Prognostic Value of Histone Modifying Enzyme EZH2 in RCHOP-Treated Diffuse Large B-Cell Lymphoma and High Grade B-Cell Lymphoma
Abstract
1. Introduction
2. Materials and Methods
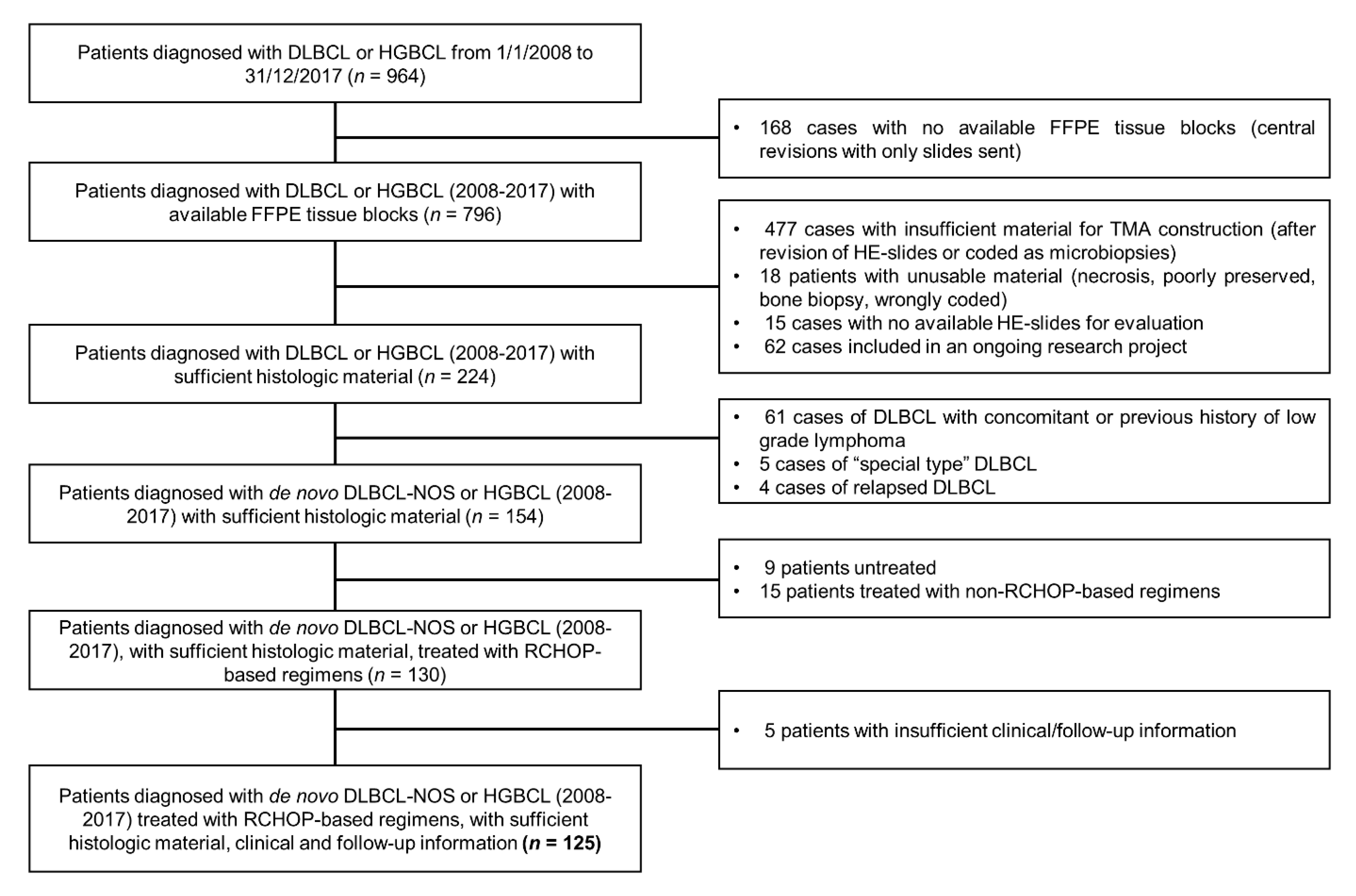
2.1. Study Population, Inclusion and Exclusion Criteria
2.2. CASE Selection and EZH2 Immunohistochemistry
2.3. Clinical, Morphological and Immunohistochemical Baseline Characteristics
2.4. Fluorescent In Situ Hybridization
2.5. Follow-Up and Endpoints
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Pathological and Cytogenetic Features
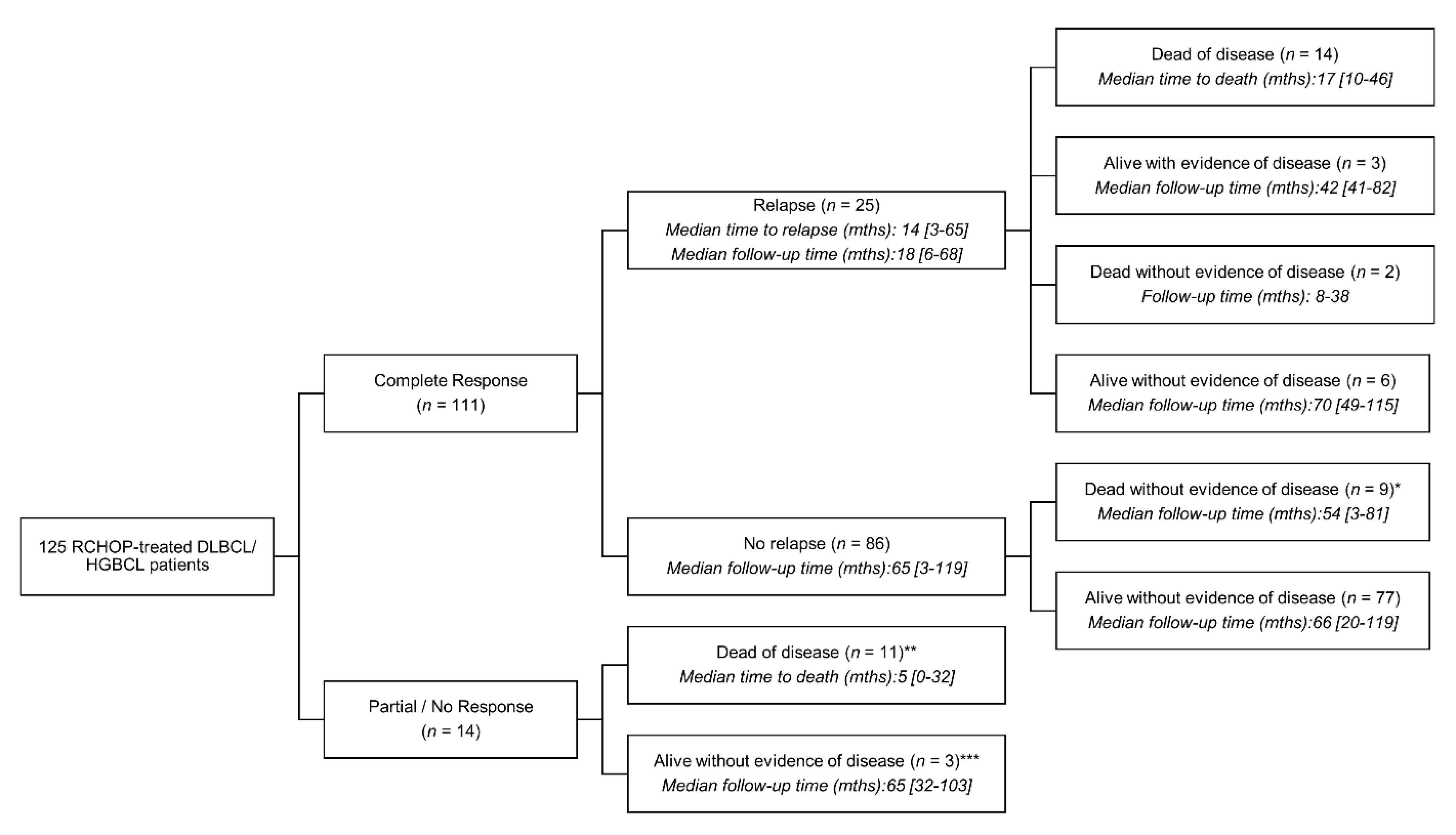
3.3. Outcomes and Follow-Up
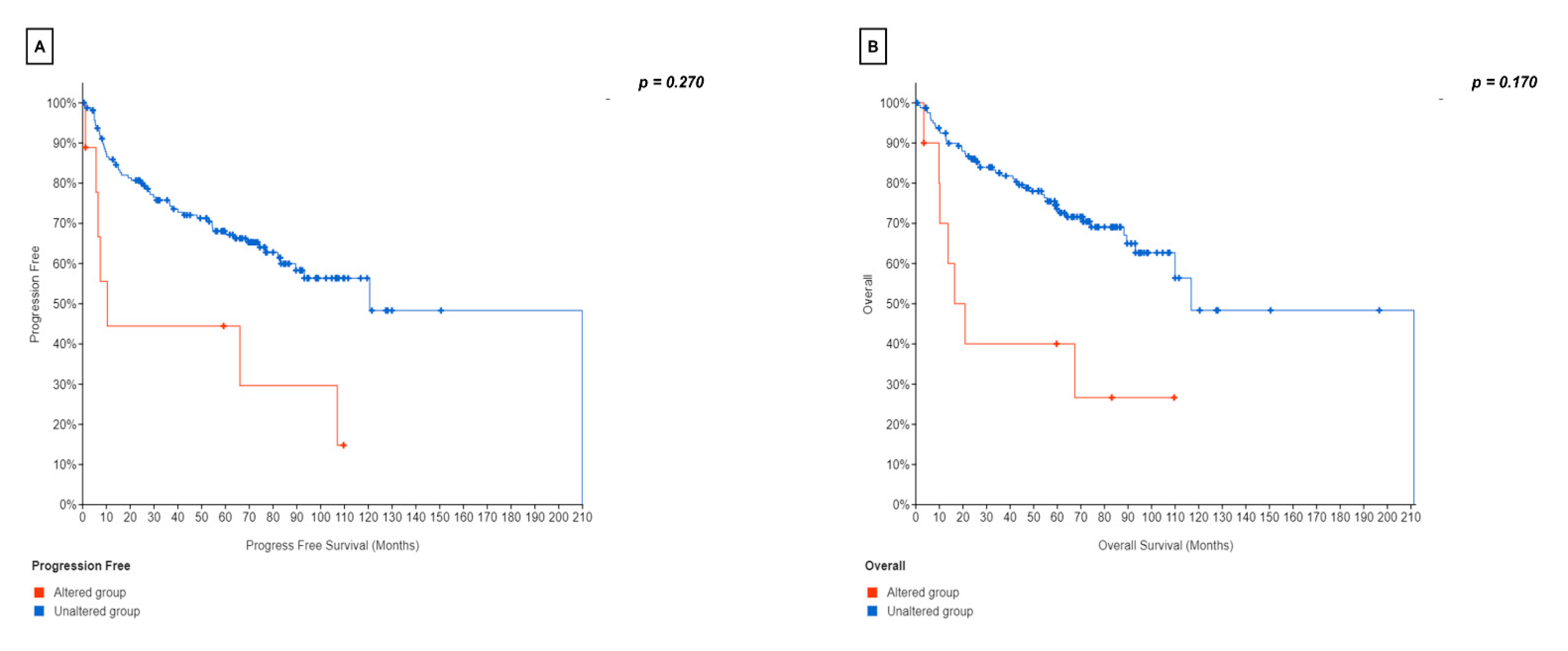
3.4. Prognostic Value of EZH2 Expression in HGBCL and DLBCL-NOS
3.5. Prognostic Value of EZH2/BCL2 Coexpression in HGBCL and DLBCL-NOS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Patients over 18 years of age | DLBCL not included in the NOS category |
| First diagnosis of de novo DLBCL NOS or HGBCL | Relapsed DLBCL/HGBCL |
| Treatment with RCHOP based regimens | Previous history or concomitant diagnosis of other hematological malignancies, including low grade B-cell lymphoma |
| Available 2-year follow-up information | Previous treatment for hematological malignancies |
| Diagnosis, treatment and follow-up at IPO Porto | Treatment with non-RCHOP based regimens/ no treatment |
| Unavailable slides or FFPE blocks | |
| Insufficient or poorly preserved histological material | |
| Bone marrow/bone biopsies. | |
| Insufficient clinical/follow-up information |
| EZH2 Low (n = 93) | EZH2 High (n = 32) | p | |
|---|---|---|---|
| Male sex | 34 (37%) | 18 (56%) | 0.051 |
| Median age (years) Age <60 years | 66 25 (27%) | 70 7 (22%) | 0.576 |
| Stage III/IV | 56 (60%) | 17 (53%) | 0.483 |
| R-IPI 3–5 * | 27 (30%) | 11 (35%) | 0.570 |
| Bone Marrow* None/Discordant Concordant | 86 (96%) 4 (4%) | 28 (87%) 4 (13%) | 0.118 |
