Predictive Biomarkers of COVID-19 Severity in SARS-CoV-2 Infected Patients with Obesity and Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Data Collection
2.3. Biochemical Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
3.2. Evaluation of Clinical Outcome of COVID-19 Regarding the Presence of Obesity and Metabolic Syndrome
3.3. Correlation between Clinical and Biochemical Variables with COVID-19 Severity
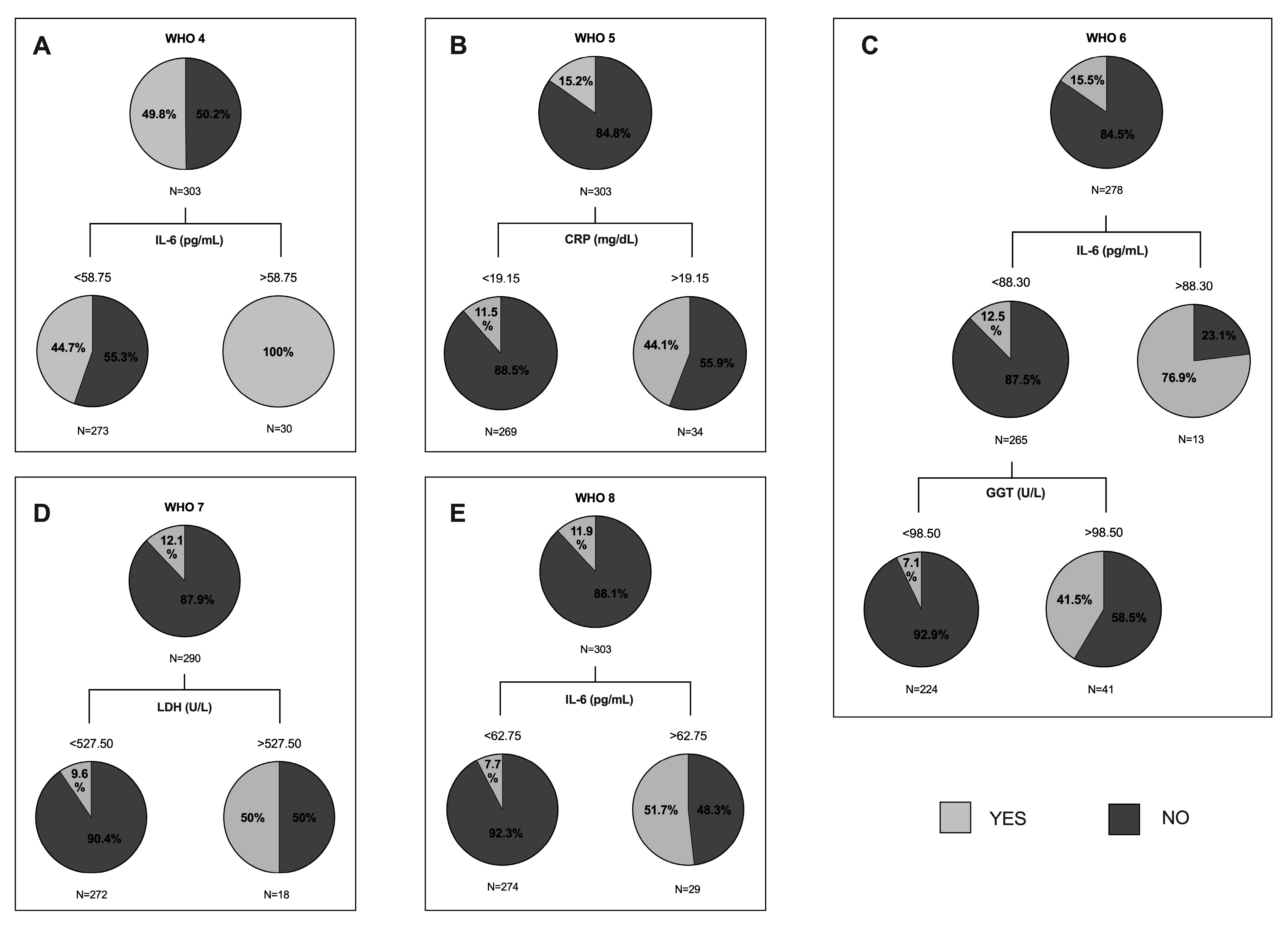
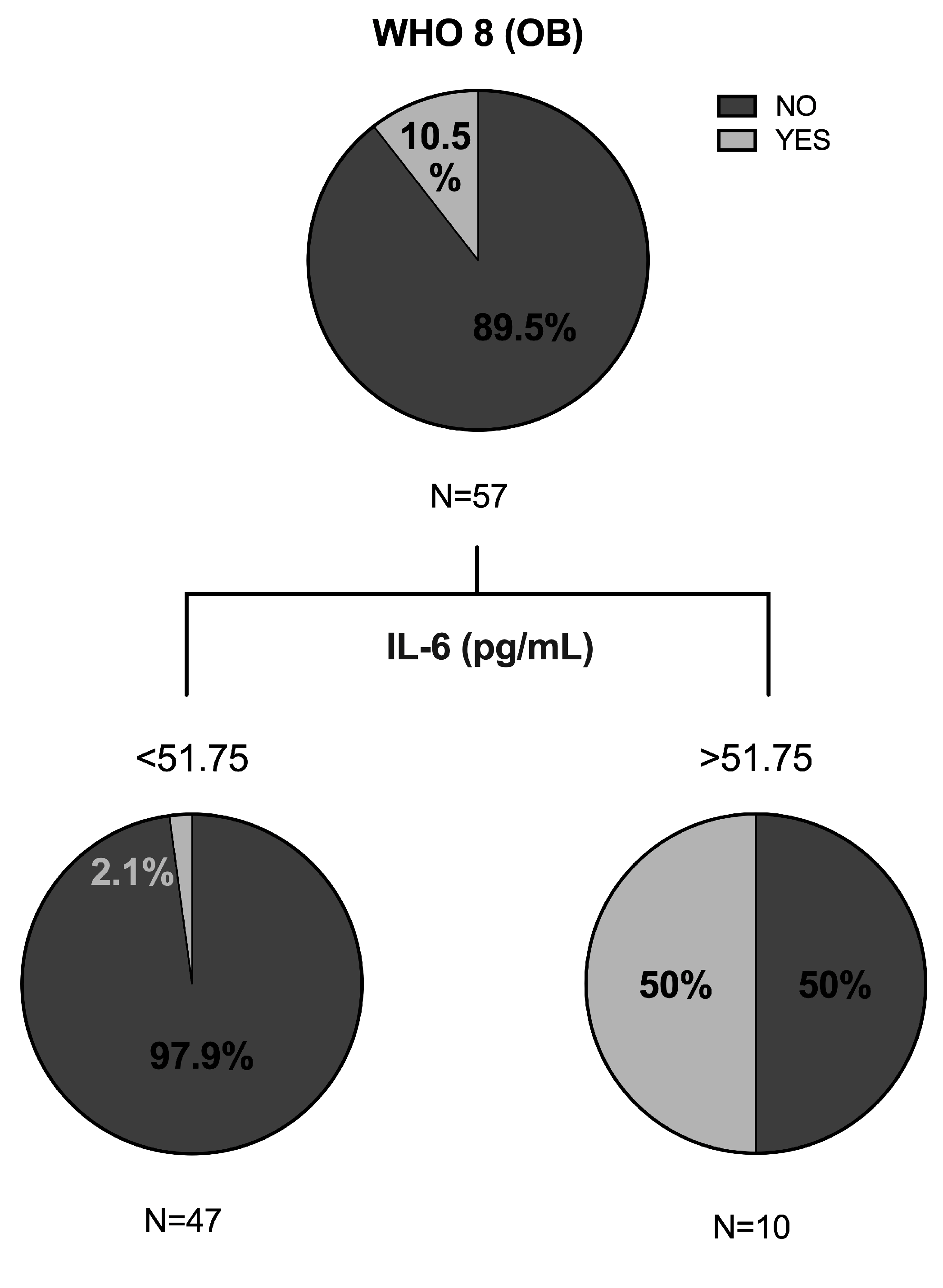
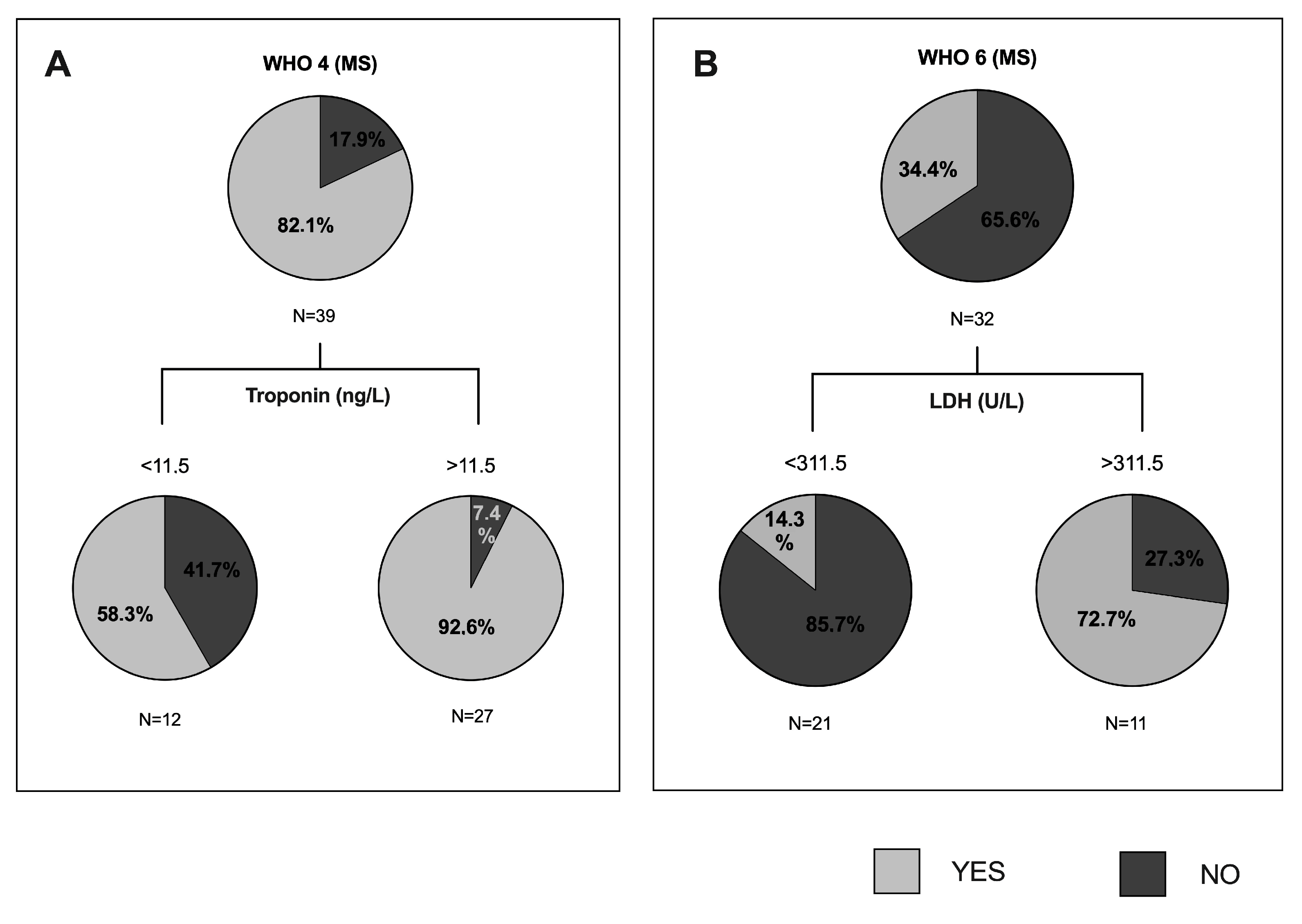
3.4. Predictive Value of Clinical and Biochemical Variables of COVID-19 Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Deng, S.-Q.; Peng, H.-J. Characteristics of and Public Health Responses to the Coronavirus Disease 2019 Outbreak in China. J. Clin. Med. 2020, 9, 575. [Google Scholar] [CrossRef]
- Gupta, R.; Ghosh, A.; Singh, A.K.; Misra, A. Clinical Considerations for Patients with Diabetes in Times of COVID-19 Epidemic. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.H.; Ravussin, E.; Heymsfield, S. COVID 19 and the Patient with Obesity—The Editors Speak Out. Obesity 2020, 28, 847. [Google Scholar] [CrossRef]
- del Rio, C.; Malani, P.N. COVID-19—New Insights on a Rapidly Changing Epidemic. JAMA J. Am. Med Assoc. 2020, 323, 1092–1093. [Google Scholar] [CrossRef]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Chen, F.; Wang, T.; Luo, F.; Liu, X.; Wu, Q.; He, Q.; Wang, Z.; Liu, Y.; Liu, L.; et al. Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China. Diabetes Care 2020, 43, 1392–1398. [Google Scholar] [CrossRef]
- Lighter, J.; Phillips, M.; Hochman, S.; Sterling, S.; Johnson, D.; Francois, F.; Stachel, A. Obesity in Patients Younger than 60 Years Is a Risk Factor for Covid-19 Hospital Admission. Clin. Infect. Dis. 2020, 71, 896–897. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with Overall and Cause-Specific Mortality: A Population-Based Cohort Study of 3·6 Million Adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef]
- Morgan, O.W.; Bramley, A.; Fowlkes, A.; Freedman, D.S.; Taylor, T.H.; Gargiullo, P.; Belay, B.; Jain, S.; Cox, C.; Kamimoto, L.; et al. Morbid Obesity as a Risk Factor for Hospitalization and Death Due to 2009 Pandemic Influenza A(H1N1) Disease. PLoS ONE 2010, 5, e9694. [Google Scholar] [CrossRef]
- Huttunen, R.; Syrjänen, J. Obesity and the Risk and Outcome of Infection. Int. J. Obes. 2013, 37, 333–340. [Google Scholar] [CrossRef]
