Multi-View Convolutional Neural Networks in Rupture Risk Assessment of Small, Unruptured Intracranial Aneurysms
Abstract
1. Introduction
2. Materials and Methods
2.1. Datasets
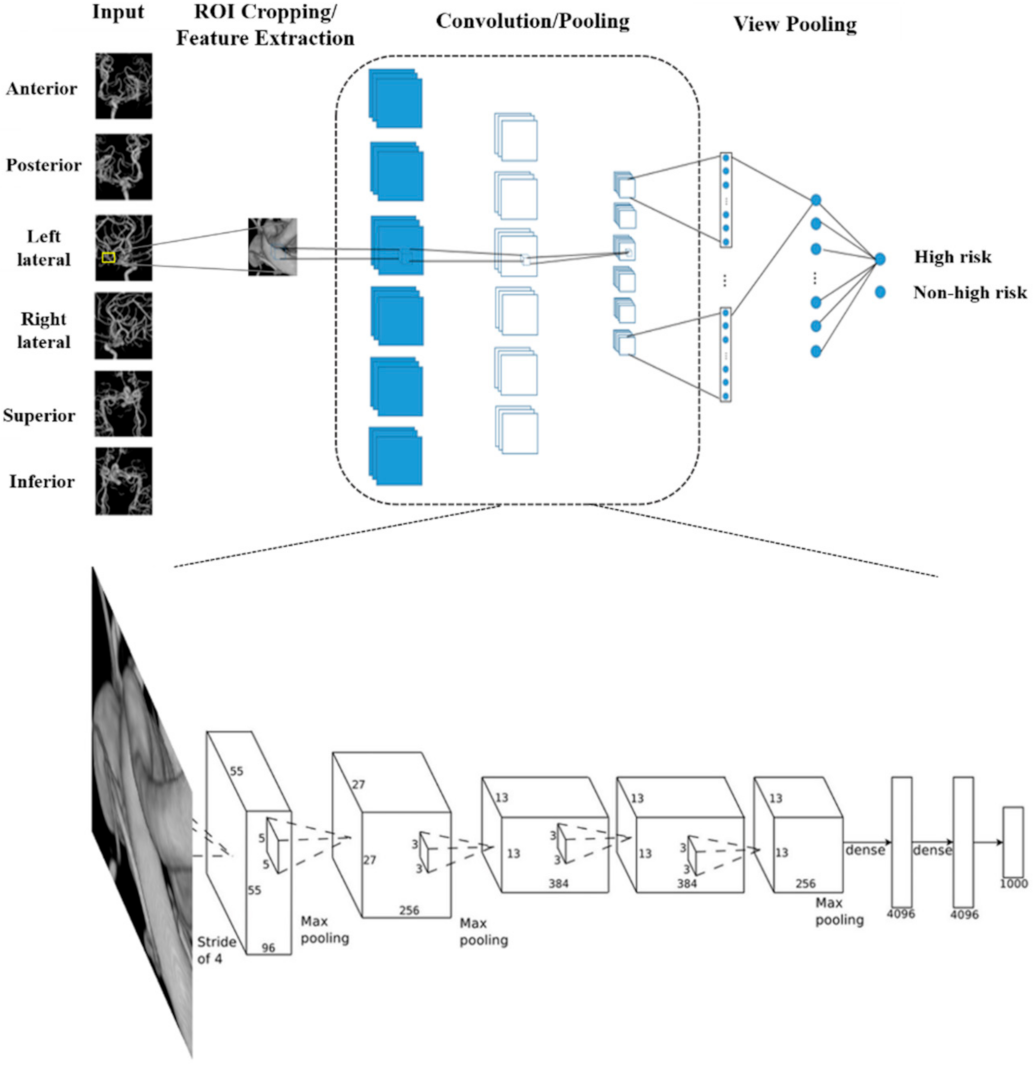
2.2. Multi-View Convolutional Neural Networks
2.3. Statistical Analysis
3. Results
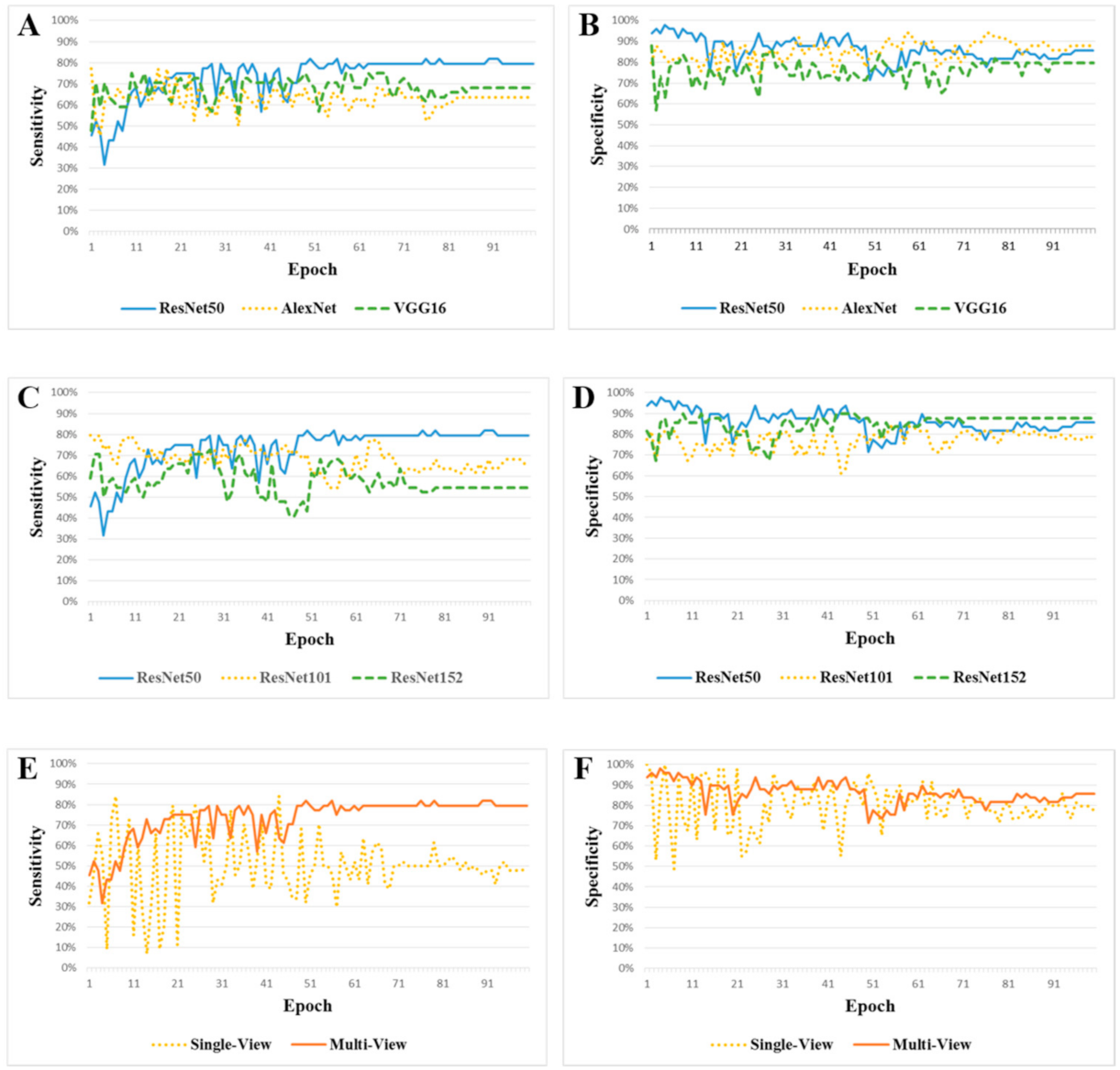
3.1. ResNet50 vs. AlexNet vs. VGG16
3.2. ResNet50 vs. ResNet101 vs. ResNet152
3.3. Multi-View CNN-ResNet50 vs. Single-View CNN-ResNet50
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakao, T.; Hanaoka, S.; Nomura, Y.; Sato, I.; Nemoto, M.; Miki, S.; Maeda, E.; Yoshikawa, T.; Hayashi, N.; Abe, O. Deep neural network-based computer-assisted detection of cerebral aneurysms in mr angiography. J. Magn. Reson. Imaging 2018, 47, 948–953. [Google Scholar] [CrossRef]
- Dai, X.; Huang, L.; Qian, Y.; Xia, S.; Chong, W.; Liu, J.; Ieva, A.D.; Hou, X.; Ou, C. Deep learning for automated cerebral aneurysm detection on computed tomography images. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 715–723. [Google Scholar] [CrossRef]
- Lantigua, H.; Ortega-Gutierrez, S.; Schmidt, J.M.; Lee, K.; Badjatia, N.; Agarwal, S.; Claassen, J.; Connolly, E.S.; Mayer, S.A. Subarachnoid hemorrhage: Who dies, and why? Crit. Care 2015, 9, 309. [Google Scholar] [CrossRef]
- Bonita, R.; Thomson, S. Subarachnoid hemorrhage: Epidemiology, diagnosis, management, and outcome. Stroke 1985, 16, 591–594. [Google Scholar] [CrossRef]
- Kim, H.C.; Rhim, J.K.; Ahn, J.H.; Park, J.J.; Moon, J.U.; Hong, E.P.; Kim, M.R.; Kim, S.G.; Lee, S.H.; Jeong, J.H.; et al. Machine learning application for rupture risk assessment in small-sized intracranial aneurysm. J. Clin. Med. 2019, 8, 683. [Google Scholar] [CrossRef]
- Katati, M.J.; Santiago-Ramajo, S.; Perez-Garcia, M.; Jofré, M.M.; Vilar-Lopez, R.; Coín-Mejias, M.A.; Cara cuel-Romero, A.; Arjona-Moron, V. Description of quality of life and its predictors in patients with aneurysmal subarachnoid hemorrhage. Cerebrovasc. Dis. 2007, 24, 66–73. [Google Scholar] [CrossRef]
