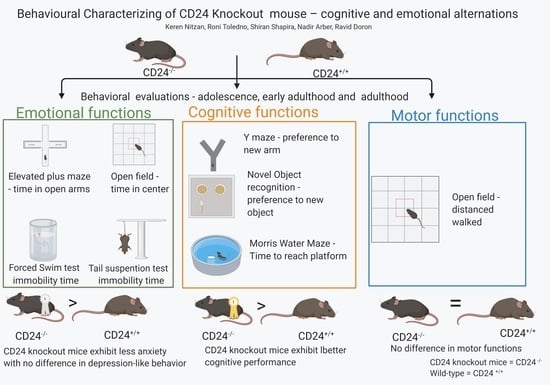
Behavioral Characterizing of CD24 Knockout Mouse—Cognitive and Emotional Alternations
Abstract
1. Introduction
2. Materials and Methods
2.1. Genotype Verification by FACS Analysis
2.2. Behavioral and Cognitive Evaluations
2.3. Data Analysis
3. Results
3.1. Anxiety-Like Behavior
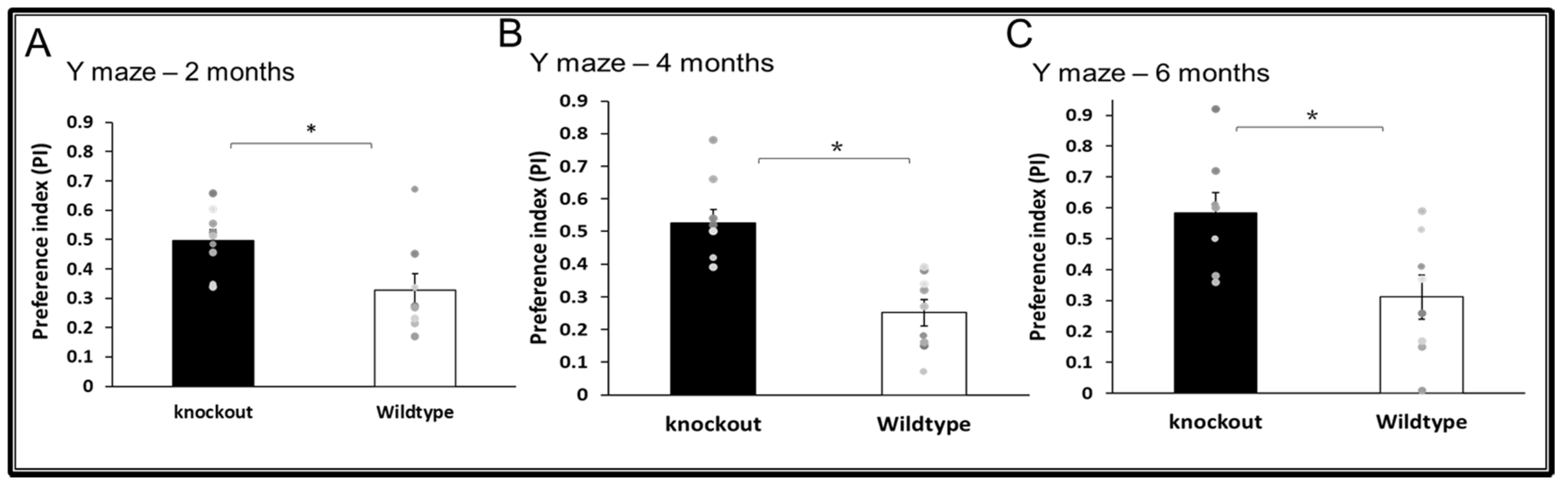
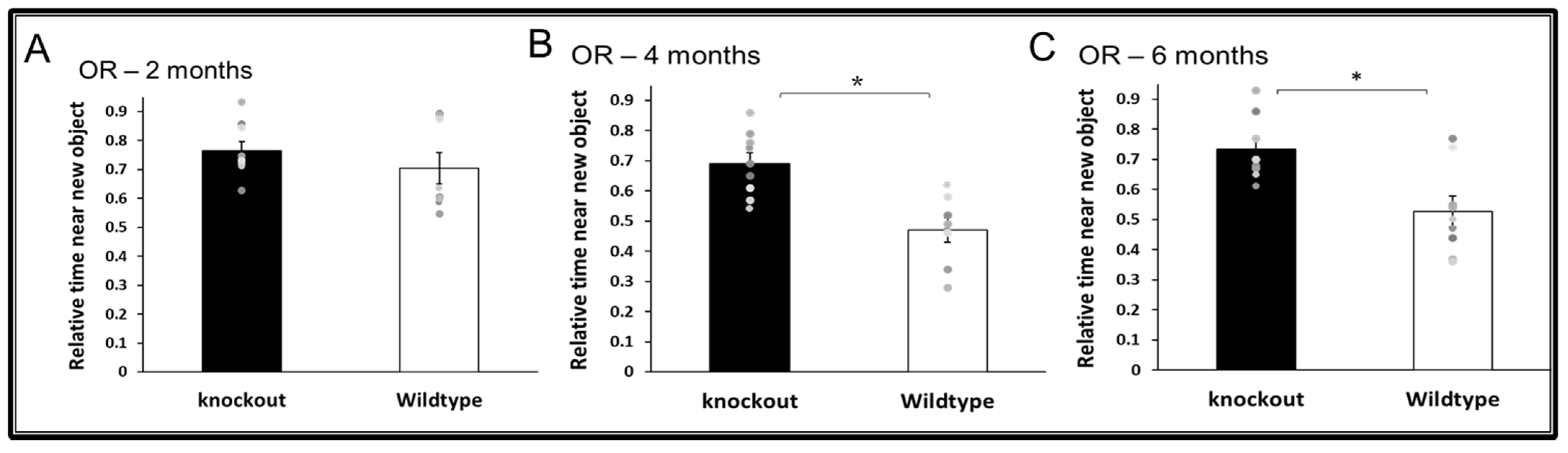
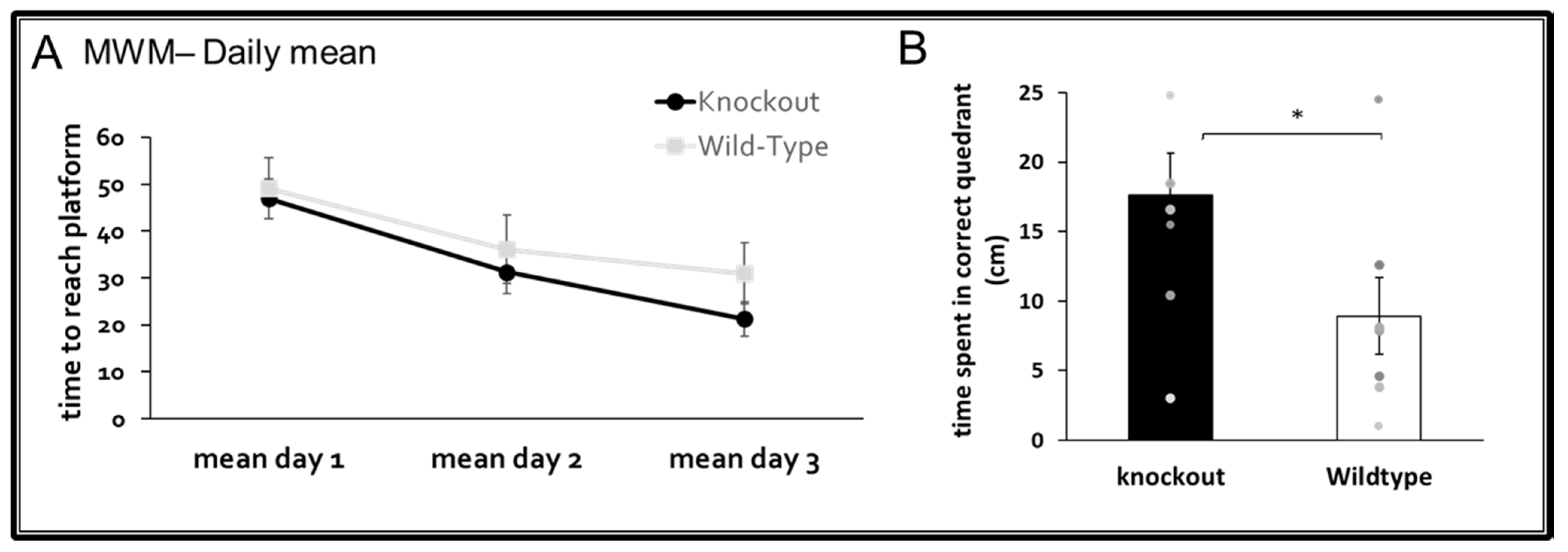