- Yang, J.K.; Feng, Y.; Yuan, M.Y.; Yuan, S.Y.; Fu, H.J.; Wu, B.Y.; Sun, G.Z.; Yang, G.R.; Zhang, X.L.; Wang, L.; et al. Plasma Glucose Levels and Diabetes Are Independent Predictors for Mortality and Morbidity in Patients with SARS. Diabet. Med. 2006, 23, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zheng, Z.; Zhang, C.; Zhang, X.; Wu, H.; Wang, J.; Wang, S.; Zheng, C. Clinical Characteristics of 145 Patients with Corona Virus Disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection 2020, 48, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Luzi, L.; Radaelli, M.G. Influenza and Obesity: Its Odd Relationship and the Lessons for COVID-19 Pandemic. Acta Diabetol. 2020, 57, 759–764. [Google Scholar] [CrossRef]
- Benetti, E.; Giliberti, A.; Emiliozzi, A.; Valentino, F.; Bergantini, L.; Fallerini, C.; Anedda, F.; Amitrano, S.; Conticini, E.; Tita, R.; et al. Clinical and Molecular Characterization of COVID-19 Hospitalized Patients. PLoS ONE 2020, 15, e0242534. [Google Scholar] [CrossRef]
- Zank, D.C.; Bueno, M.; Mora, A.L.; Rojas, M. Idiopathic Pulmonary Fibrosis: Aging, Mitochondrial Dysfunction, and Cellular Bioenergetics. Front. Med. 2018, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, N.; Oyama, Y.; Yasui, H.; Karayama, M.; Hozumi, H.; Suzuki, Y.; Kono, M.; Furuhashi, K.; Fujisawa, T.; Inui, N.; et al. Analysis of Serum Adiponectin and Leptin in Patients with Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Sci. Rep. 2019, 9, 10484. [Google Scholar] [CrossRef]
- d’Alessandro, M.; Bergantini, L.; Cameli, P.; Lanzarone, N.; Perillo, F.; Perrone, A.; Bargagli, E. BAL and Serum Multiplex Lipid Profiling in Idiopathic Pulmonary Fibrosis and Fibrotic Hypersensitivity Pneumonitis. Life Sci. 2020, 256, 117995. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO R&D Blueprint Novel Coronavirus COVID-19 Therapeutic Trial Synopsis; World Health Organization: Geneva, Switzerland, 2020; pp. 1–9. [Google Scholar]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Caussy, C.; Pattou, F.; Wallet, F.; Simon, C.; Chalopin, S.; Telliam, C.; Mathieu, D.; Subtil, F.; Frobert, E.; Alligier, M.; et al. Prevalence of Obesity among Adult Inpatients with COVID-19 in France. Lancet 2020, 1–3. [Google Scholar] [CrossRef]
- Cunningham, J.W.; Vaduganathan, M.; Claggett, B.L.; Jering, K.S.; Bhatt, A.S.; Rosenthal, N.; Solomon, S.D. Clinical Outcomes in Young US Adults Hospitalized with COVID-19. JAMA Intern. Med. 2020, 8–10. [Google Scholar] [CrossRef]
- Tamara, A.; Tahapary, D.L. Obesity as a Predictor for a Poor Prognosis of COVID-19: A Systematic Review. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Palaiodimos, L.; Kokkinidis, D.G.; Li, W.; Karamanis, D.; Ognibene, J.; Arora, S.; Southern, W.N.; Mantzoros, C.S. Severe Obesity Is Associated with Higher In-Hospital Mortality in a Cohort of Patients with COVID-19 in the Bronx, New York. Metab. Clin. Exp. 2020, 108, 154262. [Google Scholar] [CrossRef] [PubMed]