| DLBCL-NOS GC-subtype | 35 (40%) | 11 (41%) | 0.962 |
| HGBCL-DH/TH | 3 (3%) | 3 (9%) | 0.160 |
| HGBCL-NOS | 3 (3%) | 2 (6%) | 0.451 |
| CD5-positive ** | 9 (12%) | 4 (15%) | 0.743 |
| BCL2-positive | 54 (58%) | 16 (50%) | 0.428 |
| MYC-positive *** | 21 (23%) | 11 (39%) | 0.085 |
| Double-expressor *** | 14 (15%) | 6 (21%) | 0.440 |
References
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H. (Eds.) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Sehn, L.H.; Gascoyne, R.D. Diffuse large B-cell lymphoma: Optimizing outcome in the context of clinical and biologic heterogeneity. Blood 2015, 125, 22–32. [Google Scholar] [CrossRef]
- Pasqualucci, L.; Dalla-Favera, R. Genetics of DLBCL. Blood 2018, 131, 2307–2319. [Google Scholar] [CrossRef]
- Morin, R.D.; Johnson, N.A.; Severson, T.M.; Mungall, A.J.; An, J.; Goya, R.; Paul, J.E.; Boyle, M.; Woolcock, B.W.; Kuchenbauer, F.; et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010, 42, 181–185. [Google Scholar] [CrossRef]
- cBioPortal. Available online: https://www.cbioportal.org/ (accessed on 15 August 2020).
- Yap, D.B.; Chu, J.; Berg, T.; Schapira, M.; Cheng, S.W.G.; Moradian, A.; Morin, R.D.; Mungall, A.J.; Meissner, B.; Boyle, M.; et al. Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation. Blood 2011, 117, 2451–2459. [Google Scholar] [CrossRef]
- Bohers, E.; Mareschal, S.; Bertrand, P.; Viailly, P.J.; Dubois, S.; Maingonnat, C.; Ruminy, P.; Tilly, H.; Jardin, F. Activating somatic mutations in DLBCL: Lessons from next generation sequencing and key elements in the precision medicine era. Leuk. Lymph. 2015, 56, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 6 October 2019).
