Management of Hepatitis B Virus Reactivation in Malignant Lymphoma Prior to Immunosuppressive Treatment
Abstract
1. Introduction
2. Definition of HBV Reactivation
3. Risk Factors for HBV Reactivation
3.1. Host Factors
3.2. Virological Factors
3.3. Immunosuppressive Regimens
3.3.1. Corticosteroids
3.3.2. Anti-CD20 Monoclonal Antibodies
3.3.3. Other Monoclonal Antibodies
3.3.4. Other Novel Agents
3.3.5. Chimeric Antigen Receptor (CAR) T-Cell Immunotherapy
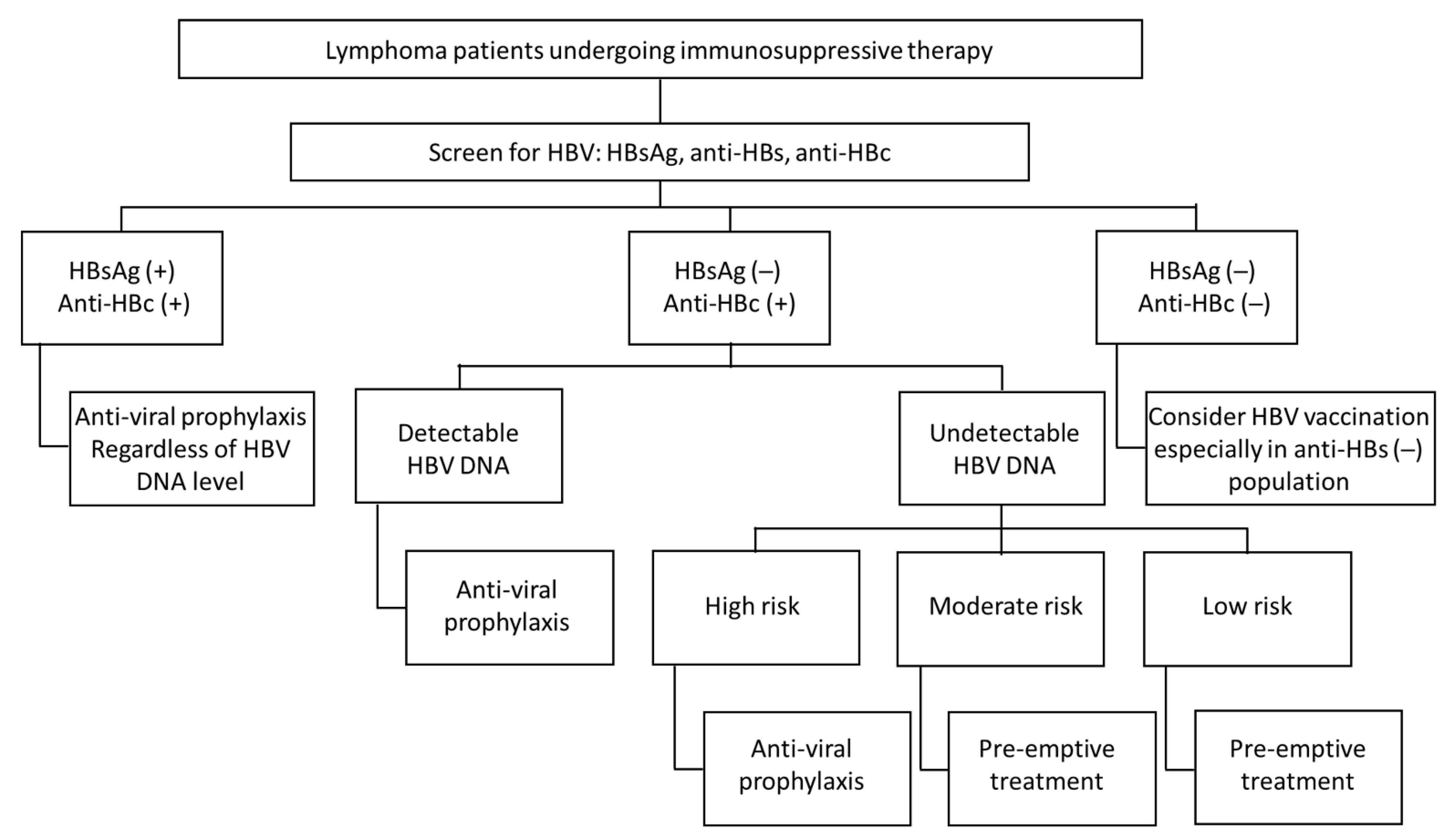
4. Screening and Management of HBV Reactivation in Lymphoma Patient Prior Immunosuppression Therapy
4.1. Screening
4.2. Strategies for HBV Reactivation
4.2.1. HBsAg-Positive Patients without Hepatitis at Baseline (Inactive Phase of CHB)
4.2.2. HBsAg-Negative and Anti-HBcAb-Positive Patients
4.2.3. HBsAg and Anti-HBcAb-Negative Patients
4.3. Choices of Antiviral Agents
4.4. Duration of Antiviral Agents
4.5. Duration of Monitoring after Cessation of Antiviral Agents
5. Summary
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schweitzer, A.; Horn, J.; Mikolajczyk, R.T.; Krause, G.; Ott, J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet 2015, 386, 1546–1555. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.; McMahon, B.J.; Chang, K.-M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL 2017. Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, G.; Pollicino, T.; Cacciola, I.; Squadrito, G. Occult hepatitis B virus infection. J. Hepatol. 2007, 46, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, G.; Locarnini, S.; Pollicino, T.; Levrero, M.; Zoulim, F.; Lok, A.S.; Taormina Workshop on Occult HBV Infection Faculty Members. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J. Hepatol. 2019, 71, 397–408. [Google Scholar] [CrossRef]
- Perrillo, R.P.; Gish, R.; Falck-Ytter, Y.T. American Gastroenterological Association Institute Technical Review on Prevention and Treatment of Hepatitis B Virus Reactivation During Immunosuppressive Drug Therapy. Gastroenterology 2015, 148, 221–244.e3. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Kumar, M.P.; Lau, G.K.; Abbas, Z.; Chan, H.L.Y.; Chen, C.J.; Chen, D.S.; Chen, H.L.; Chien, R.N.; Dokmeci, A.; et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 2016, 10, 1–98. [Google Scholar] [CrossRef] [PubMed]
- Korean Association for the Study of the Liver. KASL clinical practice guidelines for management of chronic hepatitis B. Clin. Mol. Hepatol. 2019, 25, 93–159. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.P.; Feld, J.J.; Hammond, S.P.; Wang, S.H.; Alston-Johnson, D.E.; Cryer, D.R.; Hershman, D.L.; Loehrer, A.P.; Sabichi, A.L.; Symington, B.E.; et al. Hepatitis B Virus Screening and Management for Patients with Cancer Prior to Therapy: ASCO Provisional Clinical Opinion Update. J. Clin. Oncol. 2020, 38, 3698–3715. [Google Scholar] [CrossRef]
