SOFA Score, Hemodynamics and Body Temperature Allow Early Discrimination between Porcine Peritonitis-Induced Sepsis and Peritonitis-Induced Septic Shock
Abstract
1. Introduction
2. Materials and Methods
2.1. Anesthesia and Instrumentation
2.2. Experimental Protocol
2.3. Measurements
2.4. Statistical Analysis
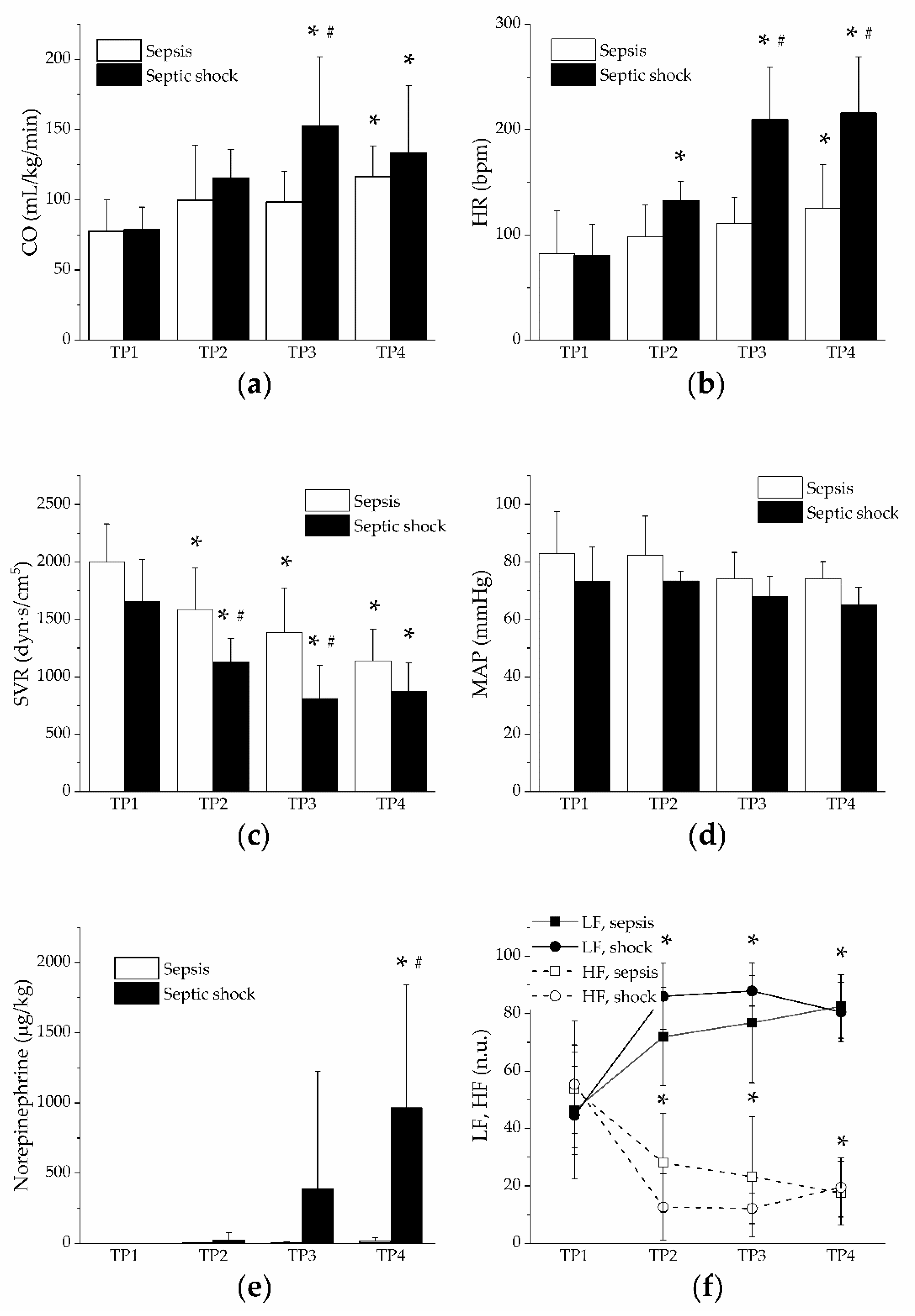
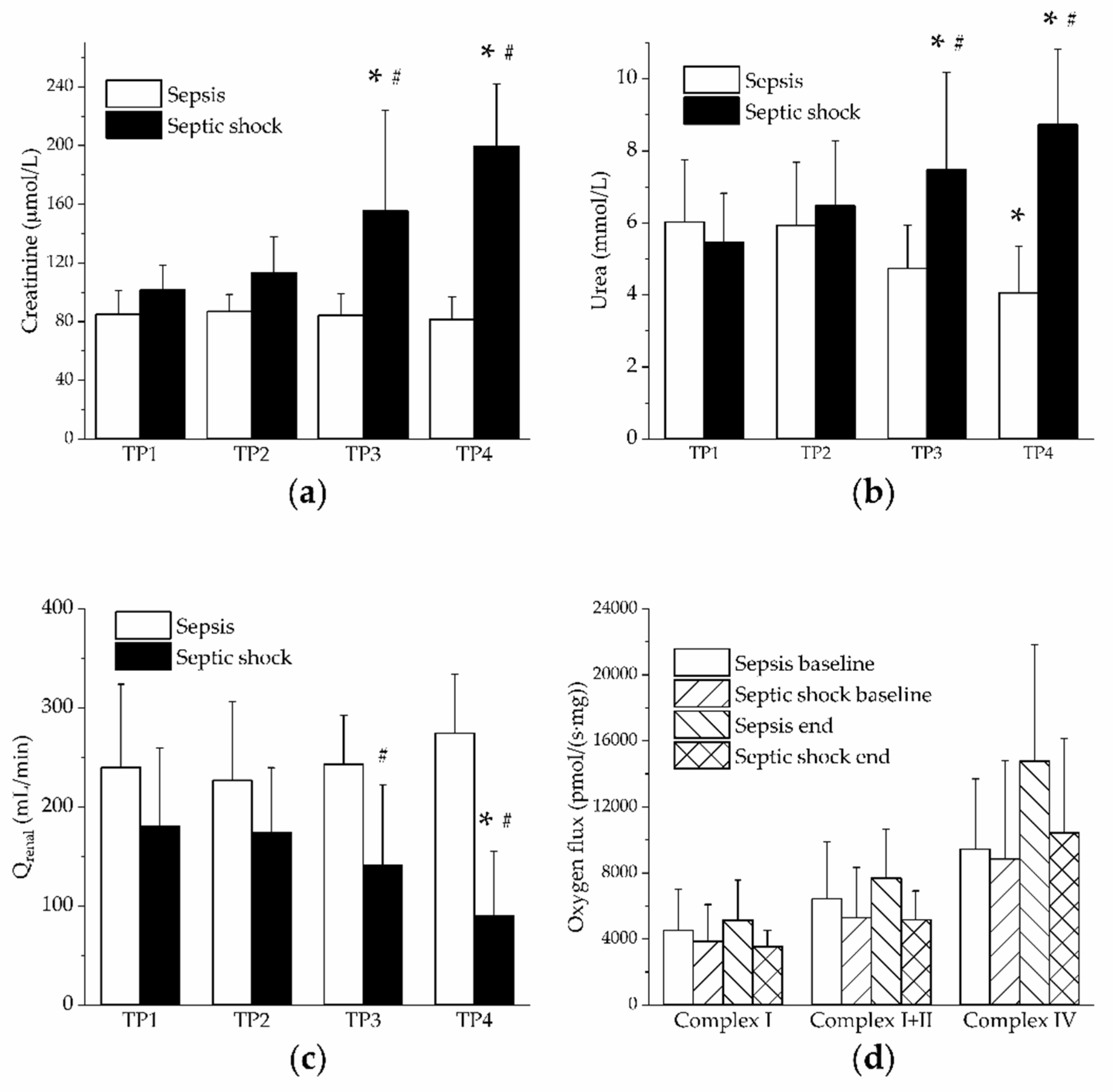
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The Epidemiology of Sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Catena, F.; Di Saverio, S.; Ansaloni, L.; Malangoni, M.; E Moore, E.; A Moore, F.; Ivatury, R.; Coimbra, R.; Leppaniemi, A.; et al. Current concept of abdominal sepsis: WSES position paper. World J. Emerg. Surg. 2014, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Mouncey, P.R.; Osborn, T.M.; Power, G.S.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Jahan, R.; Harvey, S.E.; Bell, D.; Bion, J.F.; et al. Trial of Early, Goal-Directed Resuscitation for Septic Shock. N. Engl. J. Med. 2015, 372, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- E Rudd, K.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Fleischmann, M.C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Rhee, C.; Dantes, R.; Epstein, L.; Murphy, D.J.; Seymour, C.W.; Iwashyna, T.J.; Kadri, S.S.; Angus, D.C.; Danner, R.L.; Fiore, A.E.; et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009–2014. JAMA 2017, 318, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Jones, T.M.; Hamad, Y.; Pande, A.; Varon, J.; O’Brien, C.; Anderson, D.J.; Warren, D.K.; Dantes, R.B.; Epstein, L.; et al. Prevalence, Underlying Causes, and Preventability of Sepsis-Associated Mortality in US Acute Care Hospitals. JAMA Netw. Open 2019, 2, e187571. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Thiemermann, C.; Remick, D.G. Sepsis-3 on the Block. Shock 2017, 47, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Kohoutova, M.; Horak, J.; Jarkovska, D.; Martinkova, V.; Tegl, V.; Nalos, L.; Vistejnova, L.; Benes, J.; Sviglerova, J.; Kuncova, J.; et al. Vagus Nerve Stimulation Attenuates Multiple Organ Dysfunction in Resuscitated Porcine Progressive Sepsis. Crit. Care Med. 2019, 47, e461–e469. [Google Scholar] [CrossRef] [PubMed]