- Kalligeros, M.; Shehadeh, F.; Mylona, E.K.; Benitez, G.; Beckwith, C.G.; Chan, P.A.; Mylonakis, E. Association of Obesity with Disease Severity Among Patients with Coronavirus Disease 2019. Obesity 2020, 28, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Mesas, A.E.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Cabrera, M.A.S.; de Andrade, S.M.; Sequí-Dominguez, I.; Martínez-Vizcaíno, V. Predictors of In-Hospital COVID-19 Mortality: A Comprehensive Systematic Review and Meta-Analysis Exploring Differences by Age, Sex and Health Conditions. PLoS ONE 2020, 15, e0241742. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yu, X.; Zhao, H.; Wang, H.; Zhao, R.; Sheng, J. Host Susceptibility to Severe COVID-19 and Establishment of a Host Risk Score: Findings of 487 Cases Outside Wuhan. Crit. Care 2020, 24, 2–5. [Google Scholar] [CrossRef]
- Marhl, M.; Grubelnik, V. Diabetes and Metabolic Syndrome as Risk Factors for COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 671–677. [Google Scholar] [CrossRef]
- Pasquarelli-do-Nascimento, G.; Braz-de-Melo, H.A.; Faria, S.S.; Santos, I.D.O.; Kobinger, G.P.; Magalhães, K.G. Hypercoagulopathy and Adipose Tissue Exacerbated Inflammation May Explain Higher Mortality in COVID-19 Patients With Obesity. Front. Endocrinol. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated with Mortality among Patients with COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef]
- Gupta, S.; Hayek, S.S.; Wang, W.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Factors Associated with Death in Critically Ill Patients with Coronavirus Disease 2019 in the US. JAMA Intern. Med. 2020. [Google Scholar] [CrossRef]
- da Silveira, M.P.; da Silva Fagundes, K.K.; Bizuti, M.R.; Starck, É.; Rossi, R.C.; de Resende e Silva, D.T. Physical Exercise as a Tool to Help the Immune System against COVID-19: An Integrative Review of the Current Literature. Clin. Exp. Med. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Farias, F.; Reis, W.; Ribeiro, A.C.; Guimaraes, R.; Duarte, R.-S.; Assunçao, W. Metabolic Syndrome and COVID-19: An Update on the Associated Comorbidities and Proposed Therapies. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 809–814. [Google Scholar] [CrossRef]
- Samaee, H.; Mohsenzadegan, M.; Ala, S.; Sedigh, S. Tocilizumab for Treatment Patients with COVID-19: Recommended Medication for Novel Disease. Int. Immunopharmacol. 2020, 89, 107018. [Google Scholar] [CrossRef]