- Johnston, S.C.; Zhao, S.; Dudley, R.A.; Berman, M.F.; Gress, D.R. Treatment of unruptured cerebral aneu rysms in california. Stroke 2001, 32, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, M.; Martin, C.; Elefteriades, J.A.; Sun, W. A machine learning approach to investigate the rela tionship between shape features and numerically predicted risk of ascending aortic aneurysm. Biomech. Model. Mechanobiol. 2017, 16, 1519–1533. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, A.E.; Koivisto, T.; Bjorkman, J.; Fraunberg, M.U.Z.; Helin, K.; Jääskeläinen, J.E.; Frösen, J. Irregular shape of intracranial aneurysm indicates rupture risk irrespective of size in a population-based cohort. Stroke 2016, 47, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Q.; Meng, Z.H.; Hou, Z.J.; Huang, S.; Chen, J.; Yu, H.; Feng, L.; Wang, Q.; Li, P.; Wen, Z. Geomet ric parameter analysis of ruptured and unruptured aneurysms in patients with symmetric bilateral intracranial aneurysms: A multicenter ct angiography study. AJNR Am. J. Neuroradiol. 2016, 37, 1413–1417. [Google Scholar] [CrossRef]
- Jeon, J.S.; Sheen, S.H.; Hwang, G.J.; Kim, H.C.; Kwon, B.J. Feasibility of intravenous flat panel detector ct angiography for intracranial arterial stenosis. AJNR Am. J. Neuroradiol. 2013, 34, 129–134. [Google Scholar] [CrossRef]
- Jou, L.D.; Mohamed, A.; Lee, D.H.; Mawad, M.E. 3d rotational digital subtraction angiography may under estimate intracranial aneurysms: Findings from two basilar aneurysms. AJNR Am. J. Neuroradiol. 2007, 28, 1690–1692. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, L.J.; Poon, C.S.; Wu, S.Y.; Zhou, C.S.; Luo, S.; Wang, M.; Lu, G.M. Digital subtraction ct angi ography for detection of intracranial aneurysms: Comparison with three-dimensional digital subtraction angiography. Radiology 2012, 262, 605–612. [Google Scholar] [CrossRef]
- Kingma, D.P.; Ba, J. Adam: A method for stochastic optimization. In Proceedings of the 2015 International Conference on Learning Representations (ICLR), San Diego, CA, USA, 7–9 May 2015; pp. 1–15. [Google Scholar]
- Deng, J.; Dong, W.; Socher, R.; Li, L.J.; Li, K.; Fei-Fei, L. Imagenet: A large-scale hierarchical image database. In Proceedings of the 2009 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Miami, FL, USA, 20–25 June 2009; pp. 248–255. [Google Scholar]
- Szegedy, C.; Vanhoucke, V.; Ioffe, S.; Shlens, J.; Wojna, Z. Rethinking the inception architecture for computer vision. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 26 June–1 July 2016; pp. 2818–2826. [Google Scholar]
