Predictive Model for High Coronary Artery Calcium Score in Young Patients with Non-Dialysis Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
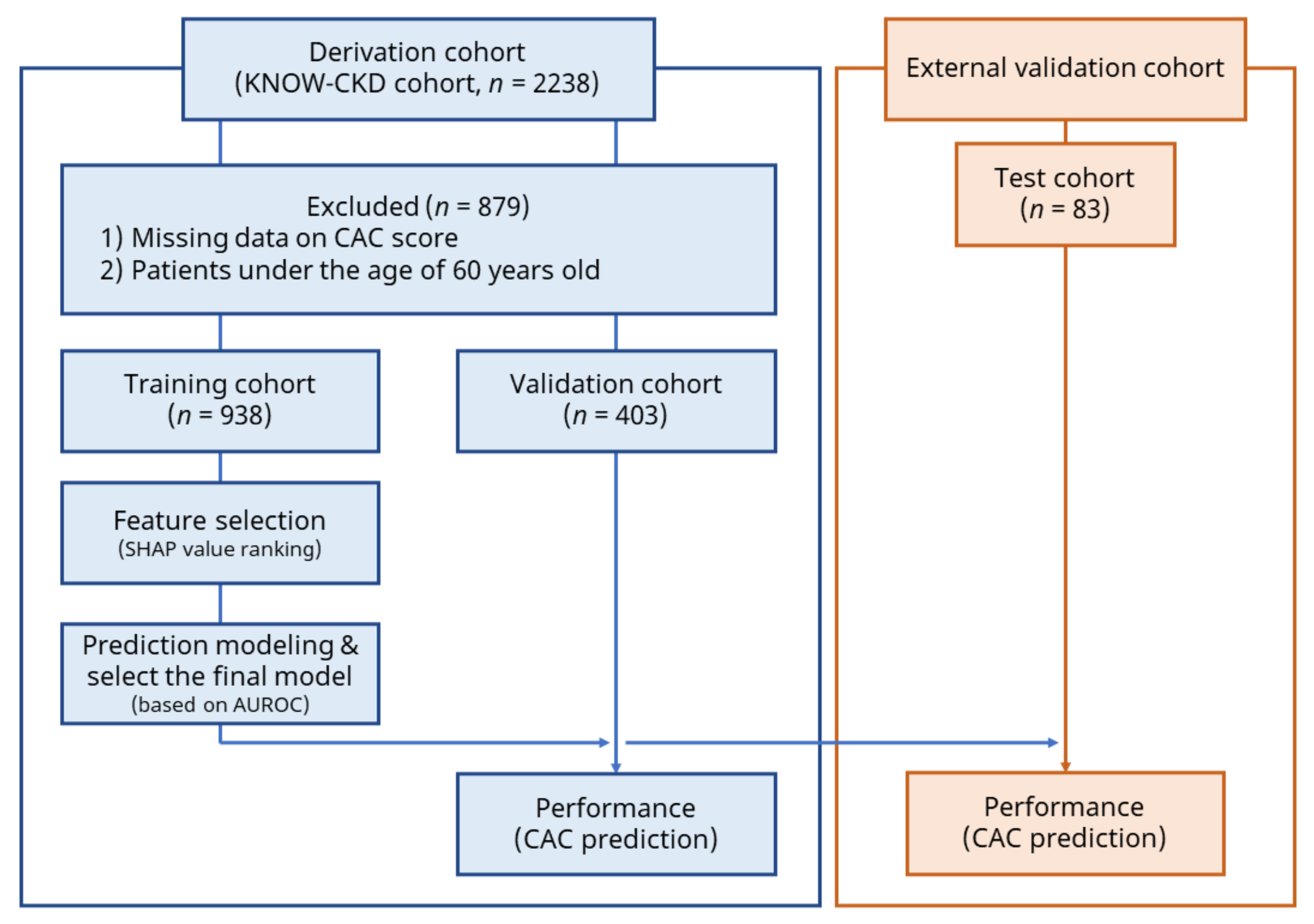
2.1. Data Source and Study Population
2.2. Measurement and Definition
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Study Population
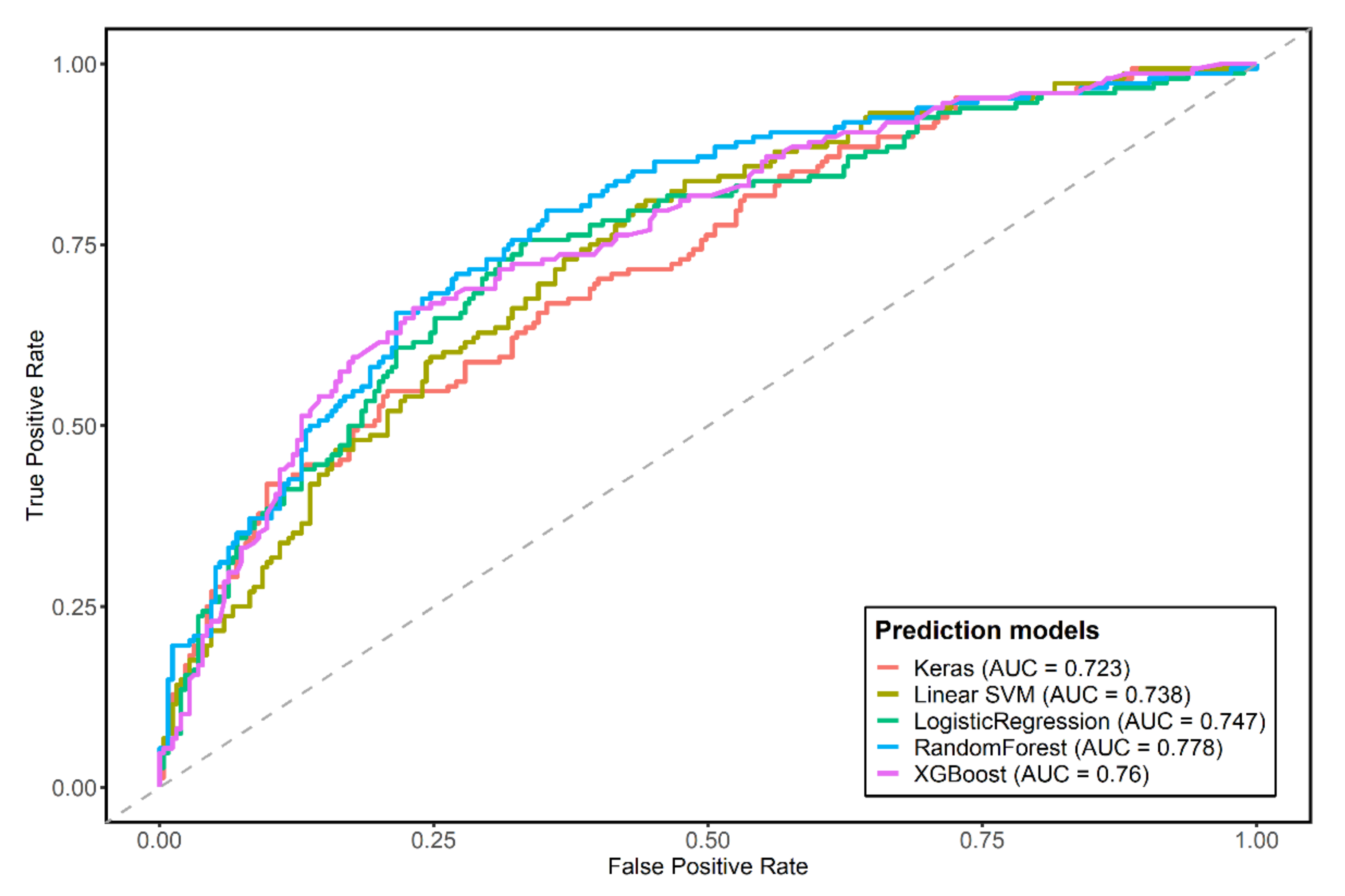
3.2. Predictive Models for Coronary Artery Calcium Score
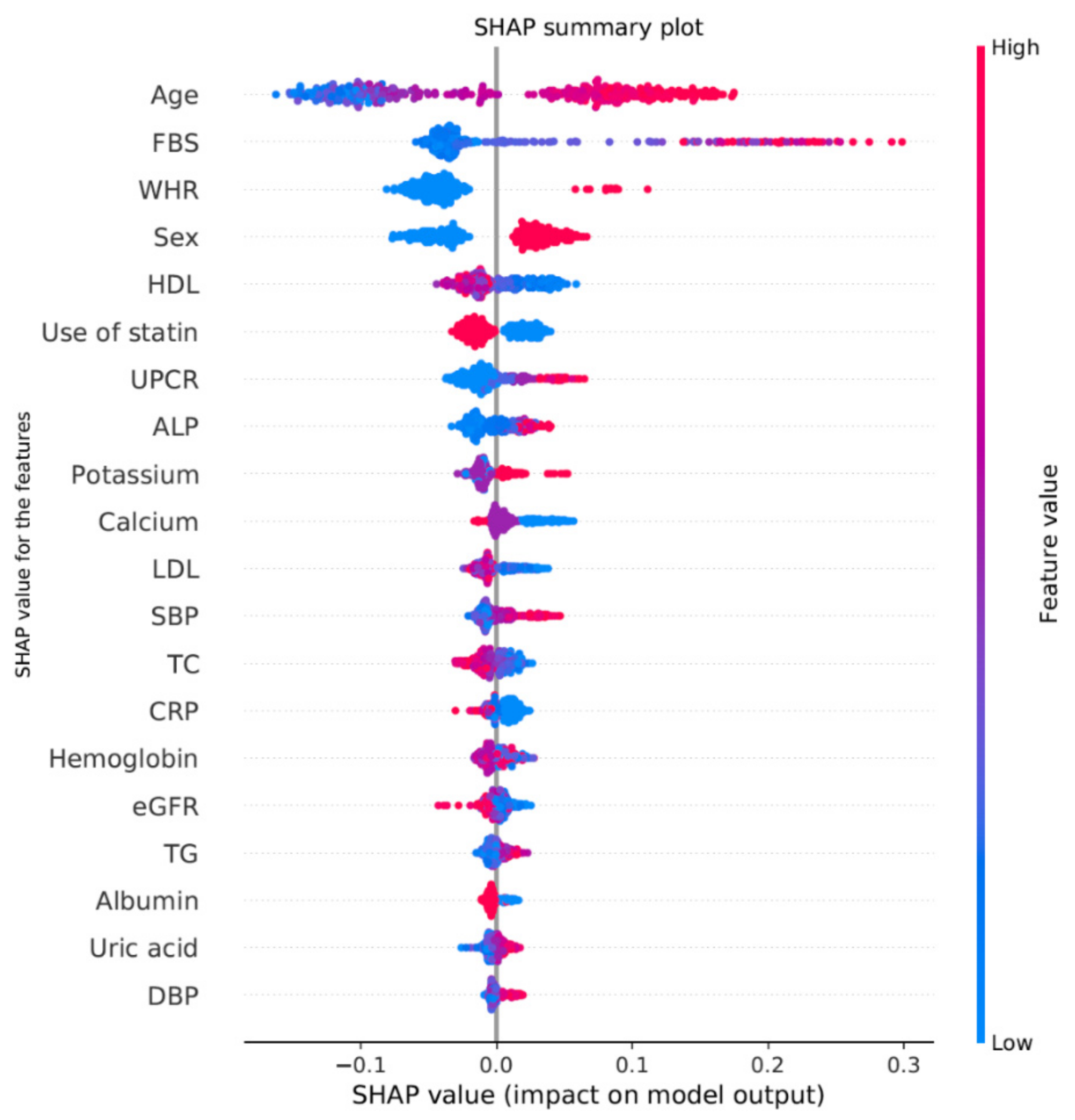
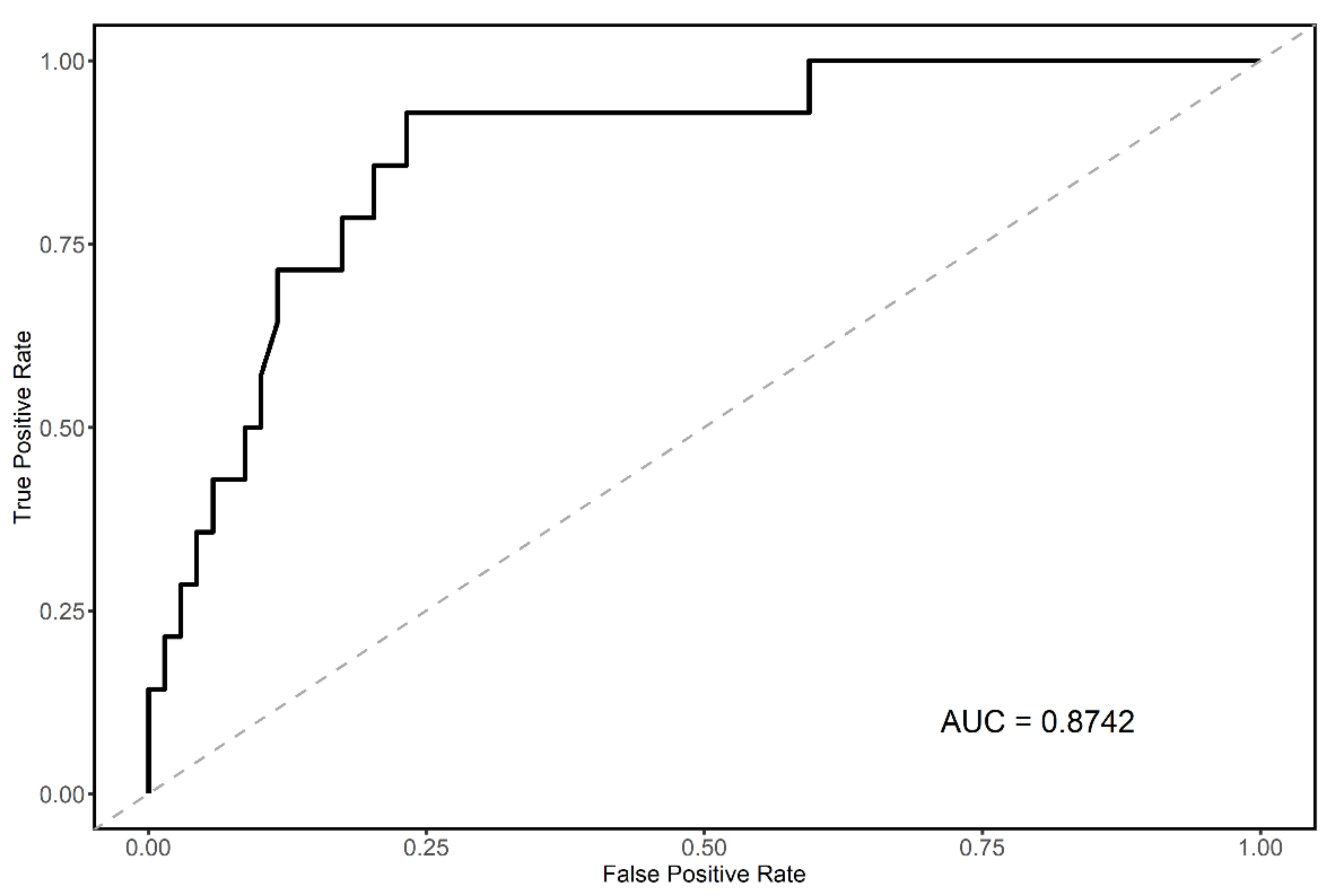
3.3. Final Predictive Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunsicker, L.G. The consequences and costs of chronic kidney disease before esrd. J. Am. Soc. Nephrol. 2004, 15, 1363–1364. [Google Scholar] [CrossRef][Green Version]
- Chadban, S.J.; Briganti, E.M.; Kerr, P.G.; Dunstan, D.W.; Welborn, T.A.; Zimmet, P.Z.; Atkins, R.C. Prevalence of kidney damage in australian adults: The ausdiab kidney study. J. Am. Soc. Nephrol. 2003, 14, S131–S138. [Google Scholar] [CrossRef]
- Thompson, S.; James, M.; Wiebe, N.; Hemmelgarn, B.; Manns, B.; Klarenbach, S.; Tonelli, M. Cause of death in patients with reduced kidney function. J. Am. Soc. Nephrol. 2015, 26, 2504–2511. [Google Scholar] [CrossRef]
- Reiss, A.B.; Miyawaki, N.; Moon, J.; Kasselman, L.J.; Voloshyna, I.; D’Avino, R., Jr.; De Leon, J. Ckd, arterial calcification, atherosclerosis and bone health: Inter-relationships and controversies. Atherosclerosis 2018, 278, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Gracia, M.; Betriu, À.; Martínez-Alonso, M.; Arroyo, D.; Abajo, M.; Fernández, E.; Valdivielso, J.M. Predictors of subclinical atheromatosis progression over 2 years in patients with different stages of ckd. Clin. J. Am. Soc. Nephrol. 2016, 11, 287–296. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Townend, J.N.; Landray, M.J. Cardiovascular risk factors in predialysis patients: Baseline data from the chronic renal impairment in birmingham (crib) study. Kidney Int. 2003, 63, S201–S203. [Google Scholar] [CrossRef][Green Version]
- McCullough, P.A.; Li, S.; Jurkovitz, C.T.; Stevens, L.; Collins, A.J.; Chen, S.C.; Norris, K.C.; McFarlane, S.; Johnson, B.; Shlipak, M.G.; et al. Chronic kidney disease, prevalence of premature cardiovascular disease, and relationship to short-term mortality. Am. Heart J. 2008, 156, 277–283. [Google Scholar] [CrossRef]
- Nasir, K.; Santos, R.D.; Tufail, K.; Rivera, J.; Carvalho, J.A.; Meneghello, R.; Brady, T.D.; Blumenthal, R.S. High-normal fasting blood glucose in non-diabetic range is associated with increased coronary artery calcium burden in asymptomatic men. Atherosclerosis 2007, 195, e155–e160. [Google Scholar] [CrossRef]
- Greenland, P.; LaBree, L.; Azen, S.P.; Doherty, T.M.; Detrano, R.C. Coronary artery calcium score combined with framingham score for risk prediction in asymptomatic individuals. JAMA 2004, 291, 210–215. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, P.G.; Taylor, A.J.; Jackson, J.L.; Doherty, T.M.; Detrano, R.C. Prognostic value of coronary electron-beam computed tomography for coronary heart disease events in asymptomatic populations. Am. J. Cardiol. 2000, 85, 945–948. [Google Scholar] [CrossRef]
- Kondos, G.T.; Hoff, J.A.; Sevrukov, A.; Daviglus, M.L.; Garside, D.B.; Devries, S.S.; Chomka, E.V.; Liu, K. Electron-beam tomography coronary artery calcium and cardiac events: A 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation 2003, 107, 2571–2576. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.W.; Kim, J.; Kim, D.K.; Oh, K.H.; Joo, K.W.; Kim, Y.S.; Han, S.S. Machine learning algorithm to predict mortality in patients undergoing continuous renal replacement therapy. Crit. Care 2020, 24, 42. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Yoon, H.K.; Nam, K.; Cho, Y.J.; Kim, T.K.; Kim, W.H.; Bahk, J.H. Derivation and validation of machine learning approaches to predict acute kidney injury after cardiac surgery. J. Clin. Med. 2018, 7, 322. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.H.; Park, S.K.; Park, H.C.; Chin, H.J.; Chae, D.W.; Choi, K.H.; Han, S.H.; Yoo, T.H.; Lee, K.; Kim, Y.S.; et al. Know-ckd (korean cohort study for outcome in patients with chronic kidney disease): Design and methods. BMC Nephrol. 2014, 15, 80. [Google Scholar] [CrossRef]