- Qiu, P.; Zhou, Y.; Wang, F.; Wang, H.; Zhang, M.; Pan, X.; Zhao, Q.; Liu, J. Clinical Characteristics, Laboratory Outcome Characteristics, Comorbidities, and Complications of Related COVID-19 Deceased: A Systematic Review and Meta-Analysis. Aging Clin. Exp. Res. 2020, 32, 1869–1878. [Google Scholar] [CrossRef]
- Martins-Filho, P.R.; Tavares, C.S.S.; Santos, V.S. Factors Associated with Mortality in Patients with COVID-19. A Quantitative Evidence Synthesis of Clinical and Laboratory Data. Eur. J. Intern. Med. 2020, 76, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Trecarichi, E.M.; Mazzitelli, M.; Serapide, F.; Pelle, M.C.; Tassone, B.; Arrighi, E.; Perri, G.; Fusco, P.; Scaglione, V.; Davoli, C.; et al. Clinical Characteristics and Predictors of Mortality Associated with COVID-19 in Elderly Patients from a Long-Term Care Facility. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Cheng, A.; Wang, Y.; Li, H.; Hu, L.; Zhao, X.; Wang, T.; He, F. Cytokines and Their Relationship with the Severity and Prognosis of Coronavirus Disease 2019 ( COVID-19 ): A Retrospective Cohort Study. BMJ Open 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Grifoni, E.; Valoriani, A.; Cei, F.; Lamanna, R.; Gelli, A.M.G.; Ciambotti, B.; Vannucchi, V.; Moroni, F.; Pelagatti, L.; Tarquini, R.; et al. Interleukin-6 as Prognosticator in Patients with COVID-19. J. Infect. 2020, 81, 452–482. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Xu, M.; Wu, J.; Luo, D.; Zhu, Y.; Li, B.; Song, X. Prognostic Value of Interleukin-6, C-Reactive Protein, and Procalcitonin in Patients. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef]
| Variables | WOB (n = 176) Mean ± SD; N (%) | OB (n = 67) Mean + SD; N (%) | p-Value | |
|---|---|---|---|---|
| Age (years) | 57 (41.25–72) | 56 (44–68) | 0.678 | |
| Sex (%) | Male | 88 (50) | 33 (49.3) | 0.917 |
| Female | 88 (50) | 34 (50.7) | ||
| BMI (kg/m2) | 25.1 (22.92–27.34) | 34.08 (31.25–37.70) | <0.001 * | |
| SBP (mmHg) | 126.62 (20.13) | 132.58 (20.19) | 0.062 | |
| DBP (mmHg) | 77 (69–85) | 80 (71–88.25) | 0.085 | |
| Background (N (%)) | ||||
| Exercise | 77 (53.5) | 17 (30.4) | 0.003 * | |
| Smoking | Active | 16 (9.4) | 5 (7.7) | 0.737 |
| Former | 19 (11.2) | 7 (10.8) | 0.687 | |
| Alcohol consumer | 32 (19.2) | 11 (17.5) | 0.938 | |
| MS | 10 (5.7) | 24 (35.8) | <0.001 * | |
| T2DM | 20 (11.4) | 15 (22.4) | 0.029 * | |
| Dyslipidemia | 51 (29) | 20 (29.9) | 0.849 | |
| Hypertension | 55 (31.3) | 32 (47.8) | 0.017 * | |
| CVD | 30 (17) | 8 (11.9) | 0.329 | |
| Respiratory diseases | 13 (7.4) | 7 (10.4) | 0.439 | |
| Cancer | 15 (8.5) | 6 (9) | 0.915 | |
| Clinical Characteristics (N (%)) | ||||
| Symptoms | Mild | 57 (32.4) | 12 (17.9) | 0.026 * |