- Jarkovska, D.; Markova, M.; Horak, J.; Nalos, L.; Benes, J.; Al-Obeidallah, M.; Tuma, Z.; Sviglerova, J.; Kuncova, J.; Matejovic, M.; et al. Cellular Mechanisms of Myocardial Depression in Porcine Septic Shock. Front. Physiol. 2018, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Kohoutová, M.; Dejmek, J.; Tůma, Z.; Kuncová, J. Variability of Mitochondrial Respiration in Relation to Sepsis-Induced Multiple Organ Dysfunction. Physiol. Res. 2018, 67, S577–S592. [Google Scholar] [CrossRef] [PubMed]
- Horak, J.; Nalos, L.; Martinkova, V.; Tegl, V.; Vistejnova, L.; Kuncova, J.; Kohoutova, M.; Jarkovska, D.; Dolejsova, M.; Benes, J.; et al. Evaluation of Mesenchymal Stem Cell Therapy for Sepsis: A Randomized Controlled Porcine Study. Front. Immunol. 2020, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Jarkovska, D.; Valesova, L.; Chvojka, J.; Benes, J.; Danihel, V.; Sviglerova, J.; Nalos, L.; Matejovic, M.; Stengl, M. Heart-rate variability depression in porcine peritonitis-induced sepsis without organ failure. Exp. Biol. Med. 2017, 242, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Tichanek, F.; Salomova, M.; Jedlicka, J.; Kuncova, J.; Pitule, P.; Macanova, T.; Petrankova, Z.; Tuma, Z.; Cendelin, J. Hippocampal mitochondrial dysfunction and psychiatric-relevant behavioral deficits in spinocerebellar ataxia 1 mouse model. Sci. Rep. 2020, 10, 5418. [Google Scholar] [CrossRef]
- Elbers, P.W.G.; Ince, C. Bench-to-bedside review: Mechanisms of critical illness—Classifying microcirculatory flow abnormalities in distributive shock. Crit. Care 2006, 10, 221. [Google Scholar] [CrossRef]
- Verdant, C.L.; De Backer, D.; Bruhn, A.; Clausi, C.M.; Su, F.; Wang, Z.; Rodriguez, H.; Pries, A.R.; Vincent, J.-L. Evaluation of sublingual and gut mucosal microcirculation in sepsis: A quantitative analysis. Crit. Care Med. 2009, 37, 2875–2881. [Google Scholar] [CrossRef]
- Siegemund, M.; Van Bommel, J.; Schwarte, L.A.; Studer, W.; Girard, T.; Marsch, S.; Radermacher, P.; Ince, C. Inducible nitric oxide synthase inhibition improves intestinal microcirculatory oxygenation and CO2 balance during endotoxemia in pigs. Intensiv. Care Med. 2005, 31, 985–992. [Google Scholar] [CrossRef]
- Ellis, C.G.; Bateman, R.M.; Sharpe, M.D.; Sibbald, W.J.; Gill, R. Effect of a maldistribution of microvascular blood flow on capillary O2 extraction in sepsis. Am. J. Physiol. Circ. Physiol. 2002, 282, H156–H164. [Google Scholar] [CrossRef]