- Miller, W.G.; Myers, G.L.; Ashwood, E.R.; Killeen, A.A.; Wang, E.; Thienpont, L.M.; Siekmann, L. Creatinine measurement: State of the art in accuracy and interlaboratory harmonization. Arch. Pathol. Lab. Med. 2005, 129, 297–304. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in python. J. Mach. Learn. Res. 2012, 12, 2825–2830. [Google Scholar]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Gulli, A.; Pal, S. Deep Learning with Keras; Packt Publishing Ltd.: Birmingham, UK, 2017. [Google Scholar]
- Buuren, S.; Groothuis-Oudshoorn, C. Mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Štrumbelj, E.; Kononenko, I. Explaining prediction models and individual predictions with feature contributions. Knowl. Inf. Syst. 2013, 41, 647–665. [Google Scholar] [CrossRef]
- Ribeiro, M.; Singh, S.; Guestrin, C. “Why should i trust you?”: Explaining the predictions of any classifier. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 1135–1144. [Google Scholar]
- Lundberg, S.; Lee, S.-I. A unified approach to interpreting model predictions. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017. [Google Scholar]
- Lundberg, S.; Erion, G.; Lee, S.-I. Consistent individualized feature attribution for tree ensembles. arXiv 2018, arXiv:1802.03888. [Google Scholar]
- Park, H.E.; Kim, M.K.; Choi, S.Y.; Lee, W.; Shin, C.S.; Cho, S.H.; Oh, B.H. The prevalence and distribution of coronary artery calcium in asymptomatic korean population. Int. J. Cardiovasc. Imaging 2012, 28, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Greenland, P.; Bonow, R.O.; Brundage, B.H.; Budoff, M.J.; Eisenberg, M.J.; Grundy, S.M.; Lauer, M.S.; Post, W.S.; Raggi, P.; Redberg, R.F.; et