- Bo, W.; Ghulam, M.; Kosh, A. Reactivation of hepatitis B virus infection in patients with hematologic disorders. Haematologica 2019, 104, 435–443. [Google Scholar]
- Lok, A.S.; Liang, R.H.; Chiu, E.K.; Wong, K.-L.; Chan, T.-K.; Todd, D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Gastroenterology 1991, 100, 182–188. [Google Scholar] [CrossRef]
- Koo, Y.X.; Tay, M.; Teh, Y.E.; Teng, D.; Tan, D.S.W.; Tan, I.B.H.; Tai, D.W.M.; Quek, R.; Tao, M.; Lim, S.T. Risk of hepatitis B virus (HBV) reactivation in hepatitis B surface antigen negative/hepatitis B core antibody positive patients receiving rituximab-containing combination chemotherapy without routine antiviral prophylaxis. Ann. Hematol. 2011, 90, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.; Chan, T.C.; Leung, N.W.Y.; Lam, W.Y.; Mo, F.K.F.; Chu, M.T.; Chan, H.L.Y.; Hui, E.P.; Lei, K.I.K.; Mok, T.S.K.; et al. Hepatitis B Virus Reactivation in Lymphoma Patients with Prior Resolved Hepatitis B Undergoing Anticancer Therapy With or Without Rituximab. J. Clin. Oncol. 2009, 27, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.; Chan, P.K.; Zhong, S.; Ho, W.M.; Steinberg, J.L.; Tam, J.S.; Hui, P.; Leung, N.W.; Zee, B.; Johnson, P.J. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: A prospective study of 626 patients with identification of risk factors. J. Med. Virol. 2000, 62, 299–307. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Tien, F.-M.; Cheng, A.; Huang, S.-Y.; Chou, W.-C.; Yao, M.; Tang, J.-L.; Tien, H.-F.; Sheng, W.-H. Hepatitis B reactivation among 1962 patients with hematological malignancy in Taiwan. BMC Gastroenterol. 2018, 18, 6. [Google Scholar] [CrossRef]
- Loomba, R.; Liang, T.J. Hepatitis B Reactivation Associated with Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology 2017, 152, 1297–1309. [Google Scholar] [CrossRef]
- Lau, G.K.K.; Leung, Y.-H.; Fong, D.Y.T.; Au, W.-Y.; Kwong, Y.-L.; Lie, A.; Hou, J.-L.; Wen, Y.-M.; Nanj, A.; Liang, R. High hepatitis B virus (HBV) DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic cell transplantation. Blood 2002, 99, 2324–2330. [Google Scholar] [CrossRef]
- Yeo, W.; Zee, B.; Zhong, S.; Chan, P.K.S.; Wong, W.-L.; Ho, W.M.; Lam, K.C.; Johnson, P.J. Comprehensive analysis of risk factors associating with Hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br. J. Cancer 2004, 90, 1306–1311. [Google Scholar] [CrossRef]
- Shibolet, O.; Shouval, D. Immunosuppression and HBV Reactivation. Semin. Liver Dis. 2013, 33, 167–177. [Google Scholar] [CrossRef]
- Seto, W.-K.; Chan, T.S.; Hwang, Y.-Y.; Wong, D.K.-H.; Man-Fung, Y.; Liu, K.S.-H.; Gill, H.; Yok-Lam, K.; Lie, A.K.; Lai, C.-L.; et al. Hepatitis B Reactivation in Patients with Previous Hepatitis B Virus Exposure Undergoing Rituximab-Containing Chemotherapy for Lymphoma: A Prospective Study. J. Clin. Oncol. 2014, 32, 3736–3743. [Google Scholar] [CrossRef]