- Bödör, C.; O’riain, C.; Wrench, D.; Matthews, J.; Iyengar, S.; Tayyib, H.; Calaminici, M.; Clear, A.; Iqbal, S.; Quentmeier, H.; et al. EZH2 Y641 mutations in follicular lymphoma. Leukemia 2011, 25, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Gao, J.; Popovic, R.; Wolniak, K.; Parimi, V.; Winter, J.N.; Licht, J.D.; Chen, Y.-H. Strong expression of EZH2 and accumulation of H3K27me3 in DLBCL independent of COO and EZH2 Y641 mutation. Leuk. Lymph. 2015, 56, 2895–2901. [Google Scholar] [CrossRef]
- Juskevicius, D.; Jucker, D.; Klingbiel, D.; Mamot, C.; Dirnhofer, S.; Tzankov, A. Mutations of CREBBP and SOCS1 are independent prognostic factors in DLBCL: Mutational analysis of the SAKK 38/07 prospective clinical trial cohort. J. Hematol. Oncol. 2017, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S.; Mareschal, S.; Picquenot, J.M.; Viailly, P.J.; Bohers, E.; Cornic, M.; Bertrand, P.; Veresezan, E.L.; Ruminy, P.; Maingonnat, C.; et al. Immunohistochemical and genomic profiles of diffuse large B-cell lymphomas: Implications for targeted EZH2 inhibitor therapy? Oncotarget 2015, 6, 16712–16724. [Google Scholar] [CrossRef][Green Version]
- Lee, H.J.; Shin, D.H.; Kim, K.B.; Shin, N.; Park, W.Y.; Lee, J.H.; Choi, K.U.; Kim, J.Y.; Lee, C.H.; Sol, M.Y. Polycomb protein EZH2 expression in diffuse large B-cell lymphoma is associated with better prognosis in patients treated with R-CHOP. Leuk. Lymph. 2014, 55, 2056–2063. [Google Scholar] [CrossRef]
- Oh, E.J.; Yang, W.I.; Cheong, J.-W.; Choi, S.-E.; Yoon, S.O. DLBCL with histone H3K27me3 another poor prognostic phenotype independent of c-Myc/Bcl2 coexpression. Hum Pathol. 2014, 45, 2043–2050. [Google Scholar] [CrossRef]
- Duncan, V.E.; Ping, Z.; Varambally, S.; Peker, D. Loss of RUNX3 expression is an independent adverse prognostic factor in diffuse large B-cell lymphoma. Leuk. Lymph. 2017, 58, 179–184. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, K.; Li, M.; Zeng, K.; Wei, J.; Li, X.; Liu, Y.; Zhao, D.; Fan, L.; Yu, Z.; et al. EZH2 overexpression in primary gastrointestinal diffuse large B-cell lymphoma and its association with the clinicopathological features. Hum. Pathol. 2017, 64, 213–221. [Google Scholar] [CrossRef] [PubMed]
