Body Composition Change, Unhealthy Lifestyles and Steroid Treatment as Predictor of Metabolic Risk in Non-Hodgkin’s Lymphoma Survivors
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Body Composition Assessmen
2.3. Metabolic Parameters’ Measurement
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morton, L.M.; Wang, S.S.; Devesa, S.S.; Hartge, P.; Weisenburger, D.D.; Linet, M.S. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006, 107, 265–276. [Google Scholar] [CrossRef]
- Denlinger, C.S.; Sanft, T.; Moslehi, J.J.; Overholser, L.; Armenian, S.; Baker, K.S.; Bro-derick, G.; Demark-Wahnefried, W.; Friedman, D.L.; Goldman, M.; et al. NCCN Guidelines Insights: Survi-vorship, Version 2.2020. JNCCN 2020, 18. [Google Scholar] [CrossRef]
- Sheldon, T. Cancer survival rates continue to rise in the Netherlands. BMJ 2001, 322, 1509. [Google Scholar] [CrossRef]
- Ciavarella, S.; Minoia, C.; Quinto, A.M.; Oliva, S.; Carbonara, S.; Cormio, C.; Cox, M.C.; Bravo, E.; Santoro, F.; Napolitano, M.; et al. Improving Provision of Care for Long-term Survivors of Lymphoma. Clin. Lymphoma Myeloma Leuk. 2017, 17, e1–e9. [Google Scholar] [CrossRef]
- Coiffier, B.; Thieblemont, C.; Van Den Neste, E.; Lepeu, G.; Plantier, I.; Castaigne, S.; Lefort, S.; Marit, G.; Macro, M.; Sebban, C.; et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d’Etudesdes Lymphomes de l’Adulte. Blood 2010, 116, 2040–2045. [Google Scholar] [CrossRef]
- Minoia, C.; Bari, A.; Nassi, L.; Banzi, R.; Gerardi, C.; Lenti, V.; Calabrese, M.; Spina, M.; Guarini, A. Management of lymphoma survivor patients in Italy: An evaluation by Fondazione Italiana Linfomi. Tumori 2020, 300891620905649. [Google Scholar] [CrossRef] [PubMed]