- Cho, Y.; Yu, S.J.; Cho, E.J.; Lee, J.H.; Kim, T.M.; Heo, D.S.; Kim, Y.J.; Yoon, J.H. High titers of anti-HBs prevent rituximab-related viral reactivation in resolved hepatitis B patient with non-Hodgkin’s lymphoma. J. Med. Virol. 2016, 88, 1010–1017. [Google Scholar] [CrossRef]
- Paul, S.; Dickstein, A.; Saxena, A.; Terrin, N.; Viveiros, K.; Balk, E.M.; Wong, J.B. Role of surface antibody in hepatitis B reactivation in patients with resolved infection and hematologic malignancy: A meta-analysis. Hepatology 2017, 66, 379–388. [Google Scholar] [CrossRef]
- Matsubara, T.; Nishida, T.; Shimoda, A.; Shimakoshi, H.; Amano, T.; Sugimoto, A.; Takahashi, K.; Mukai, K.; Yamamoto, M.; Hayashi, S.; et al. The combination of anti-HBc and anti-HBs levels is a useful predictor of the development of chemotherapy-induced reactivation in lymphoma patients with resolved HBV infection. Oncol. Lett. 2017, 14, 6543–6552. [Google Scholar] [CrossRef]
- Salpini, R.; Colagrossi, L.; Bellocchi, M.C.; Surdo, M.; Becker, C.; Alteri, C.; Aragri, M.; Ricciardi, A.; Armenia, D.; Pollicita, M.; et al. Hepatitis B surface antigen genetic elements critical for immune escape correlate with hepatitis B virus reactivation upon immunosuppression. Hepatology 2015, 61, 823–833. [Google Scholar] [CrossRef]
- Reddy, K.R.; Beavers, K.L.; Hammond, S.P.; Lim, J.K.; Falck-Ytter, Y.T. American Gastroenterological Association Institute Guideline on the Prevention and Treatment of Hepatitis B Virus Reactivation During Immunosuppressive Drug Therapy. Gastroenterology 2015, 148, 215–219. [Google Scholar] [CrossRef]
- Hui, C.-K.; Lie, A.; Au, W.-Y.; Leung, Y.-H.; Ma, S.-Y.; Cheung, W.W.W.; Zhang, H.-Y.; Chim, C.-S.; Kwong, Y.-L.; Liang, R.; et al. A long-term follow-up study on hepatitis B surface antigen–positive patients undergoing allogeneic hematopoietic stem cell transplantation. Blood 2005, 106, 464–469. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, X.; Mao, X.; Huang, L.; Meng, F.; Zhou, J. Severe early hepatitis B reactivation in a patient receiving anti-CD19 and anti-CD22 CAR T cells for the treatment of diffuse large B-cell lymphoma. J. Immunother. Cancer 2019, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.P.; Borchelt, A.M.; Ukomadu, C.; Ho, V.T.; Baden, L.R.; Marty, F.M. Hepatitis B Virus Reactivation following Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2009, 15, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Qin, B.; Yuan, Z.; Chen, L.; Zhou, H.-Y. Meta-analysis of prophylactic entecavir or lamivudine against hepatitis B virus reactivation. Ann. Hepatol. 2016, 15, 501–511. [Google Scholar] [PubMed]