- van Kemenade, F.J.; Raaphorst, F.M.; Blokzijl, T.; Fieret, E.; Hamer, K.M.; Satijn, D.P.; Otte, A.P.; Meijer, C.J. Coexpression of BMI-1 and EZH2 polycomb-group proteins is associated with cycling cells and degree of malignancy in B-cell non-Hodgkin lymphoma. Blood 2001, 97, 3896–3901. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Pelton, A.; Shahsafaei, A.; Dorfman, D.M. Differential expression of EZH2 protein in small cell and aggressive B-cell NHL and differential regulation of EZH2 expression by p-ERK1/2 and MYC in aggressive B-cell lymphomas. Mod. Pathol. 2016, 29, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Bai, Q.; Rohr, J.; Wang, Y.; Liu, Y.; Zeng, K.; Yu, K.; Zhang, X.; Wang, Z. Clinicopathological features of primary DLBCL of the central nervous system-strong EZH2 expression implying diagnostic and therapeutic implication. Apmis 2016, 124, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Hans, C.P.; Weisenburger, D.D.; Greiner, T.C.; Gascoyne, R.D.; Delabie, J.; Ott, G.; Muller-Hermelink, H.K.; Campo, E.; Braziel, R.M.; Jaffe, E.S.; et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004, 103, 275–282. [Google Scholar] [CrossRef]
- Johnson, N.A.; Slack, G.W.; Savage, K.J.; Connors, J.M.; Ben-Neriah, S.; Rogic, S.; Scott, D.W.; Tan, K.L.; Steidl, C.; Sehn, L.H.; et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. 2012, 30, 3452–3459. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.R.; Jerónimo, C.; Henrique, R.; Fonseca, D.; Oliveira, J.; Lothe, R.A.; Teixeira, M. 8q Gain Is an Independent Predictor of Poor Survival in Diagnostic Needle Biopsies from Prostate Cancer Suspects. Clin. Cancer Res. 2006, 12, 3961–3970. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, X.; Huang, C.; Chen, G.; Chen, F.; Lu, J.; Shi, X.; He, C.; Zeng, Z.; Qiu, Y.; et al. EZH2/Bcl-2 Coexpression Predicts Worse Survival in Diffuse Large B-cell Lymphomas and Demonstrates Poor Efficacy to Rituximab in Localized Lesions. J. Cancer 2019, 10, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Comet, I.; Riising, E.M.; Leblanc, B.; Helin, K. Maintaining cell identity: PRC2-mediated regulation of transcription and cancer. Nat. Rev. Cancer 2016, 16, 803–810. [Google Scholar] [CrossRef]
- Italiano, A.; Soria, J.C.; Toulmonde, M.; Michot, J.M.; Lucchesi, C.; Varga, A.; Coindre, J.M.; Blakemore, S.J.; Clawson, A.; Suttle, B.; et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: A first-in-human, open-label, phase 1 study. Lancet Oncol. 2018, 19, 649–659. [Google Scholar] [CrossRef]
- Morschhauser, F.; Salles, G.; McKay, P.; Tilly, H.; Schmitt, A.; Gerecitano, J.; Johnson, P.; Le Gouill, S.; Dickinson, M.; Fruchart, C.; et al. Interim Report from A Phase 2 Multicenter Study of Tazemetostat, an Ezh2 Inhibitor, in Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphomas. Hematol. Oncol. 2017, 35, 24–25. [Google Scholar] [CrossRef]
- Abramson, J. Hitting Back at Lymphoma: How Do Modern Diagnostics Identify High-Risk Diffuse Large B-Cell Lymphoma Subsets and Alter Treatment? Cancer 2019, 125, 3111–3120. [Google Scholar] [CrossRef] [PubMed]
- Enosr, J. Biomarker Validation: Common Data Analysis Concerns. Oncologist 2014, 19, 886–891. [Google Scholar] [CrossRef]

| All Patients (n = 125) | DLBCL-NOS (n = 114) | DLBCL-NOS GC (n = 46) | DLBCL-NOS Non-GC (n = 68) | p | |
|---|---|---|---|---|---|
| Male sex | 52 (42%) | 47 (41%) | 23 (50%) | 24 (35%) | 0.118 |
| Median age (years) <60 years | 66 (20–83) 32 (26%) | 66 (20–83) 29 (25%) | 65 (23–80) 13 (28%) | 66 (20–83) 16 (24%) | 0.815 0.569 |
| Stage III/IV | 73 (58%) | 66 (58%) | 23 (50%) | 43 (63%) | 0.160 |
| R-IPI 3–5 * | 38 (31%) | 35 (32%) | 11 (24%) | 24 (38%) | 0.131 |