- Minoia, C.; Ciavarella, S.; Lerario, G.; Daniele, A.; De Summa, S.; Napolitano, M.; Guarini, A. Improvable Lifestyle Factors in Lymphoma Survivors. Acta Haematol. 2018, 139, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Krolak, D.; Collins, B.; Weiss, L.; Harris, C.; Van der Jagt, R. Cognitive function and its relationship to other psycho social factors in lymphoma survivors. Support Care Cancer 2017, 25, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Spector, D.J.; Noonan, D.; Mayer, D.K.; Benecha, H.; Zimmerman, S.; Smith, S.K. Are lifestyle behavioral factor associated with health-related quality of life in long-term survivors of non-Hodgkin lymphoma? Cancer 2015, 121, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, K.M.; Rowland, J.H.; Arora, N.K.; Hamilton, A.S.; Miller, M.F.; Aziz, N.M. Physical activity and quality of life in adult survivors of non-Hodgkin’s lymphoma. J. Clin. Oncol. 2009, 27, 960–966. [Google Scholar] [CrossRef]
- Talvensaari, K.K.; Lanning, M.; Tapanainen, P.; Knip, M. Long-term survivors of childhood cancer have an increased risk of manifesting the metabolic syndrome. J. Clin. Endocrinol. Metab. 1996, 81, 3051–3055. [Google Scholar] [CrossRef]
- Després, J.P. Health consequences of visceral obesity. AnnMed 2001, 33, 534–541. [Google Scholar] [CrossRef]
- Fujioka, S.; Matsuzawa, Y.; Tokunaga, K.; Tarui, S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism 1987, 36, 54–59. [Google Scholar] [CrossRef]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Boyko, E.J.; Leonetti, D.L.; McNeely, M.J.; Newell-Morris, L.; Kahn, S.E.; Fujimoto, W.Y. Visceral adiposity is an independent predictor of incident hypertension in Japanese Americans. Ann. Intern. Med. 2004, 140, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, W.Y.; Bergstrom, R.W.; Boyko, E.J.; Chen, K.W.; Leonetti, D.L.; Newell-Morris, L.; Shofer, J.B.; Wahl, P.W. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care 1999, 22, 1808–1812. [Google Scholar] [CrossRef] [PubMed]
- Boyko, E.J.; Fujimoto, W.Y.; Leonetti, D.L.; Newell-Morris, L. Visceral adiposity and risk of type 2 diabetes: A prospective study among Japanese Americans. Diabetes Care 2000, 23, 465–471. [Google Scholar] [CrossRef]