| Moderate | 73 (41.5) | 39 (58.2) | 0.020 * | |
| Critical | 36 (20.5) | 14 (22.8) | 0.940 | |
| ICU admission | 33 (18.8) | 13 (20.9) | 0.908 | |
| Mortality | 13 (7.4) | 8 (11.9) | 0.260 | |
| Radiological characteristics | Bilateral interstitial pattern | 90 (51.1) | 45 (67.2) | 0.025 * |
| Pleural effusion | 2 (1.1) | 0 (0) | 0.382 | |
| Pneumonia | Mild | 17 (9.7) | 4 (6) | 0.361 |
| Moderate | 68 (38.6) | 37 (55.2) | 0.020 * | |
| Severe | 37 (21) | 17 (25.4) | 0.467 | |
| Respiratory failure | 66 (37.5) | 34 (50.7) | 0.061 | |
| PTE | 4 (2.3) | 3 (4.5) | 0.359 | |
| Treatment (N (%)) | ||||
| Hydroxychloroquine | 73 (41.5) | 30 (44.8) | 0.643 | |
| Azithromycin | 63 (35.8) | 29 (43.9) | 0.246 | |
| Lopinavir-ritonavir | 59 (33.5) | 22 (32.8) | 0.919 | |
| Tocilizumab | 3 (1.7) | 5 (7.5) | 0.025 * | |
| Interferon | 9 (5.1) | 2 (3) | 0.477 | |
| Corticosteroids | 44 (25) | 23 (34.3) | 0.147 | |
| Remdesivir | 21 (11.9) | 13 (19.4) | 0.134 | |
| Other | 101 (57.4) | 47 (70.1) | 0.069 | |
| Oxygen Therapy (N (%)) | ||||
| Oxygen mask or nasal | 80 (45.5) | 36 (53.7) | 0.249 | |
| High-flow nasal cannulas | 27 (15.3) | 10 (14.9) | 0.936 | |
| NIV | CPAP | 3 (1.7) | 3 (4.5) | 0.214 |
| BiPAP | 1 (0.6) | 1 (1.5) | 0.477 | |
| MV | Intubation | 29 (16.5) | 9 (13.4) | 0.560 |
| Mask with reservoir | 9 (5.1) | 8 (11.9) | 0.063 | |
| MV/ECMO | Vasopressors | 22 (12.5) | 9 (13.4) | 0.846 |
| Dialysis | 5 (2.8) | 2 (3) | 0.312 | |
| Biochemical Parameters (Mean (SD); Median (25th–75th Percentiles)) | ||||
| Leukocytes (x109/L) | 6.61 (4.33–3.37) | 6.37 (4.97–8.66) | 0.727 | |
| Lymphocytes (%) | 15.1 (9–25.4) | 16.85 (11–23.02) | 0.404 | |
| D-Dimer (ng/mL) | 579 (366–1190) | 591 (429–979) | 0.559 | |
| ESR (mm) | 64 (38–97.5) | 54 (32–73.5) | 0.120 | |
| IL-6 (pg/mL) | 8.46 (4.52–29.17) | 13.3 (6.7–38.5) | 0.174 | |
| Ferritin (ng/mL) | 400 (160.75–831.5) | 414 (149.25–633) | 0.836 | |
| CRP (mg/dL) | 7.1 (2.6–14) | 7.5 (3.35–14.8) | 0.568 | |
| Glucose (mg/dL) | 102.5 (86–125) | 117 (99–141) | 0.006 * | |
| Total-cholesterol (mg/dL) | 133.2 (37.37) | 144.88 (32.30) | 0.015 * | |
| HDL-c (mg/dL) | 31.5 (9.92) | 31.25 (8.73) | 0.999 | |
| LDL-c (mg/dL) | 70.46 (27.52) | 95.96 (27.95) | 0.102 | |
| Triglycerides (mg/dL) | 119 (89–224) | 142.5 (97.25–156.75) | 0.896 | |
| Creatinine (mg/dL) | 0.79 (0.61–0.95) | 0.74 (0.66–0.93) | 0.714 | |
| AST (U/L) | 31 (23–44) | 33.5 (21.75–48) | 0.347 | |
| ALT (U/L) | 28 (19–52.75) | 32.5 (21.25–58.5) | 0.191 | |
| GGT (U/L) | 49 (25–89) | 57.5 (38.5–89.75) | 0.157 | |
| AP (U/L) | 69 (54–90) | 68.5 (54.25–92.5) | 0.600 | |