al. Accf/aha 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the american college of cardiology foundation clinical expert consensus task force (accf/aha writing committee to update the 2000 expert consensus document on electron beam computed tomography) developed in collaboration with the society of atherosclerosis imaging and prevention and the society of cardiovascular computed tomography. J. Am. Coll. Cardiol. 2007, 49, 378–402. [Google Scholar] [PubMed]
- Lichtenstein, G.; Perlman, A.; Shpitzen, S.; Durst, R.; Shaham, D.; Leitersdorf, E.; Szalat, A. Correlation between coronary artery calcification by non-cardiac ct and framingham score in young patients. PLoS ONE 2018, 13, e0195061. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.A.; Navar, A.M.; Carter, R.E. Moving beyond regression techniques in cardiovascular risk prediction: Applying machine learning to address analytic challenges. Eur. Heart J. 2017, 38, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Deo, R.C. Machine learning in medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef]
- Lundberg, S.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.-I. From local explanations to global understanding with explainable ai for trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef]
- Oh, H.G.; Nallamshetty, S.; Rhee, E.J. Increased risk of progression of coronary artery calcification in male subjects with high baseline waist-to-height ratio: The kangbuk samsung health study. Diabetes Metab. J. 2016, 40, 54–61. [Google Scholar] [CrossRef]