- Janssen, I. Influence of sarcopenia on the development of physical disability: The Cardiovascular Health Study. J. Am. Geriatr. Soc. 2006, 54, 56–62. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/NationalHeart, Lung, and Blood Institute scientific statement: Executive Summary. Crit. Pathw. Cardiol. 2005, 4, 198–203. [Google Scholar]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Malhotra, J.; Tonorezos, E.S.; Rozenberg, M.; Vega, G.L.; Sklar, C.A.; Chou, J.; Moskowitz, C.S.; Eshelman-Kent, D.A.; Janiszewski, P.; Ross, R.; et al. Atherogenic low density lipoprotein phenotype in long-term survivors of childhood acute lymphoblastic leukemia. J. Lipid Res. 2012, 53, 2747–2754. [Google Scholar] [CrossRef]
- Barbosa-Cortés, L.; López-Alarcón, M.; Mejía-Aranguré, J.M.; Klünder-Klünder, M.; Del Carmen Rodríguez-Zepeda, M.; Rivera-Márquez, H.; De la Vega-Martínez, A.; Martin-Trejo, J.; Shum-Luis, J.; Solis-Labastida, K.; et al. Adipokines, insulin resistance, and adiposityas a predictors of metabolic syndrome in child survivors of lymphoma and acute lymphoblastic leukemia of a developing country. BMC Cancer 2017, 17, 125. [Google Scholar] [CrossRef]
- Morel, S.; Leahy, J.; Fournier, M.; Lamarche, B.; Garofalo, C.; Grimard, G.; Poulain, F.; Delvin, E.; Laverdière, C.; Krajinovic, M.; et al. Lipid and lipoprotein abnormalities in acute lymphoblastic leukemia survivors. J. Lipid Res. 2017, 58, 982–993. [Google Scholar] [CrossRef]
- Chueh, H.W.; Yoo, J.H. Metabolicsyndromeinduced by anticancer treatment in childhood cancer survivors. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Daniele, A.; De Summa, S.; Lerario, G.; Divella, R.; Ditonno, P.; Ciavarella, S.; Paradiso, A.V.; Sciacovelli, A.M.; De Luca, R.; Savino, E.; et al. Obesity and Metabolic Syndrome among Adult Lymphoma Survivors. Ann. Hematol. Oncol. 2019, 6, 1–7. [Google Scholar]