| LDH (U/L) | 263 (221.25–315) | 287 (237.5–344) | 0.047 * | |
| Troponin (ng/L) | 8 (3.75–19.25) | 6 (3–26) | 0.666 | |
| Variables | NMS (n = 255) Mean ± SD; N (%) | MS (n = 48) Mean + SD; N (%) | p-Value | |||
|---|---|---|---|---|---|---|
| Age (years) | 56 (41–72) | 72.5 (64.25–78) | <0.001 * | |||
| Sex (%) | Male | 126 (49.4) | 33 (68.8) | 0.014 * | ||
| Female | 129 (50.6) | 15 (31.3) | ||||
| BMI (kg/m2) | 26.06 (23.61–29.31) | 31.63 (27.27–35.12) | <0.001 * | |||
| SBP (mmHg) | 127.16 (20.03) | 136.56 (18.27) | 0.003 * | |||
| DBP (mmHg) | 78 (70.25–86) | 75 (70.5–82.5) | 0.161 | |||
| Background (N (%)) | ||||||
| Exercise | 89 (46.6) | 5 (15.6) | 0.001 * | |||
| Smoking | Active | 20 (8.4) | 6 (14.3) | 0.291 | ||
| Former | 25 (10.5) | 11 (26.2) | 0.010 * | |||
| Alcohol consumer | 44 (18.9) | 10 (25) | 0.371 | |||
| Obesity | 43 (20.6) | 24 (70.6) | <0.001 * | |||
| T2DM | 15 (5.9) | 37 (77.1) | <0.001 * | |||
| Dyslipidemia | 57 (22.4) | 43 (89.6) | <0.001 * | |||
| Hypertension | 75 (29.4) | 48 (100) | <0.001 * | |||
| CVD | 29 (11.4) | 23 (47.9) | <0.001 * | |||
| Respiratory diseases | 18 (7.1) | 11 (22.9) | 0.001 * | |||
| Cancer | 21 (8.2) | 6 (12.5) | 0.342 | |||
| Clinical Characteristics (N (%)) | ||||||
| Symptoms | Mild | 69 (27.1) | 5 (10.4) | 0.707 | ||
| Moderate | 124 (48.6) | 29 (60.4) | 0.014 * | |||
| Critical | 48 (18.8) | 12 (25) | 0.135 | |||
| ICU admission | 42 (16.5) | 9 (18.8) | 0.699 | |||
| Mortality | 22 (8.6) | 14 (29.2) | <0.001 * | |||
| Radiological characteristics | Bilateral interstitial pattern | 149 (58.4) | 32 (66.7) | 0.287 | ||
| Pleural effusion | 3 (1.2) | 1 (2.1) | 0.451 | |||
| Pneumonia | Mild | 20 (7.8) | 3 (6.3) | 0.703 | ||
| Moderate | 117 (45.9) | 22 (45.8) | 0.995 | |||
| Severe | 54 (21.2) | 18 (37.5) | 0.015 * | |||
| Respiratory failure | 103 (40.4) | 29 (60.4) | 0.01 * | |||
| PTE | 6 (2.4) | 2 (4.2) | 0.473 | |||
| Treatment (N (%)) | ||||||
| Hydroxychloroquine | 110 (43.1) | 20 (41.7) | 0.850 | |||
| Azithromycin | 103 (40.4) | 20 (42.6) | 0.782 | |||
| Lopinavir-ritonavir | 82 (32.2) | 12 (25) | 0.326 | |||
| Tocilizumab | 9 (3.5) | 4 (8.3) | 0.133 | |||
| Interferon | 13 (5.1) | 1 (2.1) | 0.362 | |||
| Corticosteroids | 71 (27.8) | 17 (35.4) | 0.290 | |||
| Remdesivir | 38 (14.9) | 10 (20.8) | 0.303 | |||
| Other | 160 (62.7) | 38 (79.2) | 0.029 * | |||
| Oxygen Therapy (N (%)) | ||||||
| Oxygen mask or nasal | 122 (47.8) | 30 (62.5) | 0.063 | |||
| High-flow nasal cannulas | 38 (14.9) | 8 (16.7) | 0.755 | |||
| NIV | CPAP | 5 (2) | 2 (4.2) | 0.351 | ||
| BiPAP | 2 (0.8) | 0 (0) | 0.539 | |||
| MV | Intubation | 35 (13.7) | 8 (16.7) | 0.593 | ||