- Herishanu, Y.; Katchman, H.; Polliack, A. Severe hepatitis B virus reactivation related to ibrutinib monotherapy. Ann. Hematol. 2017, 96, 689–690. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, I.; Morelli, F.; Autore, F.; Corbingi, A.; Pasquale, R.; Sorà, F.; Pompili, M.; Laurenti, L. HBV reactivation in CLL patients with occult HBV infection treated with ibrutinib without viral prophylaxis. Leuk. Lymphoma 2018, 60, 1340–1342. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.P.; Chen, K.; Pandit, A.; Davids, M.S.; Issa, N.C.; Marty, F.M. Risk of hepatitis B virus reactivation in patients treated with ibrutinib. Blood 2018, 131, 1987–1989. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, B.; Li, Y.; Zheng, N.; Zhou, Z.; Liu, J. Hepatitis B virus reactivation in patients with multiple myeloma receiving bortezomib-containing regimens followed by autologous stem cell transplant. Leuk. Lymphoma 2015, 56, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tur-Kaspa, R.; Shaul, Y.; Moore, D.D.; Burk, R.D.; Okret, S.; Poellinger, L.; Shafritz, D.A. The glucocorticoid receptor recognizes a specific nucleotide sequence in hepatitis B virus DNA causing increased activity of the HBV enhancer. Virology 1988, 167, 630–633. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsiung, C.A.; Su, I.J.; Chen, P.J.; Chang, M.C.; Tsao, C.J.; Kao, W.Y.; Uen, W.C.; Hsu, C.H.; Tien, H.F.; et al. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology 2003, 37, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.N.; Chen, C.H.; Lee, C.M.; Wang, M.C.; Ma, M.C.; Hu, T.H.; Kuo, C.Y. Reactivation of hepatitis B virus following rituximab-based regimens: A serious complication in both HBsAg-positive and HBsAg-negative patients. Ann. Hematol. 2010, 89, 255–262. [Google Scholar] [CrossRef]
- Evens, A.M.; Jovanovic, B.D.; Su, Y.-C.; Raisch, D.W.; Ganger, D.; Belknap, S.M.; Dai, M.-S.; Chiu, B.-C.C.; Fintel, B.; Cheng, Y.; et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: Meta-analysis and examination of FDA safety reports. Ann. Oncol. 2011, 22, 1170–1180. [Google Scholar] [CrossRef]
- Oh, M.J.; Lee, H.J. A study of hepatitis B virus reactivation associated with rituximab therapy in real-world clinical practice: A single-center experience. Clin. Mol. Hepatol. 2013, 19, 51–59. [Google Scholar] [CrossRef]
- Gutiérrez García, M.L.; Alonso Lopez, S.; Martín Rios, M.D.; Sanmartin Fenollera, P.; Agudo Fernandez, S.; Fernández Rodriguez, C.M. Hepatitis B virus reactivation in rituximab-treated patients: Incidence and risk factors. Gastroenterol. Hepatol. 2015, 38, 1–6. [Google Scholar] [CrossRef]
- Guo, Y.-F.; Pan, J.-X.; Zhuang, W.-H. Concurrent and reactivation of hepatitis B virus infection in diffuse large B-cell lymphoma: Risk factors and survival outcome. Infect. Agents Cancer 2018, 13, 40. [Google Scholar] [CrossRef]
