.; Boguniewicz, M.; Fonacier, L.; Grayson, M.H.; Ong, P.Y.; Fuxench, Z.C.; Simpson, E.L. Validation of five patient-reported outcomes for atopic dermatitis severity in adults. Br. J. Dermatol. 2020, 182, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Pinter, A.; Pulka, G.; Poulin, Y.; Bouaziz, J.D.; Wollenberg, A.; Murrell, D.F.; Alexis, A.; Lindsey, L.; Ahmad, F.; et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J. Allergy Clin. Immunol. 2020, 145, 173–182. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Blauvelt, A.; Eichenfield, L.F.; Paller, A.S.; Armstrong, A.W.; Drew, J.; Gopalan, R.; Simpson, E.L. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: A phase 2b randomized clinical trial. JAMA Dermatol. 2020, 156, 411–420. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA Approves Boxed Warning about Increased Risk of Blood Clots and Death with Higher Dose of Arthritis and Ulcerative Colitis Medicine Tofacitinib (Xeljanz, Xeljanz XR). 2019. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and (accessed on 2 April 2021).
- Brunner, P.M.; Guttman-Yassky, E. Racial differences in atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 122, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Leonard, A.; Pavel, A.B.; Malik, K.; Raja, A.; Glickman, J.; Estrada, Y.D.; Peng, X.; Del Duca, E.; Sanz-Cabanillas, J.; et al. Age-specific changes in the molecular phenotype of patients with moderate-to-severe atopic dermatitis. J. Allergy Clin. Immunol. 2019, 144, 144–156. [Google Scholar] [CrossRef]
- Løset, M.; Brown, S.J.; Saunes, M.; Hveem, K. Genetics of atopic dermatitis: From DNA sequence to clinical relevance. Dermatology 2019, 235, 355–364. [Google Scholar] [CrossRef]
- Renert-Yuval, Y.; Guttman-Yassky, E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann. Allergy Asthma Immunol. 2020, 124, 28–35. [Google Scholar] [CrossRef]
- Bieber, T. Interleukin-13: Targeting an underestimated cytokine in atopic dermatitis. Allergy 2020, 75, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Mendes, D.; Alves, C.; Batel-Marques, F. Number needed to treat (NNT) in clinical literature: An appraisal. BMC Med. 2017, 15, 112. [Google Scholar] [CrossRef]
- Citrome, L. Quantifying clinical relevance. Innov. Clin. Neurosci. 2014, 11, 26–30. [Google Scholar] [PubMed]
| Source | Clinical Trial Identifier | Study Design | Study Period | No. of Participants (Age) | Definition of AD | Severity of AD | Interventions | Mechanism of Inhibition | Endpoint | Efficacy Outcomes | Safety Outcomes | COI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bissonnette 2016 | NCT02001181 | Phase II RCT | December 2013–September 2014 | 69 (18–60 y) | Hanifin and Rajka criteria | Mild to moderate | Treatment: topical tofacitinib, 2% twice daily Placebo: topical control vehicle twice daily | JAK1 and JAK3 | Week 4 | IGA, EASI, and BSA | TEAEs and SAEs | Yes |
| Bissonnette 2019 | NCT03139981 | Phase I RCT | April 2017– November 2017 | 36 (18–75 y) | AAD guideline | Moderate to severe | Treatment: oral gusacitinib, 20 mg, 40 mg, 80 mg once daily Placebo: oral vehicle once daily | JAK1, JAK2, JAK3, TYK2, and SYK | Week 4 | IGA, EASI, pruritus NRS, and BSA | TEAEs, SAEs, and AEDC | Yes |