| Mask with reservoir | 19 (7.5) | 6 (12.5) | 0.244 | |||
| MV/ECMO | Vasopressors | 28 (11) | 7 (14.6) | 0.474 | ||
| Dialysis | 7 (2.7) | 1 (2.1) | 0.793 | |||
| Biochemical Parameters (Mean (SD); Median (25th–75th Percentiles)) | ||||||
| Leukocytes (x109/L) | 6.62 (4.78–8.70) | 75 (70.5) | 0.870 | |||
| Lymphocytes (%) | 16.8 (9.55–24) | 6.47 (4.73–8.55) | 0.219 | |||
| D-Dimer (ng/mL) | 620 (395.5–1264.5) | 764 (435.25–1329.25) | 0.295 | |||
| ESR (mm) | 60 (36–88.75) | 60 (32.25–115.5) | 0.745 | |||
| IL-6 (pg/mL) | 12.55 (5.22–30.37) | 16 (5.88–58.8) | 0.349 | |||
| Ferritin (ng/mL) | 418 (162–822) | 396 (167–556) | 0.310 | |||
| CRP (mg/dL) | 7.7 (3.1–14) | 7.9 (2.45–16.7) | 0.725 | |||
| Glucose (mg/dL) | 103 (85–124) | 132 (107.25–157) | <0.001 * | |||
| Total-cholesterol (mg/dL) | 137.35 (34.45) | 132.59 (35.43) | 0.572 | |||
| HDL-c (mg/dL) | 31.93 (8.94) | 25.33 (10.11) | 0.284 | |||
| LDL-c (mg/dL) | 75.21 (28.72) | 60.67 (23.48) | 0.529 | |||
| Triglycerides (mg/dL) | 121.5 (87.5–221.75) | 142.5 (110–165) | 0.734 | |||
| Creatinine (mg/dL) | 0.78 (0.62–0.96) | 0.92 (0.75–1.14) | <0.001 * | |||
| AST (U/L) | 31.5 (24–45) | 23 (19–48) | 0.098 | |||
| ALT (U/L) | 28 (19–52) | 28 (16.25–41.75) | 0.538 | |||
| GGT (U/L) | 49 (25–89) | 57.5 (38.5–89.75) | 0.157 | |||
| AP (U/L) | 69 (54–90) | 68.5 (54.25–92.5) | 0.600 | |||
| LDH (U/L) | 263 (221.25–315) | 287 (237.5–344) | 0.047 * | |||
| Troponin (ng/L) | 8 (3.75–19.25) | 6 (3–26) | 0.666 | |||
| Correlations | WHO 4 | WHO 5 | WHO 6 | WHO 7 | WHO 8 |
|---|---|---|---|---|---|
| Anthropometric Data | |||||
| Age (years) | 0.336 ** | 0.182 ** | 0.19 ** | 0.093 | 0.324 ** |
| BMI (kg/m2) | 0.187 ** | 0.107 | 0.154 ** | 0.103 | 0.067 |
| SBP (mmHg) | 0.112 | −0.017 | 0.052 * | 0.038 | 0.097 |
| DBP (mmHg) | −0.07 | −0.094 | −0.075 | −0.143 * | −0.112 |
| Background and Comorbidities | |||||
| Exercise | −0.308 ** | −0.194 ** | −0.287 ** | −0.22 ** | −0.275 ** |
| MS | 0.107 | 0.018 | 0.031 | 0.041 | 0.232 ** |
| T2DM | 0.191 ** | 0.125 * | 0.148 * | 0.021 | 0.212 ** |
| Dyslipidemia | 0.11 | 0.075 | 0.069 | 0.143 * | 0.176 ** |
| Hypertension | 0.286 ** | 0.062 | 0.1 | 0.091 | 0.257 ** |
| CVD | 0.069 | −0.046 | −0.048 | 0.002 | 0.157 ** |
| Respiratory diseases | −0.035 | 0.019 | −0.003 | −0.082 | 0.123 * |
| Cancer | 0.103 | 0.126* | 0.085 | 0.063 | 0.279 ** |
| COVID-19 Treatment | |||||
| Hydroxychloroquine | 0.664 ** | 0.284 ** | 0.496 ** | 0.336 ** | 0.218 ** |
| Azithromycin | 0.217 ** | 0.024 | 0.066 | −0.04 | 0.049 |
| Lopinavir−ritonavir | 0.569 ** | 0.293 ** | 0.462 ** | 0.372 ** | 0.107 |
| Tocilizumab | 0.146 * | 0.137 * | 0.011 | 0.044 | 0.124 * |