| Bone marrow * Negative/Discordant Concordant | 113 (93%) 8 (7%) | 103 (94%) 7 (6%) | 43 (93%) 3 (7%) | 60 (94%) 4 (6%) | 0.954 |
| Morphology Centroblastic Immunoblastic Anaplastic High-grade | 124 (91%) 1 (1%) 5 (4%) 5 (4%) | 108 (95%) 1 (1%) 5 (4%) NA | 45 (98%) 0 (0%) 1 (2%) NA | 63 (93%) 1 (1%) 4 (6%) NA | NA |
| CD5-positive ** | 13 (13%) | 10 (11%) | 3 (8%) | 7 (13%) | 0.449 |
| BCL2-positive | 70 (56%) | 61 (54%) | 15 (33%) | 46 (68%) | <0.001 |
| MYC-positive *** | 32 (27%) | 25 (23%) | 6 (14%) | 19 (29%) | 0.063 |
| Double-expressor *** | 20 (17%) | 15 (14%) | 1 (2%) | 14 (21%) | 0.005 |
| HGBCL-DH/TH MYC/BCL2 MYC/BCL6 MYC/BCL2/BCL6 | 6 (5%) 3 (50%) 2 (33%) 1 (17%) | NA | NA | NA | NA |
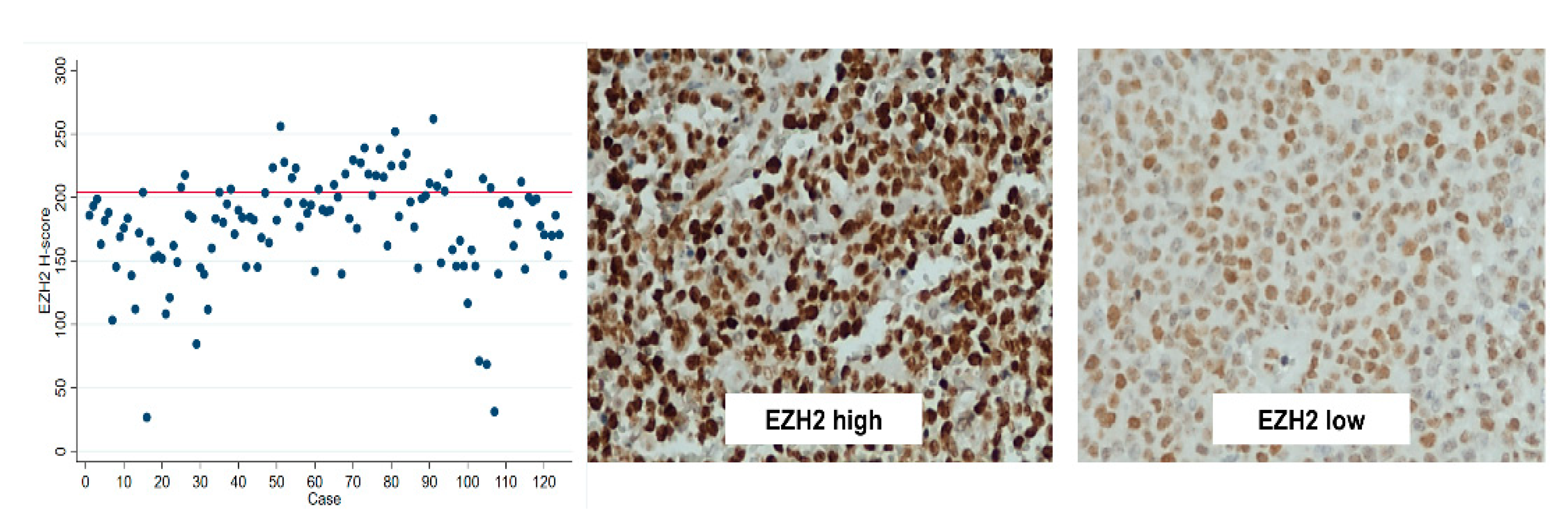
| EZH2 Mean H-score EZH2 high | 177.8 (26.7–261.9) 32 (26%) | 176.8 (31.2–261.9) 27 (24%) | 171.7 (68.5–256.0) 11 (24%) | 180.2 (31.2–261.9) 16 (24%) | 0.248 0.962 |
| EZH2/BCL2 coexp. | 16 (13%) | 12 (11%) | 2 (4%) | 10 (15%) | 0.077 |
| All Patients n = 125 | DLBCL-NOS n = 114 | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95CI) | p | HR (95CI) | p | HR (95CI) | p | HR (95CI) | p | |
| Male sex | 0.89 (0.47–1.67) | 0.711 | NA | NA | 1.20 (0.62–2.34) | 0.584 | NA | NA |
| Age (>60 years) | 1.32 (0.63–2.78) | 0.464 | NA | NA | 1.28 (0.58–2.82) | 0.541 | NA | NA |
| Stage (III/IV) | 2.46 (1.20–5.03) | 0.014 | 1.53 (0.60–3.90) | 0.373 | 2.84 (1.29–6.26) | 0.010 | 1.99 (0.73–5.40) | 0.176 |
| R-IPI (3–5) | 2.07 (1.08–3.95) | 0.028 | 1.12 (0.48–2.59) | 0.799 | 2.11 (1.05–4.22) | 0.036 | 1.03 (0.42–2.54) | 0.942 |
| BM concordant involvement | 4.53 (1.87–11.00) | 0.001 | 3.32 (1.14–9.69) | 0.028 | 4.38 (1.66–11.51) | 0.003 | 2.99 (0.91–9.88) | 0.072 |
| BCL2-positive | 2.10 (1.07–4.14) | 0.031 | 1.00 (0.44–2.25) | 0.990 | 2.14 (1.05–4.38) | 0.036 | 1.02 (0.44–2.34) | 0.967 |