- Tsai, Y.-F.; Yang, C.-I.; Du, J.-S.; Lin, M.-H.; Tang, S.-H.; Wang, H.-C.; Cho, S.-F.; Liu, Y.-C.; Su, Y.-C.; Dai, C.-Y.; et al. Rituximab increases the risk of hepatitis B virus reactivation in non-Hodgkin lymphoma patients who are hepatitis B surface antigen-positive or have resolved hepatitis B virus infection in a real-world setting: A retrospective study. PeerJ 2019, 7, e7481. [Google Scholar] [CrossRef]
- Kusumoto, S.; Arcaini, L.; Hong, X.; Jin, J.; Kim, W.S.; Kwong, Y.L.; Peters, M.G.; Tanaka, Y.; Zelenetz, A.D.; Kuriki, H.; et al. Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood 2019, 133, 137–146. [Google Scholar] [CrossRef]
- Nakaya, A.; Fujita, S.; Satake, A.; Nakanishi, T.; Azuma, Y.; Tsubokura, Y.; Hotta, M.; Yoshimura, H.; Ishii, K.; Ito, T.; et al. Delayed HBV reactivation in rituximab-containing chemotherapy: How long should we continue anti-virus prophylaxis or monitoring HBV-DNA? Leuk. Res. 2016, 50, 46–49. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Hsiao, L.-T.; Hong, Y.-C.; Chiou, T.-J.; Yu, Y.-B.; Gau, J.-P.; Liu, C.-Y.; Yang, M.-H.; Tzeng, C.-H.; Lee, P.-C.; et al. Randomized Controlled Trial of Entecavir Prophylaxis for Rituximab-Associated Hepatitis B Virus Reactivation in Patients with Lymphoma and Resolved Hepatitis B. J. Clin. Oncol. 2013, 31, 2765–2772. [Google Scholar] [CrossRef]
- Kusumoto, S.; Tanaka, Y.; Suzuki, R.; Watanabe, T.; Nakata, M.; Takasaki, H.; Fukushima, N.; Fukushima, T.; Moriuchi, Y.; Itoh, K.; et al. Monitoring of Hepatitis B Virus (HBV) DNA and Risk of HBV Reactivation in B-Cell Lymphoma: A Prospective Observational Study. Clin. Infect. Dis. 2015, 61, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.C.; Huang, Y.H.; Chu, C.J.; Lee, P.C.; Lin, H.C.; Lee, S.D. Hepatitis B virus reactivation after 23 months of rituximab-based chemotherapy in an HBsAg-negative, anti-HBs-positive patient with follicular lymphoma. J. Chin. Med. Assoc. 2010, 73, 156–160. [Google Scholar] [CrossRef][Green Version]
- Iannitto, E.; Minardi, V.; Calvaruso, G.; Mulè, A.; Ammatuna, E.; Trapani, R.D.; Ferraro, D.; Abbadessa, V.; Craxi, A.; Stefano, R.D. Hepatitis B virus reactivation and alemtuzumab therapy. Eur. J. Haematol. 2005, 74, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cao, Z.; Wang, Z.; Liu, M.; Zhou, H.; Yang, Q. Hepatitis B virus reactivation induced by Brentuximab vedotin in the treatment of Hodgkin lymphoma: A case report and literature review. Zhonghua Xue Ye Xue Za Zhi 2014, 35, 949–950. [Google Scholar]
- Pan, Z.; Scheerens, H.; Li, S.-J.; Schultz, B.E.; Sprengeler, P.A.; Burrill, L.C.; Mendonca, R.V.; Sweeney, M.D.; Scott, K.C.K.; Grothaus, P.G.; et al. Discovery of Selective Irreversible Inhibitors for Bruton’s Tyrosine Kinase. ChemMedChem 2006, 2, 58–61. [Google Scholar] [CrossRef]
- Gopal, A.K.; Kahl, B.S.; De Vos, S.; Wagner-Johnston, N.D.; Schuster, S.J.; Jurczak, W.J.; Flinn, I.W.; Flowers, C.R.; Martin, P.; Viardot, A.; et al. PI3Kδ Inhibition by Idelalisib in Patients with Relapsed Indolent Lymphoma. N. Engl. J. Med. 2014, 370, 1008–1018. [Google Scholar] [CrossRef]