- Porta, F.; Takala, J.; Weikert, C.; Bracht, H.; Kolarova, A.; Lauterburg, B.H.; Borotto, E.; Jakob, S.M. Effects of prolonged endotoxemia on liver, skeletal muscle and kidney mitochondrial function. Crit. Care 2006, 10, R118. [Google Scholar] [CrossRef]
- Makrecka-Kuka, M.; Korzh, S.; Vilks, K.; Vilskersts, R.; Cirule, H.; Dambrova, M.; Liepinsh, E. Mitochondrial Function in the Kidney and Heart, but Not the Brain, is Mainly Altered in an Experimental Model of Endotoxaemia. Shock 2019, 52, e153–e162. [Google Scholar] [CrossRef]
- Diktas, H.; Uysal, S.; Erdem, H.; Cag, Y.; Miftode, E.; Durmus, G.; Ulu-Kilic, A.; Alabay, S.; Szabo, B.G.; Lakatos, B.; et al. A novel id-iri score: Development and internal validation of the multivariable community acquired sepsis clinical risk prediction model. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 39, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Mackowiak, P.A.; Marling-Cason, M.; Cohen, R.L. Effects of Temperature on Antimicrobial Susceptibility of Bacteria. J. Infect. Dis. 1982, 145, 550–553. [Google Scholar] [CrossRef]
- Small, P.M.; Täuber, M.G.; Hackbarth, C.J.; A Sande, M. Influence of body temperature on bacterial growth rates in experimental pneumococcal meningitis in rabbits. Infect. Immun. 1986, 52, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.; Martin, E.; He, J.-R.; Frank, M.; DeTolla, L.; Hester, L.; O’Neill, T.; Manka, C.; Benjamin, I.; Nagarsekar, A.; et al. Febrile-Range Hyperthermia Augments Neutrophil Accumulation and Enhances Lung Injury in Experimental Gram-Negative Bacterial Pneumonia. J. Immunol. 2005, 174, 3676–3685. [Google Scholar] [CrossRef]
- Ejarkovska, D.; Evalesova, L.; Echvojka, J.; Ebenes, J.; Esviglerova, J.; Eflorova, B.; Enalos, L.; Ematejovic, M.; Estengl, M. Heart Rate Variability in Porcine Progressive Peritonitis-Induced Sepsis. Front. Physiol. 2016, 6, 412. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Ali, M.S.; Starke, R.M.; Jabbour, P.M.; I Tjoumakaris, S.; Gonzalez, L.F.; Rosenwasser, R.H.; Owens, G.K.; Koch, W.J.; Greig, N.H.; Dumont, A.S. TNF-α Induces Phenotypic Modulation in Cerebral Vascular Smooth Muscle Cells: Implications for Cerebral Aneurysm Pathology. Br. J. Pharmacol. 2013, 33, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Gotes, J.; Kasian, K.; Jacobs, H.; Cheng, Z.-Q.; Mink, S.N. Mechanisms of systemic vasodilation by lysozyme-c in septic shock. J. Appl. Physiol. 2012, 112, 638–650. [Google Scholar] [CrossRef][Green Version]