- Paszke, A.; Gross, S.; Massa, F.; Lerer, A.; Bradbury, J.; Chanan, G.; Killeen, T.; Lin, Z.; Gimelshein, N.; Antiga, L.; et al. Pytorch: An imperative style, high-performance deep learning library. arXiv 2019, arXiv:1912.01703. [Google Scholar]
- Cebral, J.R.; Mut, F.; Weir, J.; Putman, C.M. Association of hemodynamic characteristics and cerebral aneurysm rupture. AJNR Am. J. Neuroradiol. 2011, 32, 264–270. [Google Scholar] [CrossRef]
- Stember, J.N.; Chang, P.; Stember, D.M.; Liu, M.; Grinband, J.; Filippi, C.G.; Meyers, P.; Jambawalikar, S. Con volutional neural networks for the detection and measurement of cerebral aneurysms on magnetic resonance angiography. J. Digit. Imaging 2019, 32, 808–815. [Google Scholar] [CrossRef]
- Joo, B.; Ahn, S.S.; Yoon, P.H.; Sohi, B.; Sohn, B.; Lee, Y.E.; Bae, J.H.; Park, M.S.; Choi, H.S.; Lee, S. A deep learning algorithm may automate intracranial aneurysm detection on mr angiography with high diagnostic performance. Eur. Radiol. 2020, 30, 5785–5793. [Google Scholar] [CrossRef]
- Ueda, D.; Yamamoto, A.; Nishimori, M.; Shimono, T.; Doishita, S.; Shimazaki, A.; Katayama, Y.; Fukumoto, S.; Choppin, A.; Shimahara, Y.; et al. Deep learning for mr angiography: Automated detection of cerebral aneurysms. Radiology 2019, 290, 187–194. [Google Scholar] [CrossRef]
- Yang, J.; Xie, M.; Hu, C.; Alwalid, O.; Xu, Y.; Liu, J.; Jin, T.; Li, C.; Tu, D.; Liu, X.; et al. Deep learning for detecting cerebral aneurysms with ct angiography. Radiology 2021, 298, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Kallmes, D.F.; Erickson, B.J. Automated Aneurysm Detection: Emerging from the Shallow End of the Deep Learning Pool. Radiology 2021, 298, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Huang, Y.; Liu, L.; Dai, H.; Chen, L.; Zhou, L. Automatic detection on intracranial aneurysm from digital subtraction angiography with cascade convolutional neural networks. Biomed. Eng. Online 2019, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Yang, H.; Ni, W.; Lei, Y.; Jiang, Z.; Shi, K.; Yu, J.; Gu, Y.; Wang, Y. Automatic detection of intracranial aneurysms in 3d-dsa based on a bayesian optimized filter. Biomed. Eng. Online 2020, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Korogi, Y.; Nakashima, K.; Hamatake, S.; Honda, S.; Takahashi, M. Comparison of 2d and 3d digital subtraction angiography in evaluation of intracranial aneurysms. AJNR Am. J. Neuroradiol. 