3.2. Cognitive Functions
3.3. Depression-Like Behavior
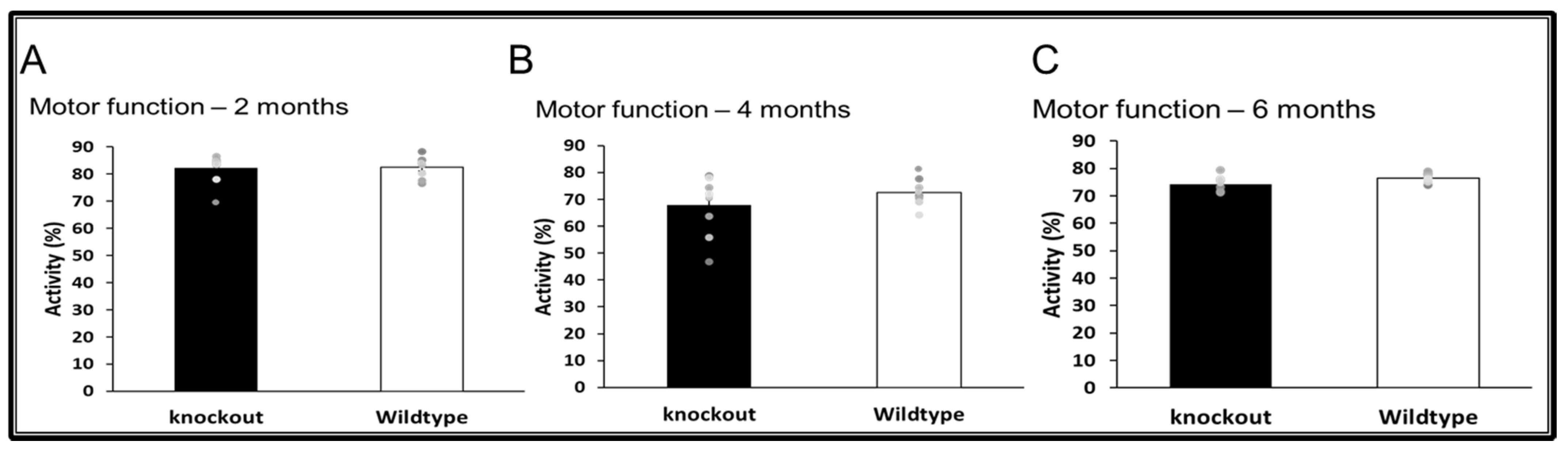
3.4. Motor Functions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Kadmon, G.; Eckert, M.; Sammar, M.; Schachner, M.; Altevogt, P. Nectadrin, the heat-stable antigen, is a cell adhesion molecule. J. Cell Biol. 1992, 118, 1245–1258. [Google Scholar] [CrossRef]
- Hunte, B.E.; Capone, M.; Zlotnik, A.; Rennick, D.; Moore, T.A. Acquisition of CD24 expression by Lin-CD43+B220(low)ckit(hi) cells coincides with commitment to the B cell lineage. Eur. J. Immunol. 1998, 28, 3850–3856. [Google Scholar] [CrossRef]
- Altevogt, P.; Sammar, M.; Hüser, L.; Kristiansen, G. Novel insights into the function of CD24: A driving force in cancer. International Journal of Cancer. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/ijc.33249 (accessed on 13 October 2020).