- Lohman, T.; Roche, A.; Martorell, R. Antropometric Standardization Reference Manual; Human Kinetics Publishers: Champaign, IL, USA, 1988; pp. 2–80. [Google Scholar]
- Durnin, J.V.; Womersley, J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br. J. Nutr. 1974, 32, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 2014, 63, 2985–3023. [Google Scholar]
- Costa, N.A.; Marinho, A.D.; Cançado, L.R. Nutritional requirements of the critically ill patient. Rev. Bras. Ter. Intensiva 2012, 24, 270–277. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Carr, M.C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef]
- Laaksonen, D.E.; Niskanen, L.; Punnonen, K.; Nyyssönen, K.; Tuomainen, T.P.; Valkonen, V.P.; Salonen, R.; Salonen, J.T. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 2004, 27, 1036–1041. [Google Scholar] [CrossRef]
- Rosen, G.P.; Nguyen, H.T.; Shaibi, G.Q. Metabolic syndrome in pediatric cancer survivors: A mechanistic review. Pediatr. Blood Cancer 2013, 60, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed]
- Stringer, A.M.; Gibson, R.J.; Bowen, J.M.; Keefe, D.M. Chemotherapy-induced modifications to gastrointestinal microflora: Evidence and implications of change. Curr. Drug Metab. 2009, 10, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.; Saarinen-Pihkala, U.M.; Hovi, L.; Lipsanen-Nyman, M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet 2000, 356, 993–997. [Google Scholar] [CrossRef]
- Didi, M.; Didcock, E.; Davies, H.A.; Ogilvy-Stuart, A.L.; Wales, J.K.; Shalet, S.M. High incidence of obesity in young adults after treatment of acute lymphoblastic leukemia in childhood. J. Pediatr. 1995, 127, 63–67. [Google Scholar] [CrossRef]
- Kourti, M.; Tragiannidis, A.; Makedou, A.; Papageorgiou, T.; Rousso, I.; Athanassiadou, F. Metabolic syndrome in children and adolescents with acute lymphoblastic leukemia after the completion of chemotherapy. J. Pediatr. Hematol. Oncol. 2005, 27, 499–501. [Google Scholar] [CrossRef]
