Personalized Approach for Obese Patients Undergoing Endoscopic Sleeve Gastroplasty
Abstract
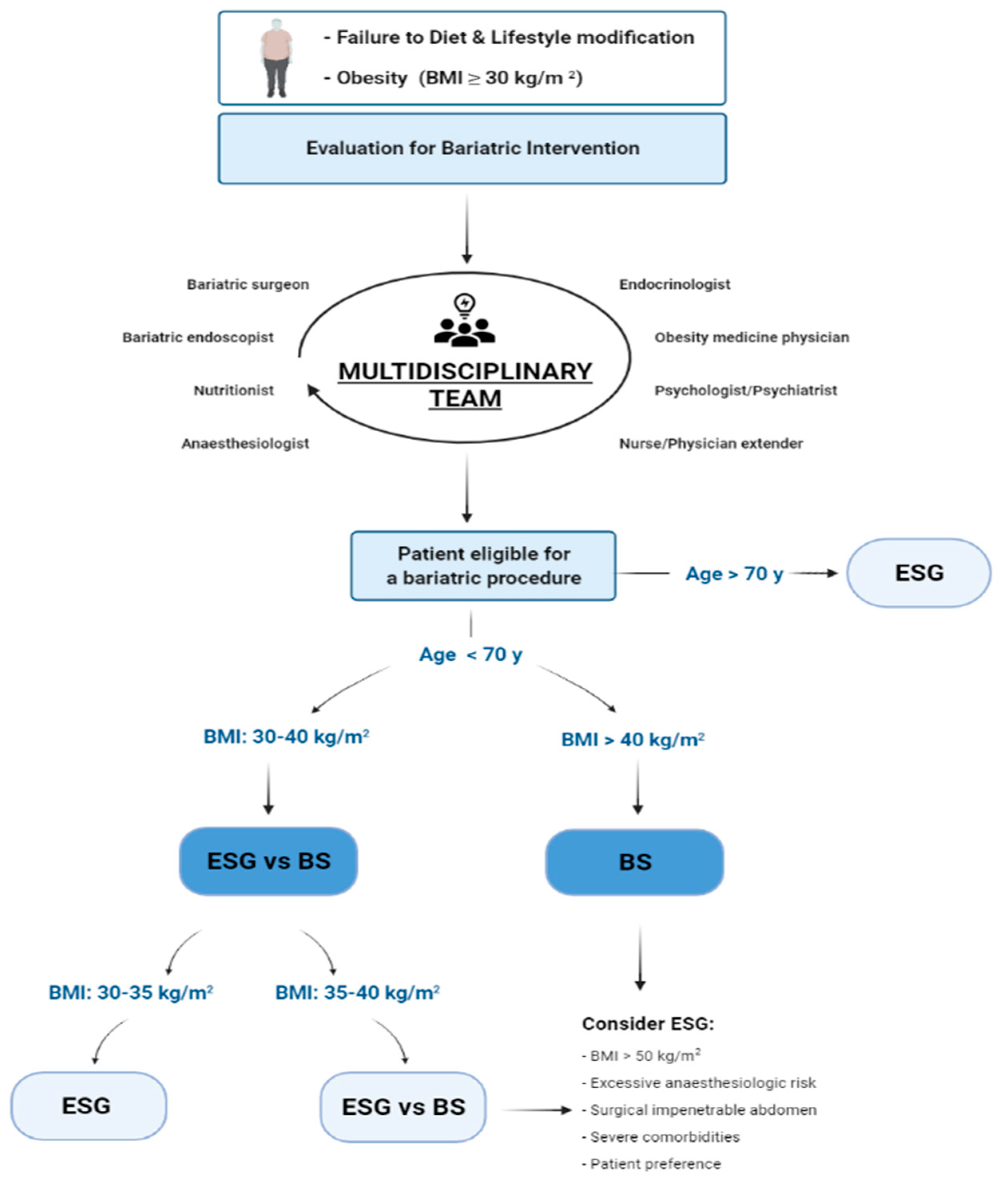
:1. Introduction
2. Materials and Methods
3. Results
3.1. Apollo Overstitch
3.2. Endomina and POSE-2 Procedure
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight Factsheet. 2018. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 December 2018).