- Flinn, I.W.; Kahl, B.S.; Leonard, J.P.; Furman, R.R.; Brown, J.R.; Byrd, J.C.; Wagner-Johnston, N.D.; Coutre, S.E.; Benson, D.M.; Peterman, S.; et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-δ, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood 2014, 123, 3406–3413. [Google Scholar] [CrossRef] [PubMed]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Coutre, S.; Seymour, J.F.; Munir, T.; Puvvada, S.D.; Wendtner, C.-M.; Roberts, A.W.; Jurczak, W.; et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 2016, 17, 768–778. [Google Scholar] [CrossRef]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, A.L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Chow, V.A.; Shadman, M.; Gopal, A.K. Translating anti-CD19 CAR T-cell therapy into clinical practice for relapsed/refractory diffuse large B-cell lymphoma. Blood 2018, 132, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Roberts, Z.J.; Better, M.; Bot, A.; Roberts, M.R.; Ribas, A. Axicabtagene ciloleucel, a first-in-class CAR T cell therapy for aggressive NHL. Leuk. Lymphoma 2017, 59, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Stirrups, R. CAR T-cell therapy in refractory large B-cell lymphoma. Lancet Oncol. 2018, 19, e19. [Google Scholar] [CrossRef]
- Lau, G.K.; Yiu, H.H.; Fong, D.Y.; Cheng, H.-C.; Au, W.-Y.; Lai, L.S.; Cheung, M.; Zhang, H.-Y.; Lie, A.; Ngan, R.; et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology 2003, 125, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Hsiung, C.A.; Su, I.J.; Hwang, W.S.; Wang, M.C.; Lin, S.F.; Lin, T.H.; Hsiao, H.H.; Young, J.H.; Chang, M.C.; et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma: A randomized trial. Hepatology 2008, 47, 844–853. [Google Scholar] [CrossRef]
- Zhang, M.-Y.; Zhu, G.-Q.; Shi, K.-Q.; Zheng, J.-N.; Cheng, Z.; Zou, Z.-L.; Huang, H.-H.; Chen, F.-Y.; Zheng, M.-H. Systematic review with network meta-analysis: Comparative efficacy of oral nucleos(t)ide analogues for the prevention of chemotherapy-induced hepatitis B virus reactivation. Oncotarget 2016, 7, 30642–30658. [Google Scholar] [CrossRef]
- Yu, S.; Luo, H.; Pan, M.; Luis, A.P.; Xiong, Z.; Shuai, P.; Zhang, Z. Comparison of entecavir and lamivudine in preventing HBV reactivation in lymphoma patients undergoing chemotherapy: A meta-analysis. Int. J. Clin. Pharm. 2016, 38, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, X.; Zhu, J.; Ye, S.; Zhang, H.; Wang, W.; Wu, X.; Peng, J.; Xu, B.; Lin, Y.; et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: A randomized clinical trial. JAMA 2014, 312, 2521–2530. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Anand, A.C.; Kumar, A.; Singh, S.P.; Aggarwal, R.; Dhiman, R.K.; Aggarwal, S.; Alam, S.; Bhaumik, P.; Dixit, V.K.; et al. INASL Guidelines on Management of Hepatitis B Virus Infection in Patients receiving Chemotherapy, Biologicals, Immunosupressants, or Corticosteroids. J. Clin. Exp. Hepatol. 2018, 8, 403–431. [Google Scholar] [CrossRef] [PubMed]