- Wang, W.; Wang, X.; Peng, L.; Deng, Q.; Liang, Y.; Qing, H.; Jiang, B. CD24-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Cancer Sci. 2010, 101, 112–119. [Google Scholar] [CrossRef]
- Pei, Z.; Zhu, G.; Huo, X.; Gao, L.; Liao, S.; He, J.; Long, Y.; Yi, H.; Xiao, S.; Yi, W.; et al. CD24 promotes the proliferation and inhibits the apoptosis of cervical cancer cells in vitro. Oncol. Rep. 2016, 35, 1593–1601. [Google Scholar] [CrossRef]
- Lee, K.; Ju, J.; Jang, K.; Yang, W.; Yi, J.Y.; Noh, D.Y.; Shin, I. CD24 regulates cell proliferation and transforming growth factor β-induced epithelial to mesenchymal transition through modulation of integrin β1 stability. Cell. Signal. 2012, 24, 2132–2142. [Google Scholar] [CrossRef] [PubMed]
- Shapira, S.; Shapira, A.; Starr, A.; Kazanov, D.; Kraus, S.; Benhar, I.; Arber, N. An Immunoconjugate of Anti-CD24 and Pseudomonas Exotoxin Selectively Kills Human Colorectal Tumors in Mice. Gastroenterology 2011, 140, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Dai, Z.; Jiang, Z.; He, Y.; Wang, L.; Liao, Q.; Sun, N.; Wang, Y.; Sun, S.; Lin, W.; et al. CD24: A marker of granulosa cell subpopulation and a mediator of ovulation. Cell Death Dis. 2019, 10, 791. [Google Scholar] [CrossRef]
- Karnan, S.; Ota, A.; Murakami, H.; Rahman, M.L.; Hasan, M.N.; Wahiduzzaman, M.; Hanamura, I.; Quang Vu, L.; Inoko, A.; Hyodo, T.; et al. Identification of CD24 as a potential diagnostic and therapeutic target for malignant pleural mesothelioma. Cell Death Discov. 2020, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zheng, P.; Tang, J.; Liu, Y. CD24: From A to Z. Cell Mol. Immunol. 2010, 7, 100–103. [Google Scholar] [CrossRef]
- Stott, S.R.W.; Hayat, S.; Carnwath, T.; Garas, S.; Sleeman, J.P.; Barker, R.A. CD24 expression does not affect dopamine neuronal survival in a mouse model of Parkinson’s disease. PLoS ONE 2017, 12, e0171748. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Carl, J.W.; Joshi, P.S.; RayChaudhury, A.; Pu, X.-A.; Shi, F.-D.; Bai, X.-F. CD24 on the Resident Cells of the Central Nervous System Enhances Experimental Autoimmune Encephalomyelitis. J. Immunol. 2007, 178, 6227–6235. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.-F.; Liu, J.-Q.; Liu, X.; Guo, Y.; Cox, K.; Wen, J.; Zheng, P.; Liu, Y. The heat-stable antigen determines pathogenicity of self-reactive T cells in experimental autoimmune encephalomyelitis. J. Clin. Invest. 2000, 105, 1227–1232. [Google Scholar] [CrossRef]
- Naboichtchikov, I.; Maharshak, N.; Shapira, S.; Fokra, A.; Arber, N.; Kraus, S. Mo1767 CD24 Plays a Role in the Pathogenesis of DSS-Induced Experimental Colitis in Mice. Gastroenterology 2015, 148, S-706. [Google Scholar] [CrossRef]
- Goris, A.; Maranian, M.; Walton, A.; Yeo, T.W.; Ban, M.; Gray, J.; Dubois, B.; Compston, A.; Sawcer, S. CD24 Ala/Val polymorphism and multiple sclerosis. J. Neuroimmunol. 2006, 175, 200–202. [Google Scholar] [CrossRef]
- Lisiansky, V.; Kraus, S.; Naumov, I.; Kazanov, D.; Nabiochtchikov, I.; Toledano, O.; Leshno, M.; Avivi, D.; Dotan, I.; Arber, N.; et al. Role of CD24 Polymorphisms in the Susceptibility to Inflammatory Bowel Disease: The International Journal of Biological Markers. Available online: https://journals.sagepub.com/doi/10.5301/jbm.5000072 (accessed on 14 October 2020).