| Gooderham 2019 | NCT02780167 | Phase II RCT | April 2016– April 2017 | 267 (18–75 y) | AAD guideline | Moderate to severe | Treatment: oral abrocitinib, 10 mg, 30 mg, 100 mg, 200 mg once daily Placebo: oral control vehicle once daily | JAK1 | Week 12 | IGA, EASI, pruritus NRS, BSA, SCORAD, DLQI, HADS, and POEM | TEAEs and SAEs | Yes |
| Guttman-Yassky 2018 | NCT02576938 | Phase II RCT | February 2016– March 2017 | 124 (≥18 y) | Hanifin and Rajka criteria | Moderate to severe | Treatment: oral baricitinib, 2 mg and 4 mg once daily plus TCSPlacebo: oral control vehicle once daily | JAK1 and JAK2 | Week 16 | IGA, EASI, pruritus NRS, SCORAD, DLQI, and POEM | TEAEs, SAEs, and AEDC | Yes |
| Guttman-Yassky 2019 | NCT02925117 | Phase II RCT | November 2016– April 2017 | 167 (18–75 y) | Hanifin and Rajka criteria | Moderate to severe | Treatment: oral upadacitinib, 7.5 mg, 15 mg, 30 mg once daily Placebo: oral control vehicle once daily | JAK1 | Week 16 | IGA, EASI, pruritus NRS, BSA, SCORAD, and POEM | TEAEs, SAEs, and AEDC | Yes |
| Kim 2020 | NCT03011892 | Phase II RCT | January 2017– November 2017 | 307 (18–70 y) | NA | Mild to moderate | Treatment: topical ruxolitinib, 0.15%, 0.5%, 1.5% once daily, and 1.5% twice daily; Placebo: topical control vehicle twice daily | JAK1 and JAK2 | Week 8 | pruritus NRS, and Skindex-16 | TEAEs, SAEs, and AEDC | Yes |
| Nakagawa 2017 | JapicCTI-152887 | Phase II RCT | April 2015– May 2016 | 327 (16–65 y) | JDA guideline | Moderate to severe | Treatment: topical delgocitinib, 0.25%, 0.5%, 1%, 3% twice daily Placebo: topical control vehicle twice daily | JAK1, JAK2, JAK3, and TYK2 | Week 4 | IGA, mEASI, pruritus NRS, and BSA | SAEs and AEDC | Yes |
| Nakagawa 2019 | JapicCTI-173553 | Phase II RCT | March 2017– February 2018 | 103 (2–15 y) | JDA guideline | Mild to moderate | Treatment: topical delgocitinib, 0.25%, 0.5% twice daily Placebo: topical control vehicle twice daily | JAK1, JAK2, JAK3, and TYK2 | Week 4 | IGA, mEASI, pruritus NRS, and BSA | SAEs and AEDC | Yes |
| Nakagawa 2020 | JapicCTI-173554 | Phase III RCT | March 2017– September 2018 | 158 (≥16 y) | JDA guideline | Moderate to severe | Treatment: topical delgocitinib, 0.5% twice daily Placebo: topical control vehicle twice daily | JAK1, JAK2, JAK3, and TYK2 | Week 4 | IGA, mEASI, pruritus NRS, BSA, and Skindex-16 | TEAEs, SAEs, and AEDC | Yes |
| Reich 2020 | NCT03733301 (BREEZE-AD7) | Phase III RCT | November 2018–August 2019 | 329 (≥18 y) | AAD guideline | Moderate to severe | Treatment: oral baricitinib, 2 mg, 4 mg once daily plus TCS Placebo: oral control vehicle once daily | JAK1 and JAK2 | Week 16 | IGA, EASI-50, EASI-75, EASI-90, pruritus NRS, pain NRS, SCORAD, ADSS, POEM, HADS, DLQI, and WPAI | TEAEs, SAEs, and AEDC | Yes |
| Silverberg 2020 | NCT03575871 (JADE MONO-2) | Phase III RCT | June 2018– August 2019 | 391 (≥12 y) | Hanifin and Rajka criteria | Moderate to severe | Treatment: oral abrocitinib, 100 mg and 200 mg once daily Placebo: oral control vehicle once daily | JAK1 | Week 12 | IGA, EASI, pruritus NRS, PSAAD, DLQI, CDLQI, POEM, and HADS | SAEs and AEDC | Yes |