2002, 23, 1545–1552. [Google Scholar] [PubMed]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. In Proceedings of the 2012 Advances in Neural Information Processing Systems (NIPS), Lake Tahoe, NV, USA, 3–8 December 2012; pp. 1097–1105. [Google Scholar]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. In Proceedings of the 2015 International Conference on Learning Representations (ICLR), San Diego, CA, USA, 7–9 May 2015. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 26 June–1 July 2016; pp. 770–778. [Google Scholar]
- Abrol, A.; Bhattarai, M.; Fedorov, A.; Du, Y.; Plis, S.; Calhoun, V. Deep residual learning for neuroimaging: An application to predict progression to alzheimer’s disease. J. Neurosci. Methods 2020, 339, 108701. [Google Scholar] [CrossRef]
- Ramzan, F.; Khan, M.U.G.; Rehmat, A.; Iqbal, S.; Saba, T.; Rehman, A.; Mehmood, Z. A deep learning ap proach for automated diagnosis and multi-class classification of alzheimer’s disease stages using resting-state fmri and residual neural networks. J. Med. Syst. 2019, 44, 37. [Google Scholar] [CrossRef]
- Kang, G.; Liu, K.; Hou, B.; Zhang, N. 3D multi-view convolutional neural networks for lung nodule classifica tion. PLoS ONE 2017, 12, e0188290. [Google Scholar] [CrossRef]
- Tykocki, T.; Kostkiewicz, B. Aneurysms of the anterior and posterior cerebral circulation: Comparison of the morphometric features. Acta Neurochir. 2014, 156, 1647–1654. [Google Scholar] [CrossRef]
- Williams, L.N.; Brown, R.D., Jr. Management of unruptured intracranial aneurysms. Neurol. Clin. Pract. 2013, 3, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Investigators, U.J.; Morita, A.; Kirino, T.; Hashi, K.; Aoki, N.; Fukuhara, S.; Hashimoto, N.; Nakayama, T.; Sakai, M.; Teramoto, A.; et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N. Engl. J. Med. 2012, 366, 2474–2482. [Google Scholar] [CrossRef]
- Wiebers, D.O.; Whisnant, J.P.; Huston, J., 3rd; Meissner, I.; Brown, R.D., Jr.; Forbes, P.G.S.; Thielen, K.; Nichols, D.; O’Fallon, W.M.; Peacock, J.; et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003, 362, 103–110. [Google Scholar] [CrossRef]
- Dolati, P.; Pittman, D.; Morrish, W.F.; Wong, J.; Sutherland, G.R. The frequency of subarachnoid hemorrhage from very small cerebral aneurysms (<5 mm): A population-based study. Cureus 2015, 7, e279. [Google Scholar] [PubMed]