- Geven, C.; Kox, M.; Pickkers, P. Adrenomedullin and Adrenomedullin-Targeted Therapy As Treatment Strategies Relevant for Sepsis. Front. Immunol. 2018, 9, 292. [Google Scholar] [CrossRef]
- Kuncová, J.; Sykora, R.; Chvojka, J.; Švíglerová, J.; Stengl, M.; Krouzecky, A.; Nalos, L.; Matejovic, M. Plasma and Tissue Levels of Neuropeptide Y in Experimental Septic Shock: Relation to Hemodynamics, Inflammation, Oxidative Stress, and Hemofiltration. Artif. Organs 2011, 35, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, S.M.; Cunnion, R.E.; Zimmerberg, J. Nitric oxide synthase inhibition reverses arteriolar hyporesponsiveness to catecholamines in septic rats. Am. J. Physiol. Circ. Physiol. 1993, 264, H660–H663. [Google Scholar] [CrossRef]
- Hollenberg, S.M.; Piotrowski, M.J.; Parrillo, J.E. Nitric oxide synthase inhibition reverses arteriolar hyporesponsiveness to endothelin-1 in septic rats. Am. J. Physiol. Integr. Comp. Physiol. 1997, 272, R969–R974. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, S.M.; Tangora, J.J.; Piotrowski, M.J.; Easington, C.; Parrillo, J.E. Impaired microvascular vasoconstrictive responses to vasopressin in septic rats. Crit. Care Med. 1997, 25, 869–873. [Google Scholar] [CrossRef]
- Marshall, J.M. Interactions between local dilator and sympathetic vasoconstrictor influences in skeletal muscle in acute and chronic hypoxia. Exp. Physiol. 2015, 100, 1400–1411. [Google Scholar] [CrossRef]
- Jolly, L.; E March, J.; A Kemp, P.; Bennett, T.; Gardiner, S.M. Regional haemodynamic responses to adenosine receptor activation vary across time following lipopolysaccharide treatment in conscious rats. Br. J. Pharmacol. 2008, 154, 1600–1610. [Google Scholar] [CrossRef]
- Lee, H.T.; Kim, M.; Joo, J.D.; Gallos, G.; Chen, J.-F.; Emala, C.W. A3 adenosine receptor activation decreases mortality and renal and hepatic injury in murine septic peritonitis. Am. J. Physiol. Integr. Comp. Physiol. 2006, 291, R959–R969. [Google Scholar] [CrossRef]