- Gallagher, D.; DeLegge, M. Body composition (sarcopenia) in obese patients: Implications for care in the intensive care unit. JPEN J. Parenter. Enteral Nutr. 2011, 35 (Suppl. 5), 21S–28S. [Google Scholar] [CrossRef]
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Consitt, L.A.; Bell, J.A.; Houmard, J.A. Intramuscular lipid metabolism, insulin action, and obesity. IUBMB Life 2009, 61, 47–55. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Oldervoll, L.M.; Loge, J.H.; Kaasa, S.; Lydersen, S.; Hjermstad, M.J.; Thorsen, L.; Holte, H., Jr.; Jacobsen, A.B.; Fosså, S.D. Physical activity in Hodgkin’s lymphoma survivors with and without chronic fatigue compared with the general population—A cross-sectional study. BMC Cancer 2007, 7, 210. [Google Scholar] [CrossRef] [PubMed]
| Years | (Range) | |
|---|---|---|
| Male | (25–82) | |
| Female | (24–76) | |
| Hystotypes | n° | % |
| DLBCL | 57 | 95 |
| FL | 3 | 5 |
| Treatment | n° | % |
| Chemotherapy | 60 | 100 |
| Autologous transplantation | 9 | 15 |
| Radiotherapy | 54 | 90 |
| High-dose steroids | n° | % |
| Yes | 50 | 83 |
| No | 10 | 17 |
| Hypertension | n° | % |
| Yes (BP > 130/85 mmHg) | 32 | 53 |
| No | 28 | 47 |
| Weight Status | n° | % |
| Obesity/Overweight | 43 | 72 |
| Normal weight | 8 | 13 |
| Underweight | 9 | 15 |
| Biotype | n° | % |
| Ginoid | 27 | 45 |
| Android | 17 | 28 |
| Intermediate | 16 | 27 |
| Metabolic syndrome | n° | % |
| Yes | 36 | 60 |
| No | 24 | 40 |
| Excess malnutrition | n° | % |
| Yes | 36 | 60 |
| No | 24 | 40 |
| Unbalanced Diet | n° | % |
| Yes | 35 | 58 |
| No | 25 | 42 |
| Metabolic risk | n° | % |
| High | 32 | 53 |
| Low | 28 | 47 |
| Sarcopenia | n° | % |
| Yes | 38 | 63 |
| No | 22 | 37 |
| Smoke | n° | % |
| Smoker | 15 | 25 |
| Nonsmoker | 13 | 22 |
| Ex-smoker | 32 | 53 |