- Chien, R.-N.; Kao, J.-H.; Peng, C.-Y.; Chen, C.-H.; Liu, C.-J.; Huang, Y.-H.; Hu, T.-H.; Yang, H.-I.; Lu, S.-N.; Ni, Y.-H.; et al. Taiwan consensus statement on the management of chronic hepatitis B. J. Formos. Med. Assoc. 2019, 118, 7–38. [Google Scholar] [CrossRef]
- Charlton, M.R.; Alam, A.; Shukla, A.; Dashtseren, B.; Lesmana, C.R.A.; Duger, D.; Payawal, D.A.; Cuong, D.D.; Jargalsaikhan, G.; Cua, I.H.Y.; et al. An expert review on the use of tenofovir alafenamide for the treatment of chronic hepatitis B virus infection in Asia. J. Gastroenterol. 2020, 55, 811–823. [Google Scholar] [CrossRef]
- Sagnelli, C.; Pisaturo, M.; Calò, F.; Martini, S.; Sagnelli, E.; Coppola, N. Reactivation of hepatitis B virus infection in patients with hemo-lymphoproliferative diseases, and its prevention. World J. Gastroenterol. 2019, 25, 3299–3312. [Google Scholar] [CrossRef]
- Hwang, J.P.; Somerfield, M.R.; Alston-Johnson, D.E.; Cryer, D.R.; Feld, J.J.; Kramer, B.S.; Sabichi, A.L.; Wong, S.L.; Artz, A.S. Hepatitis B Virus Screening for Patients with Cancer Before Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update. J. Clin. Oncol. 2015, 33, 2212–2220. [Google Scholar] [CrossRef]
- Myint, A.; Tong, M.J.; Beaven, S.W. Reactivation of Hepatitis B Virus: A Review of Clinical Guidelines. Clin. Liver Dis. 2020, 15, 162–167. [Google Scholar] [CrossRef]
| Phase | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| HBeAg-positive chronic infection, also called Immune tolerant phase | HBeAg-positive chronic hepatitis, also called immune reactive phase | HBeAg-negative chronic hepatitis | HBeAg-negative chronic infection, also called inactive carrier phase | HBsAg-negative, also called resolved HBV infection or occult HBV infection a | |
| Serological marker | HBeAg (+); Anti-HBe (−) | HBeAg (+); may develop anti-HBe | HBeAg (−); Anti-HBe (+/−) | HBeAg (−); Anti-HBe (+) | HBsAg (−); Anti-HBc (+) a; Anti-HBs (+/−) |
| HBV DNA | very high levels, generally ≥107 IU/mL | 104–107 IU/mL | >2000 IU/mL | Generally <2000 IU/mL or negative | <200 IU/mL or negative |
| ALT | Normal | Elevated | Elevated | Normal | Normal |
| Liver disease | None/Minimal | Moderate/severe | Moderate/severe | None/minimal | None |
| Society | Reactivaion of CHB | Reactivation of Resolved HBV |
|---|---|---|
| American Association for the Study of Liver Diseases (AASLD) 2018 guideline [2] | Any of the following:
| Any of the following:
|
| American Gastroenterological Association (AGA) 2015 guideline [6] |
|
|
| The Asian Pacific Association for the Study of the Liver (APASL) 2016 guideline [7] |
|
|