- Huang, S.; Sun, C.; Hou, Y.; Tang, Y.; Zhu, Z.; Zhang, Z.; Zhang, Y.; Wang, L.; Zhao, Q.; Chen, M.-G.; et al. A comprehensive bioinformatics analysis on multiple Gene Expression Omnibus datasets of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Sci. Rep. 2018, 8, 7630. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol. Rev. 2018, 98, 477–504. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, H.; del Rey, A.; Sorkin, E.; Dinarello, C. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 1986, 233, 652–654. [Google Scholar] [CrossRef]
- Wolf, G.; Gabay, E.; Tal, M.; Yirmiya, R.; Shavit, Y. Genetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in mice. Pain 2006, 120, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Shavit, Y.; Fridel, K.; Beilin, B. Postoperative Pain Management and Proinflammatory Cytokines: Animal and Human Studies. J. Neuroimmune Pharm. 2006, 1, 443–451. [Google Scholar] [CrossRef]
- Yirmiya, R.; Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.; Pinteaux, E. The Interleukin-1 System: An Attractive and Viable Therapeutic Target in Neurodegenerative Disease. CDTCNSND 2003, 2, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.W.; Duman, R.S. Evidence for IL-1 receptor blockade as a therapeutic strategy for the treatment of depression. Curr. Opin. Investig. Drugs 2009, 10, 664–671. [Google Scholar]
- Calaora, V.; Chazal, G.; Nielsen, P.J.; Rougon, G.; moreau, H. mCD24 expression in the developing mouse brain and in zones of secondary neurogenesis in the adult. Neuroscience 1996, 73, 581–594. [Google Scholar] [CrossRef]
- Bai, X.-F.; Li, O.; Zhou, Q.; Zhang, H.; Joshi, P.S.; Zheng, X.; Liu, Y.; Wang, Y.; Zheng, P.; Liu, Y. CD24 Controls Expansion and Persistence of Autoreactive T Cells in the Central Nervous System during Experimental Autoimmune Encephalomyelitis. J. Exp. Med. 2004, 200, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Doron, R.; Lotan, D.; Rak-Rabl, A.; Raskin-Ramot, A.; Lavi, K.; Rehavi, M. Anxiolytic effects of a novel herbal treatment in mice models of anxiety. Life Sci. 2012, 90, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Doron, R.; Lotan, D.; Versano, Z.; Benatav, L.; Franko, M.; Armoza, S.; Kately, N.; Rehavi, M. Escitalopram or novel herbal mixture treatments during or following exposure to stress reduce anxiety-like behavior through corticosterone and BDNF modifications. PLoS ONE 2014, 9, e91455. [Google Scholar] [CrossRef] [PubMed]
- Sarne, Y.; Toledano, R.; Rachmany, L.; Sasson, E.; Doron, R. Reversal of age-related cognitive impairments in mice by an extremely low dose of tetrahydrocannabinol. Neurobiol. Aging 2018, 61, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Doron, R.; Lotan, D.; Einat, N.; Yaffe, R.; Winer, A.; Marom, I.; Meron, G.; Kately, N.; Rehavi, M. A novel herbal treatment reduces depressive-like behaviors and increases BDNF levels in the brain of stressed mice. Life Sci. 2014, 94, 151–157. [Google Scholar] [CrossRef]
- Kleene, R.; Yang, H.; Kutsche, M.; Schachner, M. The Neural Recognition Molecule L1 Is a Sialic Acid-binding Lectin for CD24, Which Induces Promotion and Inhibition of Neurite Outgrowth. J. Biol. Chem. 2001, 276, 21656–21663. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.J.; Munchow, A.H.; Rios, L.M.; Zhang, G.; Ásgeirsdóttir, H.N.; Stackman, R.W. The Rodent Hippocampus Is Essential for Nonspatial Object Memory. Curr. Biol. 2013, 23, 1685–1690. [Google Scholar] [CrossRef]
- Jarrard, L.E. On the role of the hippocampus in learning and memory in the rat. Behav. Neural Biol. 1993, 60, 9–26. [Google Scholar] [CrossRef]
- Baptista, P.; Andrade, J.P. Adult Hippocampal Neurogenesis: Regulation and Possible Functional and Clinical Correlates. Front. Neuroanat. 2018, 12, 44. [Google Scholar] [CrossRef]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—Linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Belvindrah, R.; Rougon, G.; Chazal, G. Increased Neurogenesis in Adult mCD24-Deficient Mice. J. Neurosci. 2002, 22, 3594–3607. [Google Scholar] [CrossRef]
- Conrad, C.D.; Galea, L.A.M.; Kuroda, Y.; McEwen, B.S. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav. Neurosci. 1996, 110, 1321–1334. [Google Scholar] [CrossRef]