| Physical Activity | n° | % |
| Yes | 22 | 37 |
| No | 38 | 63 |
| DLBCL (N = 60) | |||
|---|---|---|---|
| MetSyn/yes (N = 36) | MetSyn/no (N = 24) | p-Value | |
| Anthropometry | |||
| Weight (Kg) | 76.3 ± 14.0 | 73.0 ± 13.0 | n.s |
| Height (cm) | 169.5 ± 2.5 | 166.5 ± 6.7 | n.s |
| BMI (kg/h2) | 27.5 ± 4.5 | 26.6 ± 4.2 | n.s |
| Waist circumference | |||
| Women | 98.0 ± 17.0 | 84.0 ± 11.0 | 0.001 |
| Men | 104.0 ± 9.0 | 93.3 ± 8.1 | 0.005 |
| Percentage body fat | |||
| Women | 39.5 ± 5.0 | 38.4 ± 2.6 | ns |
| Men | 32.1 ± 4.2 | 28.2 ± 5.0 | 0.04 |
| Percentage fat-free mass | |||
| Women | 60.6 ± 4.0 | 61.5 ± 2.5 | 0.45 |
| Men | 67.9 ± 4.1 | 71.7 ± 5.0 | 0.002 |
| TDEE | 2113 ± 309 | 2185 ± 275 | ns |
| Inflammatory markers | |||
| CRP (mg/dL) | 2.10 ± 2.02 | 1.90 ± 1.01 | n.s |
| β2-microglobulin (mg/L) | 1.08 ± 0.45 | 1.82 ± 0.45 | 0.001 |
| Metabolic parameters | |||
| Glycemia (mg/dL) | 101.5 ± 17.0 | 99.3 ± 7.2 | n.s |
| Total cholesterol (mg/dL) | 201.0 ± 30.8 | 199.0 ± 21.0 | n.s |
| HDL-cholesterol (mg/dL) | 52.4 ± 11.9 | 53.0 ± 11.0 | n.s |
| Triglycerides(mg/dL) | 133.2 ± 12 | 130.3 ± 6.0 | n.s |
| Albumin (%) | 55.5 ± 5.3 | 56.1 ± 4.7 | n.s |
| TFR | 250.5 ± 35.0 | 270.7 ± 31.0 | n.s |
| HCT | 41.7 ± 3.8 | 43.5 ± 2.5 | n.s |
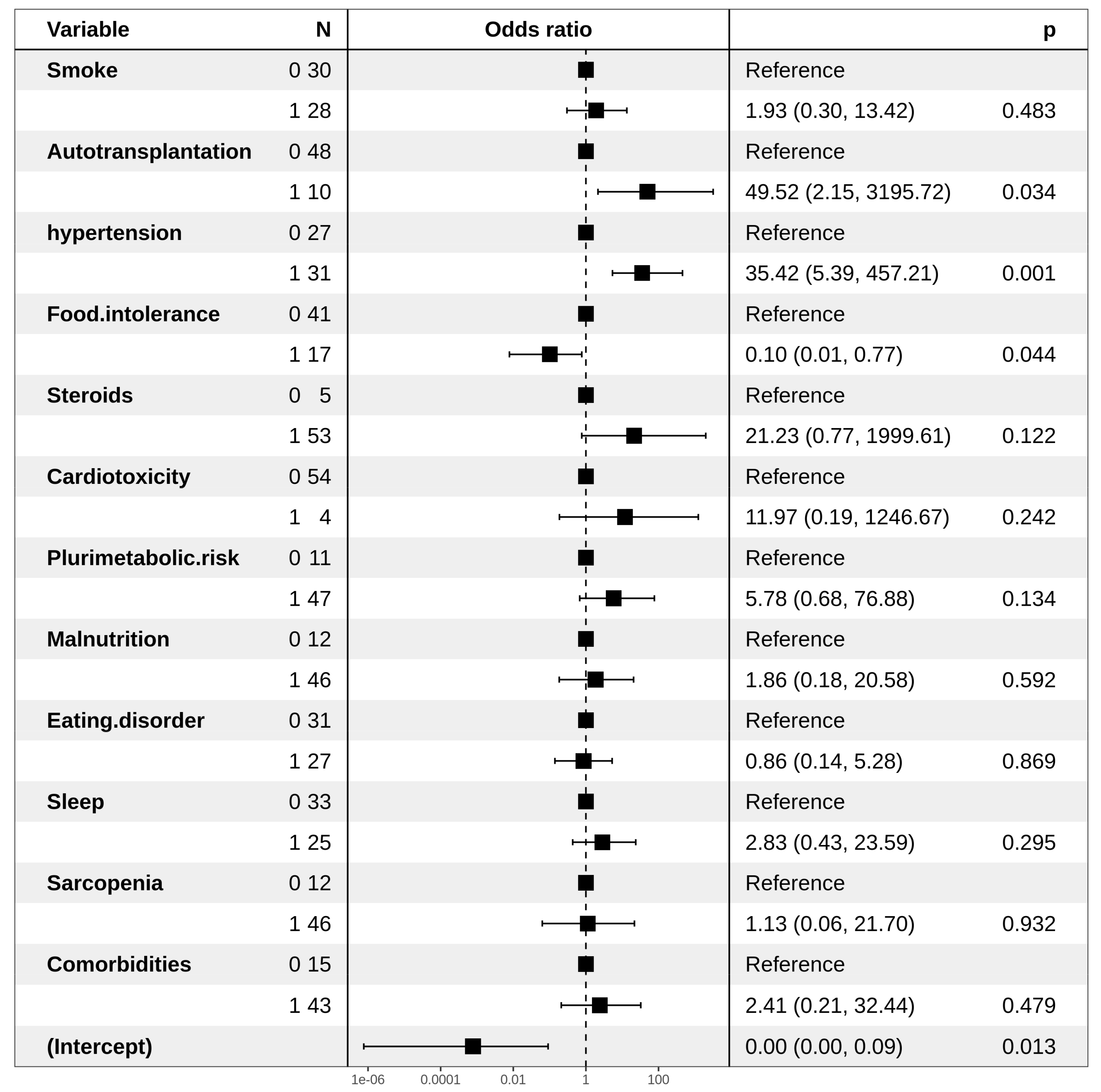
| Odds Ratio (95% CI) | p-Value | |
|---|---|---|
| MetSyn | ||
| Smoke | ||
| No | Ref | |
| Yes | 2.67 (0.92–8.16) | ns |
| ASCT | ||
| No | Ref | |
| Yes | 7.96 (1.34–152.57) | ns |
| Hypertension | ||
| No | Ref | |
| Yes | 10.98 (3.38–41.74) | 0.0001 |
| Food Intolerance | ||
| No | Ref | |
| Yes | 0.35 (0.11–1.10) | ns |
| Steroids | ||
| No | Ref | |
| Yes | 6.80 (0.93–138.07) | 0.096 |
| Unbalanced Diet | ||
| No | Ref | |
| Yes | 0.30 (0.10–0.82) | 0.02 |
| Physical Activity | ||
| No | Ref | |
| Yes | 0.32 (0.11–0.89) | 0.01 |
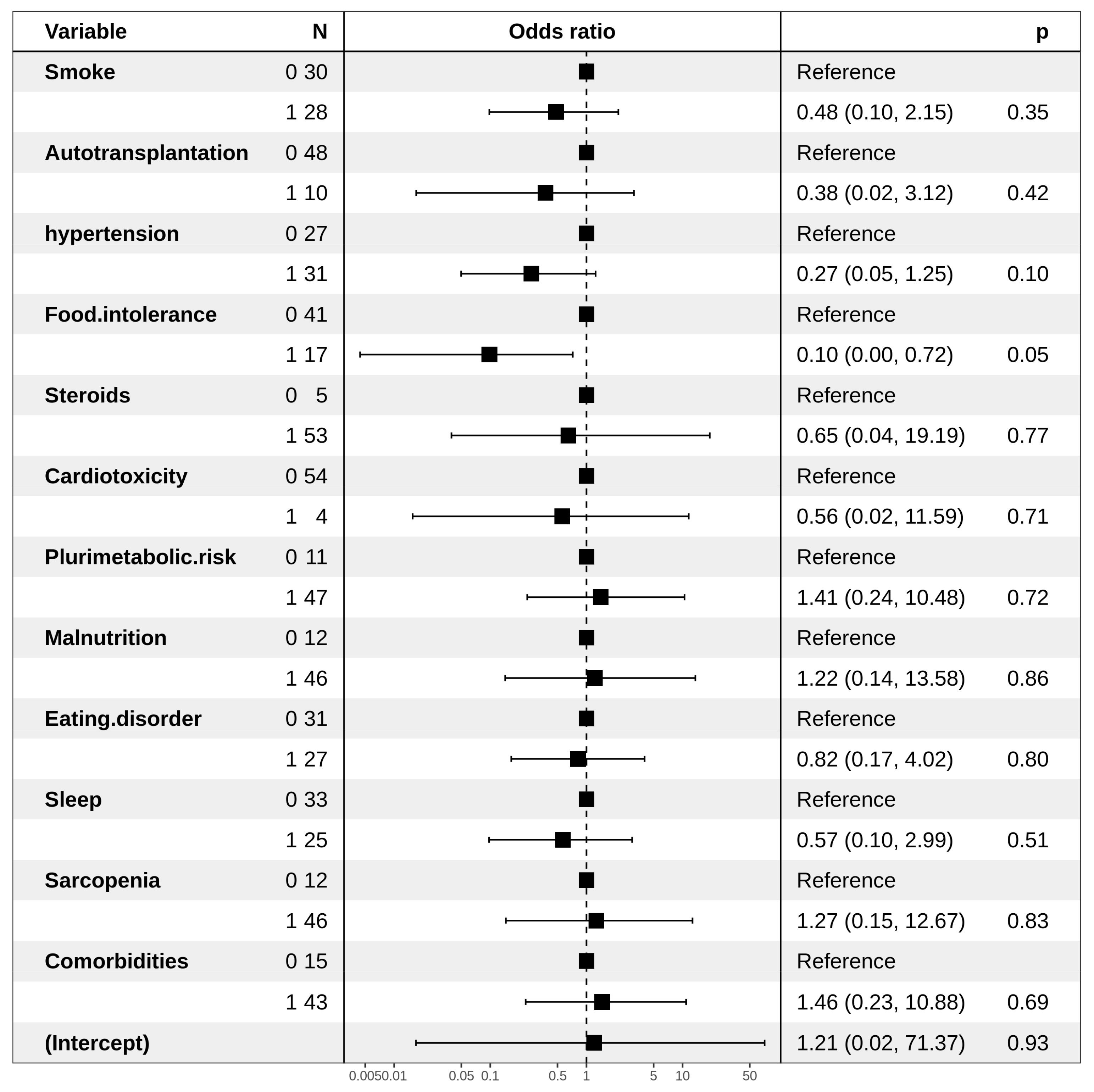
| Dyslipidemia IIa | ||
| Hypertension | ||