| Accuracy | Sensitivity | Specificity | AUROC (95% CI) | p-Value for DeLong Test | |
|---|---|---|---|---|---|
| Logistic regression | 0.7022 | 0.5068 | 0.8157 | 0.7467 (0.696–0.797) | reference |
| XGboost | 0.737 | 0.5405 | 0.8510 | 0.7599 (0.711–0.809) | 0.3809 |
| RandomForest | 0.727 | 0.5068 | 0.8549 | 0.7776 (0.731–0.825) | 0.0220 |
| Support vector machine | 0.6799 | 0.17568 | 0.97255 | 0.7379 (0.689–0.787) | 0.4711 |
| Multilayer perceptron neural network | 0.6923 | 0.4527 | 0.8314 | 0.7233 (0.672–0.775) | 0.1014 |
| Variables | Odds Ratio | Confidence Interval | p-Value |
|---|---|---|---|
| Age | 1.107 | 1.081–1.135 | <0.001 |
| Male | 4.066 | 2.617–6.405 | <0.001 |
| Estimated glomerular filtration rate | 1.008 | 1.001–1.015 | 0.0174 |
| C-reactive protein | 0.941 | 0.889–0.989 | 0.0256 |
| Fasting blood glucose | 1.01 | 1.005–1.015 | 0.0002 |
| High density lipid | 0.99 | 0.978–1.002 | 0.1219 |
| Total cholesterol | 0.995 | 0.99–1 | 0.0434 |
| Marital status: Never | 1.798 | 1.043–3.102 | 0.0345 |
| Marital status: DW | 1.515 | 0.712–3.236 | 0.2799 |
| Unemployed | 1.499 | 1–2.257 | 0.0509 |
| Non-use of statin | 0.675 | 0.479–0.952 | 0.025 |
| Phosphate | 1.249 | 0.945–1.656 | 0.1207 |
| Waist–hip ratio | 39.309 | 2.587–621.509 | 0.0086 |
| Hemoglobin | 0.921 | 0.822–1.031 | 0.1544 |