| European Association for the Study of the Liver (EASL) 2017 guideline [3] |
|
|
| Korean Association for the Study of the Liver (KASL) 2019 guideline [8] |
|
|
| American Society of Clinical Oncology (ASCO) 2020 update [9] |
|
|
| Risk Group | Immunosuppressive Agents | |
|---|---|---|
| HBsAg-Positive, Anti-HBc-Positive | HBsAg-Negative, Anti-HBc-Positive | |
| High risk (>10%) | ||
| Moderate risk (1–10%) |
|
|
| Low risk (<1%) |
|
|
| Society | Who Should Be Screened? | Screening Tests | Strategy | Choice of NAs | NAs Duration | Monitoring after Prophylaxis |
|---|---|---|---|---|---|---|
| American Association for the Study of Liver Diseases (AASLD) 2018 guideline [2] | All patients | HBsAg and anti-HBcAb |
| ETV, TDF, TAF |
| Patients should be monitored for up to 12 months after cessation of anti-HBV therapy |
| American Gastroenterological Association (AGA) 2015 guideline [25] | Moderate or high risk of HBV reactivation | HBsAg and anti-HBc, HBV DNA test if either positive |
| Antivirals with highbarrier to resistance over lamivudine |
| Not mentioned |
| The Asian Pacific Association for the Study of the Liver (APASL) 2016 guideline [7] | All patients | HBsAg and anti-HBcAb, HBsAg (−), anti-HbcAb (+): HBV DNA |
| ETV, TDF | At least 12 months after cessation of therapy | Not mentioned |
| European Association for the Study of the Liver (EASL) 2017 guideline [3] | All patients | HBsAg, anti-HbcAb, and anti-HbsAb |
| ETV, TDF, TAF |
| Liver function tests and HBV DNA should be tested every 3 to 6 months and for at least 12 months after NAs withdrawal |
| Korean Association for the Study of the Liver (KASL) 2019 guideline [8] | All patients | HBsAg and anti-HbcAb, HBV DNA test if either positive |
| ETV, TDF |
| Not mentioned |
| American Society of Clinical Oncology (ASCO) 2020 update [9] | All patients | HBsAg, anti-HBcAb, and anti-HBsAb |
| ETV, TDF, TAF |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-F.; Hsu, C.-M.; Hsiao, H.-H. Management of Hepatitis B Virus Reactivation in Malignant Lymphoma Prior to Immunosuppressive Treatment. J. Pers. Med. 2021, 11, 267. https://doi.org/10.3390/jpm11040267
Tsai Y-F, Hsu C-M, Hsiao H-H. Management of Hepatitis B Virus Reactivation in Malignant Lymphoma Prior to Immunosuppressive Treatment. Journal of Personalized Medicine. 2021; 11(4):267. https://doi.org/10.3390/jpm11040267
Chicago/Turabian StyleTsai, Yu-Fen, Chin-Mu Hsu, and Hui-Hua Hsiao. 2021. "Management of Hepatitis B Virus Reactivation in Malignant Lymphoma Prior to Immunosuppressive Treatment" Journal of Personalized Medicine 11, no. 4: 267. https://doi.org/10.3390/jpm11040267
APA StyleTsai, Y.-F., Hsu, C.-M., & Hsiao, H.-H. (2021). Management of Hepatitis B Virus Reactivation in Malignant Lymphoma Prior to Immunosuppressive Treatment. Journal of Personalized Medicine, 11(4), 267. https://doi.org/10.3390/jpm11040267