- Suzuki, T.; Takao, H.; Rapaka, S.; Fujimura, S.; Nita, C.I.; Uchiyama, Y.; Ohno, H.; Otani, K.; Dahmani, C.; Mihalef, V.; et al. Rupture risk of small unruptured intracranial aneurysms in japanese adults. Stroke 2020, 51, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lu, M.; Shi, Z.; Xia, S.; Ren, Y.; Liu, Z.; Liu, X.; Li, Z.; Mao, L.; Li, X.L.; et al. Development and validation of machine learning prediction model based on computed tomography angiography-derived hemodynamics for rupture status of intracranial aneurysms: A chinese multicenter study. Eur. Radiol. 2020, 30, 5170–5182. [Google Scholar] [CrossRef] [PubMed]
| Variables | Training Dataset (n = 364) | Test Dataset (n = 93) |
|---|---|---|
| Clinical findings | ||
| Female | 206 (56.6%) | 54 (58.1%) |
| Age, years | 57.2 ± 14.5 | 56.8 ± 15.3 |
| Hypertension | 97 (26.7%) | 28 (30.1%) |
| Diabetes mellitus | 35 (9.6%) | 10 (10.8%) |
| Hyperlipidemia | 42 (11.5%) | 10 (10.8%) |
| Smoking | 48 (13.2%) | 12 (12.9%) |
| Radiologic findings | ||
| High-risk UIA | 133 (36.5%) | 44 (47.3%) |
| Size (mm) | 5.2 ± 1.2 | 5.3 ± 1.3 |
| Aneurysm location | ||
| Anterior cerebral artery | 78 (21.4%) | 15 (16.1%) |
| Middle cerebral artery | 115 (31.6%) | 40 (43.0%) |
| Internal carotid artery | 171 (47.0%) | 38 (40.9%) |
| Imaging platform | ||
| Siemens Healthcare | 210 (57.7%) | 41 (44.1%) |
| Philips Medical Systems | 154 (42.3%) | 52 (55.9%) |
| CNN | ResNet50 Classification | |||
|---|---|---|---|---|
| Diagnosis | High Risk | Non-High Risk | Total | |
| High risk | 36 | 8 | 44 | |
| Non-high risk | 9 | 40 | 49 | |
| Total | 45 | 48 | 93 | |
| Type | Models | Sensitivity (95% CI) | Specificity (95% CI) | Overall Accuracy (95% CI) | F1 Score (95% CI) |
|---|---|---|---|---|---|
| Multi-view | ResNet50 | 81.82 (66.76–91.2)% | 81.63 (67.50–90.76)% | 81.72 (66.98–90.92)% | 80.90 (67.29–91.81)% |
| Multi-view | AlexNet | 63.64 (47.74–77.17)% | 87.76 (74.54–94.92)% | 76.34 (62.31–88.19)% | 71.79 (55.13–85.00)% |
| Multi-view | VGG16 | 68.18 (52.29–80.93)% | 79.59 (65.24–89.28)% | 74.19 (58.93–85.60)% | 71.43 (55.42–84.28)% |
| Multi-view | ResNet101 | 68.18 (52.29–80.93)% | 77.55 (63.01–87.75)% | 73.12 (57.71–84.66)% | 70.59 (55.42–84.28)% |
| Multi-view | ResNet152 | 54.55 (39.00–69.31)% | 87.76 (74.54–94.92)% | 72.04 (58.18–85.68)% | 64.86 (47.46–79.79)% |