| Urine protein to creatinine ratio | 1.139 | 1.043–1.248 | 0.0044 |
| Serum potassium | 1.405 | 0.993–1.992 | 0.0555 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, T.R.; Song, S.H.; Choi, H.S.; Suh, S.H.; Kim, C.S.; Jung, J.Y.; Choi, K.H.; Oh, K.-H.; Ma, S.K.; Bae, E.H.; et al. Predictive Model for High Coronary Artery Calcium Score in Young Patients with Non-Dialysis Chronic Kidney Disease. J. Pers. Med. 2021, 11, 1372. https://doi.org/10.3390/jpm11121372
Oh TR, Song SH, Choi HS, Suh SH, Kim CS, Jung JY, Choi KH, Oh K-H, Ma SK, Bae EH, et al. Predictive Model for High Coronary Artery Calcium Score in Young Patients with Non-Dialysis Chronic Kidney Disease. Journal of Personalized Medicine. 2021; 11(12):1372. https://doi.org/10.3390/jpm11121372
Chicago/Turabian StyleOh, Tae Ryom, Su Hyun Song, Hong Sang Choi, Sang Heon Suh, Chang Seong Kim, Ji Yong Jung, Kyu Hun Choi, Kook-Hwan Oh, Seong Kwon Ma, Eun Hui Bae, and et al. 2021. "Predictive Model for High Coronary Artery Calcium Score in Young Patients with Non-Dialysis Chronic Kidney Disease" Journal of Personalized Medicine 11, no. 12: 1372. https://doi.org/10.3390/jpm11121372
APA StyleOh, T. R., Song, S. H., Choi, H. S., Suh, S. H., Kim, C. S., Jung, J. Y., Choi, K. H., Oh, K.-H., Ma, S. K., Bae, E. H., & Kim, S. W. (2021). Predictive Model for High Coronary Artery Calcium Score in Young Patients with Non-Dialysis Chronic Kidney Disease. Journal of Personalized Medicine, 11(12), 1372. https://doi.org/10.3390/jpm11121372