| Simpson 2020a | NCT03334396 (BREEZE-AD1) | Phase III RCT | November 2017–January 2019 | 624 (≥18 y) | AAD guideline | Moderate to severe | Treatment: oral baricitinib, 1 mg, 2 mg, 4 mg once daily Placebo: oral control vehicle once daily | JAK1 and JAK2 | Week 16 | IGA, EASI, pruritus NRS, pain NRS, SCORAD, and ADSS | TEAEs, SAEs, and AEDC | Yes |
| Simpson 2020b | NCT03334422 (BREEZE-AD2) | Phase III RCT | November 2017–December 2018 | 615 (≥18 y) | AAD guideline | Moderate to severe | Treatment: oral baricitinib, 1 mg, 2 mg, 4 mg once daily Placebo: oral control vehicle once daily | JAK1 and JAK2 | Week 16 | IGA, EASI, pruritus NRS, pain NRS, SCORAD, and ADSS | TEAEs, SAEs, and AEDC | Yes |
| Simpson 2020c | NCT03349060 (JADE MONO-1) | Phase III RCT | December 2017–March 2019 | 387 (≥12 y) | Hanifin and Rajka criteria | Moderate to severe | Treatment: oral abrocitinib, 100 mg and 200 mg once daily Placebo: oral control vehicle once daily | JAK1 | Week 12 | IGA, EASI, pruritus NRS, PSAAD, DLQI, CDLQI, and POEM | TEAEs, SAEs, and AEDC | Yes |
| BREEZE-AD4 2020 * | NCT03428100 (BREEZE-AD4) | Phase III RCT | May 2018 | 463 (≥18 y) | AAD guideline | Moderate to severe | Treatment: oral baricitinib, 1 mg, 2 mg, 4 mg once daily Placebo: oral control vehicle once daily | JAK1 and JAK2 | Week 16 | NA | TEAEs, SAEs, and AEDC | Yes |
| Treatment-Emergent Adverse Events | Meta-Regression | Adverse Events Leading to Drug Discontinuation | Meta-Regression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroups | No. of Studies | Pooled RR (95% CI) | p-Value | I2 (%) | τ2 | p-Value | No. of Studies | Pooled RR (95% CI) | p-Value | I2 (%) | τ2 | p-Value |
| Overall | 12 | 1.14 (1.02 to 1.28) * | 0.023 | 52.0 | 14 | 0.89 (0.57 to 1.38) | 0.621 | 0.0 | ||||
| Route of administration | 0.013 | 0.033 | 0 | 0.064 | ||||||||
| Oral | 9 | 1.18 (1.06 to 1.32) ** | 0.003 | 48.3 | 9 | 1.03 (0.64 to 1.64) | 0.917 | 0.0 | ||||
| Topical | 3 | 0.77 (0.49 to 1.20) | 0.255 | 25.2 | 5 | 0.26 (0.07 to 1.02) | 0.054 | 0.0 | ||||
| Severity of atopic dermatitis | 0.012 | 0.021 | 0 | 0.036 | ||||||||
| Mild to moderate | 2 | 0.73 (0.47 to 1.13) | 0.163 | 33.1 | 3 | 0.16 (0.03 to 0.84) * | 0.031 | 0.0 | ||||
| Moderate to severe | 10 | 1.18 (1.06 to 1.31) ** | 0.002 | 43.7 | 11 | 1.01 (0.64 to 1.60) | 0.929 | 0.0 | ||||
| Age of participants | 0.019 | 0.483 | 0 | 0.200 | ||||||||
| Adults only | 10 | 1.12 (0.99 to 1.26) | 0.068 | 55.3 | 9 | 1.10 (0.63 to 1.93) | 0.728 | 0.0 | ||||
| Contain children or adolescents | 2 | 1.41 (1.06 to 1.88) * | 0.019 | 0.0 | 5 | 0.60 (0.29 to 1.26) | 0.193 | 0.0 | ||||
| Mechanism of action | 0.012 | 0.062 | 0 | 0.750 | ||||||||
| Selective for JAK1 inhibition | 3 | 1.29 (1.11 to 1.50) * | 0.001 | 0.0 | 3 | 0.77 (0.39 to 1.52) | 0.456 | 0.0 | ||||
| Selective for JAK1/JAK2 inhibition | 6 | 1.14 (0.98 to 1.31) | 0.082 | 59.4 | 6 | 1.22 (0.60 to 2.51) | 0.582 | 13.3 | ||||
| Selective for JAK1/JAK3 inhibition | 1 | 0.56 (0.32 to 1.00) * | 0.049 | NA | 1 | 0.14 (0.01 to 2.59) | 0.186 | NA | ||||
| Pan-JAK inhibition | 2 | 0.99 (0.66 to 1.48) | 0.917 | 0.0 | 4 | 0.55 (0.11 to 2.63) | 0.504 | 0.0 | ||||
| Treatment duration | 0.013 | 0.026 | 0 | 0.134 | ||||||||
| <12 weeks | 4 | 0.84 (0.63 to 1.11) | 0.223 | 8.2 | 6 | 0.37 (0.11 to 1.26) | 0.120 | 0.0 | ||||
| ≥12 weeks | 8 | 1.20 (1.07 to 1.34) ** | 0.002 | 52.5 | 8 | 1.01 (0.63 to 1.63) | 0.965 | 0.0 | ||||