- Yang, X.; Lu, G.-P.; Cai, X.-D.; Lu, Z.-J.; Kissoon, N. Alterations of complex IV in the tissues of a septic mouse model. Mitochondrion 2019, 49, 89–96. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Martin, G.S.; Levy, M.M. qSOFA does not replace SIRS in the definition of sepsis. Crit. Care 2016, 20, 1–3. [Google Scholar] [CrossRef]
- Zanza, C.; Thangathurai, J.; Audo, A.; A Muir, H.; Candelli, M.; Pignataro, G.; Thangathurai, D.; Cicchinelli, S.; Racca, F.; Longhitano, Y.; et al. Oxidative stress in critical care and vitamins supplement therapy: “A beneficial care enhancing”. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7703–7712. [Google Scholar] [PubMed]
- Longhitano, Y.; Zanza, C.; Thangathurai, D.; Taurone, S.; Kozel, D.; Racca, F.; Audo, A.; Ravera, E.; Migneco, A.; Piccioni, A.; et al. Gut Alterations in Septic Patients: A Biochemical Literature Review. Rev. Recent Clin. Trials 2021, 15, 289–297. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Sepsis | Septic Shock | ||||||
|---|---|---|---|---|---|---|---|---|
| TP1 | TP2 | TP3 | TP4 | TP1 | TP2 | TP3 | TP4 | |
| SV (mL) | 40 ± 12 | 39 ± 6 | 35 ± 6 | 39 ± 12 | 44 ± 19 | 35 ± 7 | 33 ± 15 | 29 ± 17 |
| PAOP (mmHg) | 10 ± 3 | 9 ± 2 | 11±2 | 13±3 | 9 ± 2 | 10 ± 1 | 12 ± 3 | 11 ± 6 |
| CVP (mmHg) | 10 ± 2 | 10 ± 3 | 11 ± 3 | 12 ± 4 | 10 ± 3 | 11 ± 2 | 15 ± 2 *,# | 14 ± 3 * |
| MPAP (mmHg) | 22 ± 5 | 24 ± 3 | 24 ± 3 | 26 ± 6 | 23 ± 3 | 24 ± 3 | 33 ± 11 *,# | 34 ± 11 *,# |
| DO2 (mL/(min·kg)) | 9.5 ± 3.6 | 12.3 ± 4.3 | 10.5 ± 3.7 | 11.8 ± 3.5 | 9.1±1.1 | 15.2 ± 2.3 | 21.5 ± 7.1 *,# | 18.6 ± 6.4 * |
| VO2 (mL/(min·kg)) | 4.7 ± 1.4 | 4.2 ± 1.6 | 4.6±1.3 | 4.7 ± 0.9 | 4.1 ± 1.2 | 5.2 ± 1.5 | 6.9 ± 1.5 | 6.1 ± 1.3 |
| O2ER | 0.51 ± 0.07 | 0.35 ± 0.12 | 0.44±0.05 | 0.41 ± 0.06 | 0.45±0.13 | 0.34 ± 0.08 | 0.35 ± 0.13 | 0.33 ± 0.17 |
| Fluid resuscitation (mL) | 1883 ± 273 | 3999 ± 1058 | 3042 ± 491 | 2554 ± 498 | 1889 ± 466 | 4223 ± 738 | 2488 ± 916 | 1628 ± 1325 # |
| Hemoglobin (g/L) | 77 ± 31 | 81 ± 33 | 56 ± 40 | 66 ± 28 | 59 ± 33 | 72 ± 43 | 83 ± 50 | 44 ± 52 *,# |
| Urine output (mL) | 267 ± 85 | 409 ± 248 | 468 ± 346 | 537 ± 315 | 248 ± 59 | 303 ± 114 | 332 ± 243 | 314 ± 371 |
| ALP (μkat/L) | 1.9 ± 0.2 | 1.9 ± 0.4 | 1.7 ± 0.3 | 1.3 ± 0.3 | 1.9 ± 0.4 | 2.1 ± 0.5 | 3.1 ± 1.6 | 3.4±1.5 *,# |
| AST (μkat/L) | 1.3 ± 0.5 | 1.4 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.6 | 0.7 ± 0.1 | 1.8 ± 0.8 | 3.0 ± 2.2 * | 3.3±2.0 * |