- Cohen, S.J.; Stackman, R.W., Jr. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 2015, 285, 105–117. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, X.-M.; Xu, W.-D.; Tao, T.; Liu, G.-J.; Gao, Y.-Y.; Lu, Y.; Wu, L.-Y.; Yu, Z.; Yuan, B.; et al. Inhibition of Elevated Hippocampal CD24 Reduces Neurogenesis in Mice with Traumatic Brain Injury. J. Surg. Res. 2020, 245, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Salani, F.; Sterbini, V.; Sacchinelli, E.; Garramone, M.; Bossù, P. Is Innate Memory a Double-Edge Sword in Alzheimer’s Disease? A Reappraisal of New Concepts and Old Data. Front. Immunol. 2019, 10, 1768. [Google Scholar] [CrossRef] [PubMed]
- Revest, J.-M.; Dupret, D.; Koehl, M.; Funk-Reiter, C.; Grosjean, N.; Piazza, P.-V.; Abrous, D.N. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol. Psychiatry 2009, 14, 959–967. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Tang, J.; Zheng, P.; Liu, Y. CD24 and Siglec-10 Selectively Repress Tissue Damage-Induced Immune Responses. Science 2009, 323, 1722–1725. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ling, H.-P.; You, W.-C.; Liu, H.-D.; Sun, Q.; Zhou, M.-L.; Shen, W.; Zhao, J.-B.; Zhu, L.; Hang, C.-H. Elevated Cerebral Cortical CD24 Levels in Patients and Mice with Traumatic Brain Injury: A Potential Negative Role in Nuclear Factor Kappa B/Inflammatory Factor Pathway. Mol. Neurobiol. 2014, 49, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.W.; Russo, S.J.; Ferguson, D.; Nestler, E.J.; Duman, R.S. Nuclear factor- B is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl. Acad. Sci. 2010, 107, 2669–2674. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.-Q.; Huang, H.-J.; Wang, Y.-L.; Yang, L.; Pilot, A.; Zhu, X.-C.; Yu, R.; Wang, J.; Chen, X.-R.; Liu, Q.; et al. Ghrelin exhibited antidepressant and anxiolytic effect via the p38-MAPK signaling pathway in hippocampus. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 93, 11–20. [Google Scholar] [CrossRef]
- Kwatra, M.; Ahmed, S.; Gawali, B.; Panda, S.R.; Naidu, V. Hesperidin alleviates chronic restraint stress and lipopolysaccharide-induced Hippocampus and Frontal cortex damage in mice: Role of TLR4/NF-κB, p38 MAPK/JNK, Nrf2/ARE signaling. Neurochem. Int. 2020, 140, 104835. [Google Scholar] [CrossRef]
- Dai, H.; Hu, W.; Jiang, L.; Li, L.; Gaung, X.; Xiao, Z. p38 MAPK Inhibition Improves Synaptic Plasticity and Memory in Angiotensin II-dependent Hypertensive Mice. Sci. Rep. 2016, 6, 27600. [Google Scholar] [CrossRef]
- Sharma, V.; Gilhotra, R.; Dhingra, D.; Gilhotra, N. Possible Underlying Influence of p38MAPK and NF-κB in the Diminished Anti-anxiety Effect of Diazepam in Stressed Mice. J. Pharmacol. Sci. 2011, 116, 257–263. [Google Scholar] [CrossRef]
- Gates, P.; Gough, K.; Dhillon, H.; Wilson, C.; Hawkes, E.; Dore, V.; Perchyonok, Y.; Rowe, C.C.; Walker, A.K.; Vardy, J.L.; et al. Longitudinal exploration of cancer-related cognitive impairment in patients with newly diagnosed aggressive lymphoma: Protocol for a feasibility study. BMJ Open 2020, 10, e038312. [Google Scholar] [CrossRef]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.B.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.F. Bi-directional immune–brain communication: Implications for understanding stress, pain, and cognition. Brain Behav. Immun. 2003, 17, 69–85. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitzan, K.; Toledano, R.; Shapira, S.; Arber, N.; Doron, R. Behavioral Characterizing of CD24 Knockout Mouse—Cognitive and Emotional Alternations. J. Pers. Med. 2021, 11, 105. https://doi.org/10.3390/jpm11020105
Nitzan K, Toledano R, Shapira S, Arber N, Doron R. Behavioral Characterizing of CD24 Knockout Mouse—Cognitive and Emotional Alternations. Journal of Personalized Medicine. 2021; 11(2):105. https://doi.org/10.3390/jpm11020105
Chicago/Turabian StyleNitzan, Keren, Roni Toledano, Shiran Shapira, Nadir Arber, and Ravid Doron. 2021. "Behavioral Characterizing of CD24 Knockout Mouse—Cognitive and Emotional Alternations" Journal of Personalized Medicine 11, no. 2: 105. https://doi.org/10.3390/jpm11020105
APA StyleNitzan, K., Toledano, R., Shapira, S., Arber, N., & Doron, R. (2021). Behavioral Characterizing of CD24 Knockout Mouse—Cognitive and Emotional Alternations. Journal of Personalized Medicine, 11(2), 105. https://doi.org/10.3390/jpm11020105