| JAK Inhibitors by Mechanism | No. of Patients | No. (%) of Patients with Common TEAEs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nasopharyngitis | URTI | Headache | Nausea | Diarrhea | Blood CPK Increase | Acne | Herpes Viral Infection | ||
| Selective for JAK1 inhibition | |||||||||
| Abrocitinib | 834 | 73 (8.8) | 85 (10.2) | 64 (7.7) | 94 (11.3) | 10 (1.2) | 8 (1.0) | 11 (1.3) | 10 (1.2) |
| Upadacitinib | 126 | 9 (7.1) | 17 (13.5) | 10 (7.9) | 7 (5.6) | 4 (3.2) | 7 (5.6) | 12 (9.5) | 0 |
| Selective for JAK1/JAK2 inhibition | |||||||||
| Baricitinib | 1318 | 118 (9.0) | 36 (2.7) | 64 (4.9) | 2 (0.2) | 25 (1.9) | 27 (2.0) | 5 (0.4) | 74 (5.6) |
| Ruxolitinib | 204 | 10 (4.9) | 5 (2.5) | 4 (2.0) | 0 | 0 | 0 | 0 | 0 |
| Selective for JAK1/JAK3 inhibition | |||||||||
| Tofacitinib | 35 | 2 (5.7) | 1 (2.9) | 1 (2.9) | 1 (2.9) | 0 | 0 | 0 | 0 |
| Pan-JAK inhibition | |||||||||
| Gusacitinib | 27 | 3 (11.1) | 0 | 7 (25.9) | 5 (18.5) | 3 (11.1) | 0 | 0 | 0 |
| Delgocitinib | 439 | 28 (6.4) | 0 | 0 | 0 | 0 | 0 | 4 (0.9) | 0 |
| Certainty Assessment | Summary of Findings | |||||||
|---|---|---|---|---|---|---|---|---|
| Participants (Studies) Follow Up | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Overall Certainty of Evidence | Relative Effect (95% CI) | NNTs or NNHs |
| EASI-75 response | ||||||||
| 3498 (12 RCTs) | Not serious | Not serious | Not serious | Not serious | Likely a | ⨁⨁⨁◯ MODERATE | 2.84 (2.20 to 3.67) | 3.97 |
| IGA response | ||||||||
| 2417 (11 RCTs) | Not serious | Not serious | Not serious | Not serious | Likely a | ⨁⨁⨁◯ MODERATE | 2.99 (2.26 to 3.95) | 5.72 |
| Pruritus-NRS response | ||||||||
| 3036 (8 RCTs) | Not serious | Not serious | Not serious | Not serious | Likely a | ⨁⨁⨁◯ MODERATE | 2.52 (1.90 to 3.35) | 4.91 |
| TEAEs | ||||||||
| 3402 (12 RCTs) | Serious b | Not serious | Not serious | Not serious | Likely a | ⨁⨁◯◯ LOW | 1.14 (1.02 to 1.28) | 14.80 |
| AEs leading to drug discontinuation | ||||||||
| 3926 (14 RCTs) | Serious b | Not serious | Not serious | Not serious | Likely a | ⨁⨁◯◯ LOW | 0.89 (0.57 to 1.38) | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.-R.; Lu, J.-W.; Chen, L.-Y.; Chen, T.-L. Application of Janus Kinase Inhibitors in Atopic Dermatitis: An Updated Systematic Review and Meta-Analysis of Clinical Trials. J. Pers. Med. 2021, 11, 279. https://doi.org/10.3390/jpm11040279
Tsai H-R, Lu J-W, Chen L-Y, Chen T-L. Application of Janus Kinase Inhibitors in Atopic Dermatitis: An Updated Systematic Review and Meta-Analysis of Clinical Trials. Journal of Personalized Medicine. 2021; 11(4):279. https://doi.org/10.3390/jpm11040279
Chicago/Turabian StyleTsai, Hou-Ren, Jing-Wun Lu, Li-Yu Chen, and Tai-Li Chen. 2021. "Application of Janus Kinase Inhibitors in Atopic Dermatitis: An Updated Systematic Review and Meta-Analysis of Clinical Trials" Journal of Personalized Medicine 11, no. 4: 279. https://doi.org/10.3390/jpm11040279
APA StyleTsai, H.-R., Lu, J.-W., Chen, L.-Y., & Chen, T.-L. (2021). Application of Janus Kinase Inhibitors in Atopic Dermatitis: An Updated Systematic Review and Meta-Analysis of Clinical Trials. Journal of Personalized Medicine, 11(4), 279. https://doi.org/10.3390/jpm11040279