| CD5-positive | 0.96 (0.34–2.72) | 0.933 | NA | NA | 0.93 (0.28–3.07) | 0.905 | NA | NA |
| GC subtype | 0.38 (0.19–0.78) | 0.009 | 0.44 (0.20–0.98) | 0.046 | 0.26 (0.11–0.62) | 0.003 | 0.31 (0.12–0.80) | 0.016 |
| HGBCL-DH/TH | 2.39 (0.73–7.79) | 0.148 | 2.25 (0.53–9.53) | 0.272 | NA | NA | NA | NA |
| HGBCL-NOS | 1.77 (0.43–7.40) | 0.431 | NA | NA | NA | NA | NA | NA |
| Double- expressor | 1.72 (0.82–3.62) | 0.155 | 0.97 (0.37–2.58) | 0.958 | 1.42 (0.59–3.42) | 0.439 | 0.79 (0.25–2.47) | 0.686 |
| EZH2 high | 1.59 (0.82–3.09) | 0.173 | NA | NA | 1.69 (0.82–3.46) | 0.153 | NA | NA |
| EZH2/BCL2 coexp. | 2.40 (1.10–5.26) | 0.029 | 1.93 (0.74–5.05) | 0.178 | 2.50 (1.03–6.09) | 0.043 | 2.01 (0.69–5.85) | 0.200 |
| All Patients n = 125 | DLBCL-NOS n = 114 | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95CI) | p | HR (95CI) | p | HR (95CI) | p | HR (95CI) | p | |
| Male sex | 1.02 (0.52–1.98) | 0.955 | NA | NA | 1.34 (0.67–2.68) | 0.413 | NA | NA |
| Age (>60 years) | 3.58 (1.26–10.19) | 0.017 | 4.58 (1.25–16.65) | 0.021 | 4.33 (1.31–14.30) | 0.016 | 7.33 (1.55–34.67) | 0.012 |
| Stage (III/IV) | 3.92 (1.63–9.45) | 0.002 | 3.54 (1.13–11.11) | 0.030 | 4.30 (1.65–11.19) | 0.003 | 4.94 (1.43–17.03) | 0.011 |
| R-IPI (3–5) | 2.88 (1.45–5.74) | 0.003 | 0.69 (0.31–2.04) | 0.628 | 2.88 (1.38–6.01) | 0.005 | 0.73 (0.27–1.98) | 0.535 |
| BM concordant involvement | 4.42 (1.67–11.69) | 0.003 | 1.94 (0.60–6.29) | 0.269 | 4.26 (1.45–12.52) | 0.008 | 1.40 (0.37–5.31) | 0.625 |
| BCL2-positive | 2.72 (1.28–5.80) | 0.009 | 1.22 (0.50–2.97) | 0.654 | 2.47 (1.14–5.34) | 0.021 | 1.18 (0.48–2.89) | 0.722 |
| CD5-positive | 1.24 (0.43–3.59) | 0.686 | NA | NA | 1.13 (0.34–3.77) | 0.845 | NA | NA |
| GC subtype | 0.40 (0.19–0.85) | 0.017 | 0.50 (0.21–1.16) | 0.107 | 0.31 (0.13–0.75) | 0.009 | 0.38 (0.15–0.99) | 0.048 |
| HGBCL–DH/TH | 2.79 (0.85–9.18) | 0.092 | 1.78 (0.41–7.66) | 0.441 | NA | NA | NA | NA |