| ALT (μkat/L) | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.2 | 0.7 ± 0.32 |
| Bilirubin (µmol/L) | 3 ± 0 | 3 ± 0 | 3 ± 0 | 3 ± 0 | 3 ± 0 | 3 ± 0 | 3 ± 0.49 | 3 ± 0.49 |
| Thrombocytes (109/L) | 331 ± 116 | 280 ± 111 | 219 ± 102 | 161 ± 72 * | 393 ± 144 | 270 ± 63 | 219 ± 76 * | 112 ± 55 * |
| Isoprostane (ng/L) | 64 ± 50 | 65 ± 44 | 60 ± 27 | 59 ± 23 | 110 ± 18 | 59 ± 15 | 89 ± 50 | 86 ± 50 |
| TBARs (µmol/L) | 0.23 ± 0.1 | 0.16 ± 0.1 | 0.16 ± 0.1 | 0.17 ± 0.03 | 0.20 ± 0.04 | 0.14 ± 0.04 | 0.20 ± 0.1 | 0.20 ± 0.1 |
| NOx (µmol/g prot.) | 1.1 ± 0.6 | 1.2 ± 0.5 | 2.2 ± 0.5 * | 1.8 ± 0.7 | 0.8 ± 0.5 | 1.5 ± 1.1 | 1.8 ± 0.9 * | 1.8 ± 0.9 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Obeidallah, M.; Jarkovská, D.; Valešová, L.; Horák, J.; Jedlička, J.; Nalos, L.; Chvojka, J.; Švíglerová, J.; Kuncová, J.; Beneš, J.; et al. SOFA Score, Hemodynamics and Body Temperature Allow Early Discrimination between Porcine Peritonitis-Induced Sepsis and Peritonitis-Induced Septic Shock. J. Pers. Med. 2021, 11, 164. https://doi.org/10.3390/jpm11030164
Al-Obeidallah M, Jarkovská D, Valešová L, Horák J, Jedlička J, Nalos L, Chvojka J, Švíglerová J, Kuncová J, Beneš J, et al. SOFA Score, Hemodynamics and Body Temperature Allow Early Discrimination between Porcine Peritonitis-Induced Sepsis and Peritonitis-Induced Septic Shock. Journal of Personalized Medicine. 2021; 11(3):164. https://doi.org/10.3390/jpm11030164
Chicago/Turabian StyleAl-Obeidallah, Mahmoud, Dagmar Jarkovská, Lenka Valešová, Jan Horák, Jan Jedlička, Lukáš Nalos, Jiří Chvojka, Jitka Švíglerová, Jitka Kuncová, Jan Beneš, and et al. 2021. "SOFA Score, Hemodynamics and Body Temperature Allow Early Discrimination between Porcine Peritonitis-Induced Sepsis and Peritonitis-Induced Septic Shock" Journal of Personalized Medicine 11, no. 3: 164. https://doi.org/10.3390/jpm11030164
APA StyleAl-Obeidallah, M., Jarkovská, D., Valešová, L., Horák, J., Jedlička, J., Nalos, L., Chvojka, J., Švíglerová, J., Kuncová, J., Beneš, J., Matějovič, M., & Štengl, M. (2021). SOFA Score, Hemodynamics and Body Temperature Allow Early Discrimination between Porcine Peritonitis-Induced Sepsis and Peritonitis-Induced Septic Shock. Journal of Personalized Medicine, 11(3), 164. https://doi.org/10.3390/jpm11030164