| Interferon | 0.219 ** | 0.214 ** | 0.329 ** | 0.416 ** | 0.065 |
| Remdesivir | −0.038 | 0.043 | −0.133 * | −0.042 | −0.02 |
| Biochemical Parameters | |||||
| Leukocytes (x109/L) | 0.057 | 0.024 | 0.16 * | 0.101 | 0.087 |
| Lymphocytes (%) | −0.221 ** | −0.217 ** | −0.277 ** | −0.204 ** | −0.158 * |
| D-Dimer (ng/mL) | 0.246 ** | 0.119 | 0.192 * | 0.166 ** | 0.236 ** |
| ESR (mm) | 0.19 * | 0.097 | 0.114 | 0.138 | 0.036 |
| IL-6 (pg/mL) | 0.285 ** | 0.114 | 0.32 ** | 0.222 ** | 0.262 ** |
| Ferritin (ng/mL) | 0.194 * | 0.162 * | 0.204 ** | 0.15 * | 0.082 |
| CRP (mg/dL) | 0.334 ** | 0.223 ** | 0.323 ** | 0.177 ** | 0.085 |
| Glucose (mg/dL) | 0.129 * | 0.038 | 0.116 | 0.106 | 0.165 ** |
| Triglycerides (mg/dL) | 0.501 ** | 0.122 | 0.289 | 0.188 | 0.074 |
| Creatinine (mg/dL) | 0.056 | −0.026 | 0.065 | 0.091 | 0.212 ** |
| AST (U/L) | 0.104 | 0.106 | 0.137 * | 0.111 | 0.02 |
| GGT (U/L) | 0.243 ** | 0.121 | 0.15 * | 0.186 ** | −0.054 |
| AP (U/L) | 0.092 | 0.021 | 0.146 * | 0.117 | 0.09 |
| LDH (U/L) | 0.299 ** | 0.22 ** | 0.286 ** | 0.21 ** | 0.15 * |
| Troponin (ng/L) | 0.281 ** | 0.124 | 0.27 ** | 0.244 ** | 0.319 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perpiñan, C.; Bertran, L.; Terra, X.; Aguilar, C.; Lopez-Dupla, M.; Alibalic, A.; Riesco, D.; Camaron, J.; Perrone, F.; Rull, A.; et al. Predictive Biomarkers of COVID-19 Severity in SARS-CoV-2 Infected Patients with Obesity and Metabolic Syndrome. J. Pers. Med. 2021, 11, 227. https://doi.org/10.3390/jpm11030227
Perpiñan C, Bertran L, Terra X, Aguilar C, Lopez-Dupla M, Alibalic A, Riesco D, Camaron J, Perrone F, Rull A, et al. Predictive Biomarkers of COVID-19 Severity in SARS-CoV-2 Infected Patients with Obesity and Metabolic Syndrome. Journal of Personalized Medicine. 2021; 11(3):227. https://doi.org/10.3390/jpm11030227
Chicago/Turabian StylePerpiñan, Carles, Laia Bertran, Ximena Terra, Carmen Aguilar, Miguel Lopez-Dupla, Ajla Alibalic, David Riesco, Javier Camaron, Francesco Perrone, Anna Rull, and et al. 2021. "Predictive Biomarkers of COVID-19 Severity in SARS-CoV-2 Infected Patients with Obesity and Metabolic Syndrome" Journal of Personalized Medicine 11, no. 3: 227. https://doi.org/10.3390/jpm11030227
APA StylePerpiñan, C., Bertran, L., Terra, X., Aguilar, C., Lopez-Dupla, M., Alibalic, A., Riesco, D., Camaron, J., Perrone, F., Rull, A., Reverté, L., Yeregui, E., Marti, A., Vidal, F., & Auguet, T. (2021). Predictive Biomarkers of COVID-19 Severity in SARS-CoV-2 Infected Patients with Obesity and Metabolic Syndrome. Journal of Personalized Medicine, 11(3), 227. https://doi.org/10.3390/jpm11030227