| Single-view | ResNet50 | 50.00 (34.79–65.21)% | 77.55 (63.01–87.75)% | 64.52 (48.93–78.45)% | 57.14 (40.82–73.69)% |
| Block | Output Size | ResNet50 | ResNet101 | ResNet152 | VGG16 | Output Size | AlexNet |
|---|---|---|---|---|---|---|---|
| 1 | × 112 | 7 × 7, 64, stride 2 | 7 × 7, 64, stride 2 | 7 × 7, 64, stride 2 | × 55 | 11 × 11, 96, stride 4 | |
| 2 | × 56 | 3 × 3 max-pooling, stride 2 | 3 × 3 max-pooling, stride 2 | 3 × 3 max-pooling, stride 2 | 2 × 2 max-pooling, stride 2 | × 27 | 3 × 3 max-pooling, stride 2 |
| 5 × 5, 256, stride 1 | |||||||
| 3 | × 28 | 2 × 2 max-pooling, stride 2 | × 13 | 3 × 3 max-pooling, stride 2 | |||
| , stride 1 | |||||||
| 4 | × 14 | 2 × 2 max-pooling, stride 2 | × 1 | 3 × 3 max-pooling, stride 2 | |||
| average pooling | |||||||
| [4096 fully connected, | |||||||
| n-class fully connected, softmax | |||||||
| 5 | × 7 | ||||||
| 6 | × 1 | average-pooling | 2 × 2 max-pooling, stride 2 | ||||
| [4096 fully connected, ReLU] | |||||||
| n-class fully connected, softmax | n-class fully connected, softmax | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.H.; Kim, H.C.; Rhim, J.K.; Park, J.J.; Sigmund, D.; Park, M.C.; Jeong, J.H.; Jeon, J.P. Multi-View Convolutional Neural Networks in Rupture Risk Assessment of Small, Unruptured Intracranial Aneurysms. J. Pers. Med. 2021, 11, 239. https://doi.org/10.3390/jpm11040239
Ahn JH, Kim HC, Rhim JK, Park JJ, Sigmund D, Park MC, Jeong JH, Jeon JP. Multi-View Convolutional Neural Networks in Rupture Risk Assessment of Small, Unruptured Intracranial Aneurysms. Journal of Personalized Medicine. 2021; 11(4):239. https://doi.org/10.3390/jpm11040239
Chicago/Turabian StyleAhn, Jun Hyong, Heung Cheol Kim, Jong Kook Rhim, Jeong Jin Park, Dick Sigmund, Min Chan Park, Jae Hoon Jeong, and Jin Pyeong Jeon. 2021. "Multi-View Convolutional Neural Networks in Rupture Risk Assessment of Small, Unruptured Intracranial Aneurysms" Journal of Personalized Medicine 11, no. 4: 239. https://doi.org/10.3390/jpm11040239
APA StyleAhn, J. H., Kim, H. C., Rhim, J. K., Park, J. J., Sigmund, D., Park, M. C., Jeong, J. H., & Jeon, J. P. (2021). Multi-View Convolutional Neural Networks in Rupture Risk Assessment of Small, Unruptured Intracranial Aneurysms. Journal of Personalized Medicine, 11(4), 239. https://doi.org/10.3390/jpm11040239