| HGBCL-NOS | 0.95 (0.13–6.95) | 0.956 | NA | NA | NA | NA | NA | NA |
| Double-expressor | 1.70 (0.77–3.74) | 0.188 | 1.00 (0.36–2.80) | 0.999 | 1.26 (0.48–3.28) | 0.636 | 0.65 (0.18–2.31) | 0.503 |
| EZH2 high | 1.57 (0.77–3.23) | 0.215 | NA | NA | 1.56 (0.72–3.40) | 0.263 | NA | NA |
| EZH2/BCL2 coexp. | 3.53 (1.63–7.65) | 0.001 | 2.70 (0.98–3.38) | 0.054 | 3.70 (1.57–8.72) | 0.003 | 3.43 (1.16–10.18) | 0.026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petronilho, S.; Sequeira, J.P.; Paulino, S.; Lopes, P.; Lisboa, S.; Chacim, S.; Lobo, J.; Teixeira, M.; Jerónimo, C.; Henrique, R. Prognostic Value of Histone Modifying Enzyme EZH2 in RCHOP-Treated Diffuse Large B-Cell Lymphoma and High Grade B-Cell Lymphoma. J. Pers. Med. 2021, 11, 1384. https://doi.org/10.3390/jpm11121384
Petronilho S, Sequeira JP, Paulino S, Lopes P, Lisboa S, Chacim S, Lobo J, Teixeira M, Jerónimo C, Henrique R. Prognostic Value of Histone Modifying Enzyme EZH2 in RCHOP-Treated Diffuse Large B-Cell Lymphoma and High Grade B-Cell Lymphoma. Journal of Personalized Medicine. 2021; 11(12):1384. https://doi.org/10.3390/jpm11121384
Chicago/Turabian StylePetronilho, Sara, José Pedro Sequeira, Sofia Paulino, Paula Lopes, Susana Lisboa, Sérgio Chacim, João Lobo, Manuel Teixeira, Carmen Jerónimo, and Rui Henrique. 2021. "Prognostic Value of Histone Modifying Enzyme EZH2 in RCHOP-Treated Diffuse Large B-Cell Lymphoma and High Grade B-Cell Lymphoma" Journal of Personalized Medicine 11, no. 12: 1384. https://doi.org/10.3390/jpm11121384
APA StylePetronilho, S., Sequeira, J. P., Paulino, S., Lopes, P., Lisboa, S., Chacim, S., Lobo, J., Teixeira, M., Jerónimo, C., & Henrique, R. (2021). Prognostic Value of Histone Modifying Enzyme EZH2 in RCHOP-Treated Diffuse Large B-Cell Lymphoma and High Grade B-Cell Lymphoma. Journal of Personalized Medicine, 11(12), 1384. https://doi.org/10.3390/jpm11121384