| No | Ref | |
| Yes | 0.27 (0.06–0.93) | 0.05 |
| Food intolerance | ||
| No | Ref | |
| Yes | 0.14 (0.007–0.80) | ns |
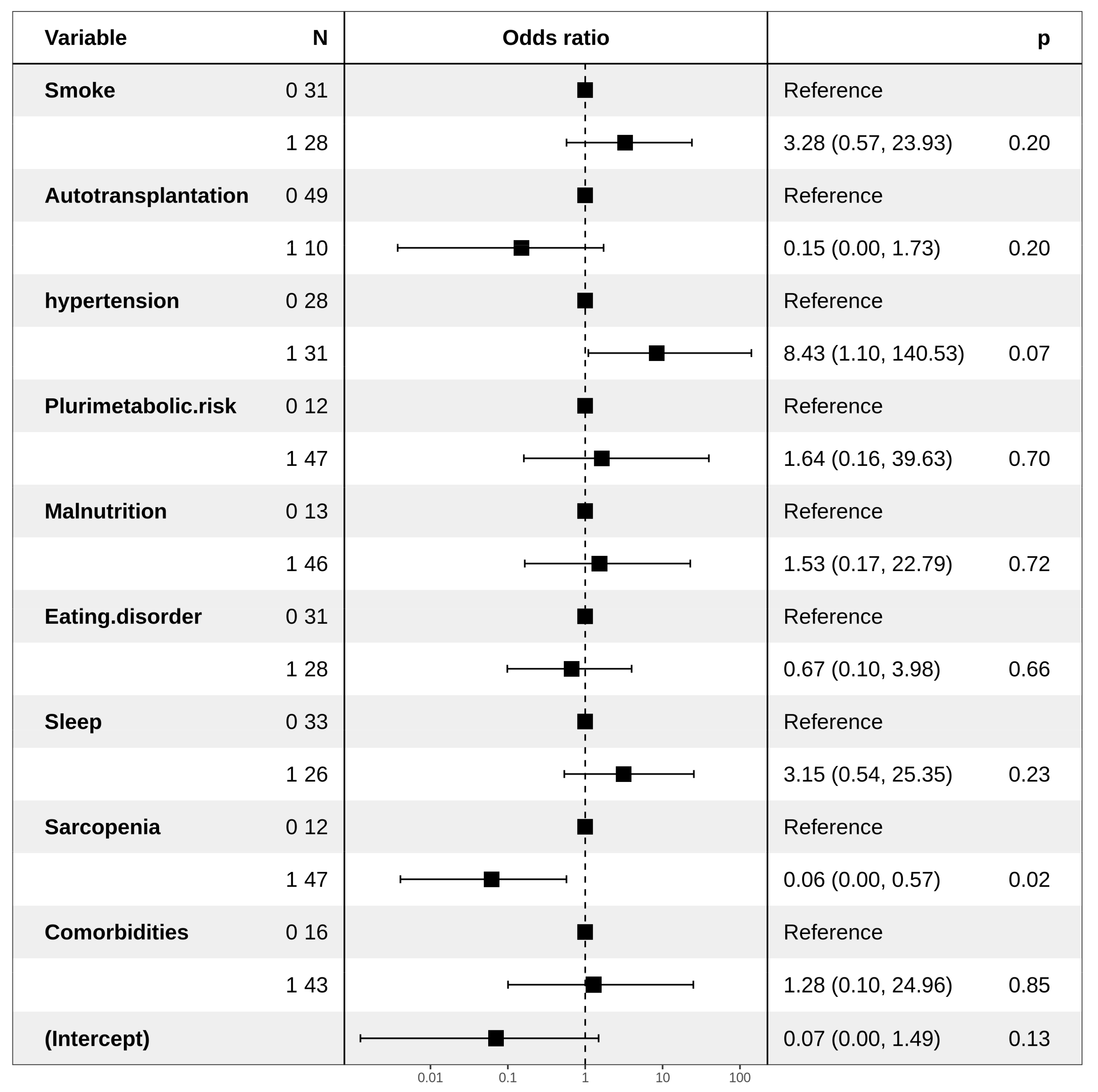
| Dyslipidemia IIb | ||
| Hypertension | ||
| No | Ref | |
| Yes | 4.52 (1.01–32.04) | ns |
| Smoke | ||
| No | Ref | |
| Ex-Smoker | 7.17 (1.94–30.49) | 0.003 |
| Yes | 4.09 (0.89–19.23) | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniele, A.; Guarini, A.; Summa, S.D.; Dellino, M.; Lerario, G.; Ciavarella, S.; Ditonno, P.; Paradiso, A.V.; Divella, R.; Casamassima, P.; et al. Body Composition Change, Unhealthy Lifestyles and Steroid Treatment as Predictor of Metabolic Risk in Non-Hodgkin’s Lymphoma Survivors. J. Pers. Med. 2021, 11, 215. https://doi.org/10.3390/jpm11030215
Daniele A, Guarini A, Summa SD, Dellino M, Lerario G, Ciavarella S, Ditonno P, Paradiso AV, Divella R, Casamassima P, et al. Body Composition Change, Unhealthy Lifestyles and Steroid Treatment as Predictor of Metabolic Risk in Non-Hodgkin’s Lymphoma Survivors. Journal of Personalized Medicine. 2021; 11(3):215. https://doi.org/10.3390/jpm11030215
Chicago/Turabian StyleDaniele, A., A. Guarini, S. De Summa, M. Dellino, G. Lerario, S. Ciavarella, P. Ditonno, A. V. Paradiso, R. Divella, P. Casamassima, and et al. 2021. "Body Composition Change, Unhealthy Lifestyles and Steroid Treatment as Predictor of Metabolic Risk in Non-Hodgkin’s Lymphoma Survivors" Journal of Personalized Medicine 11, no. 3: 215. https://doi.org/10.3390/jpm11030215
APA StyleDaniele, A., Guarini, A., Summa, S. D., Dellino, M., Lerario, G., Ciavarella, S., Ditonno, P., Paradiso, A. V., Divella, R., Casamassima, P., Savino, E., Carbonara, M. D., & Minoia, C. (2021). Body Composition Change, Unhealthy Lifestyles and Steroid Treatment as Predictor of Metabolic Risk in Non-Hodgkin’s Lymphoma Survivors. Journal of Personalized Medicine, 11(3), 215. https://doi.org/10.3390/jpm11030215