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric surgery: A systematic review and meta-analysis. JAMA 2004, 292, 1724–1737. [Google Scholar] [CrossRef]
- Chang, S.H.; Stoll, C.R.T.; Song, J.; Varela, J.E.; Eagon, C.J.; Colditz, G.A. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003-2012. JAMA 2014, 149, 275–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ASGE/ASMBS Task Force on Endoscopic Bariatric Therapy. A pathway to endoscopic Bariatric Therapy. Gastrointest. Endosc. 2011, 74, 943–953. [Google Scholar] [CrossRef]
- Abu Dayyeh, B.K.; Rajan, E.; Gostout, C.J. Endoscopic sleeve gastroplasty: A potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest. Endosc. 2013, 78, 530–535. [Google Scholar] [CrossRef]
- Acosta, A.; Streett, S.; Kroh, M.D.; Cheskin, L.J.; Saunders, K.H.; Kurian, M.; Schofield, M.; Barlow, S.E.; Aronne, L. White Paper AGA: POWER—Practice Guide on Obesity and Weight Management, Education and Resources. Clin. Gastroenterol. Hepatol. 2016, 15, 631–649.e10. [Google Scholar] [CrossRef]
- ASGE Bariatric Endoscopy Task Force; Sullivan, A.; Kumar, N.; Edmundowicz, S.A.; Dayyeh, B.K.A.; Jonnalagadda, S.S.; Larsen, M.; Thompson, C.C. ASGE position statement on endoscopic bariatric therapies in clinical practice. Gastrointest. Endosc. 2015, 82, 767–772. [Google Scholar] [CrossRef] [Green Version]
- ASGE EndoVators Task Force; Ryou, M.; McQuaid, K.R.; Thompson, C.C.; Edmundowicz, S.; Mergener, K.; Dayyeh, B.A.; Apovian, C.; Burke, C.; Chand, B.; et al. ASGE EndoVators Summit: Defining the role and value of endoscopic therapies in obesity management. Gastrointest. Endosc. 2017, 86, 757–767. [Google Scholar] [CrossRef]
- Orlandini, B.; Gallo, C.; Boskoski, I.; Bove, V.; Costamagna, G. Procedures and devices for bariatric and metabolic endoscopy. Ther. Adv. Gastrointest. Endosc. 2020, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nava, G.; Galvão, M.P.; Bautista-Castaño, I.; Jimenez, A.; Grado, T.D.; Fernandez-Corbelle, J.P. Endoscopic sleeve gastroplasty for the treat ment of obesity. Endoscopy 2015, 47, 449–452. [Google Scholar] [CrossRef]
- Sharaiha, R.Z.; Kedia, P.; Kumta, N.; DeFilippis, E.M.; Gaidhane, M.; Shukla, A.; Aronne, L.J.; Kahaleh, M. Initial experience with endoscopic sleeve gastroplasty: Technical success and reproducibility in the bariatric population. Endoscopy 2015, 47, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nava, G.; Galvão, M.P.; Bautista-Castaño, I.; Jimenez-Baños, A.; Fernandez-Corbelle, J.P. Endoscopic sleeve gastroplasty: How I do it? Obes. Surg. 2015, 25, 1534–1538. [Google Scholar] [CrossRef]
- Lopez-Nava, G.; Galvao, M.P.; Bautista-Castaño, I.; Fernandez-Corbelle, J.P.; Trell, M. Endoscopic sleeve gastroplasty with 1-year follow-up: Factors predictive of success. Endosc. Int. Open 2016, 04, E222–E227. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Abu Dayyeh, B.K.; Lopez-Nava, G.B.; Neto, M.P.G.; Sahdala, N.P.; Shaikh, S.N.; Hawes, R.H.; Gostout, C.J.; Goenka, M.K.; Orillac, J.R.; et al. Endoscopic sutured gastroplasty: Procedure evolution from first-in-man cases through current technique. Surg. Endosc. 2017, 32, 2159–2164. [Google Scholar] [CrossRef]
- Sharaiha, R.Z.; Kumta, N.A.; Saumoy, M.; Desai, A.P.; Sarkisian, A.M.; Benevenuto, A.; Tyberg, A.; Kumar, R.; Igel, L.; Verna, E.C.; et al. Endoscopic sleeve gastroplasty significantly reduces bodymass index and metabolic complications in obese patients. Clin. Gastroenterol. Hepatol. 2017, 15, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Abu Dayyeh, B.K.; Acosta, A.; Camilleri, M.; Mundi, M.S.; Rajan, E.; Topazian, M.D.; Gostout, C.J. Endoscopic Sleeve Gastroplasty Alters Gastric Physiology and Induces Loss of Body Weight in Obese Individuals. Clin. Gastroenterol. Hepatol. 2017, 15, 37–43.e1. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Nava, G.; Galvão, M.P.; Bautista-Castaño, I.; Fernandez-Corbelle, J.P.; Trell, M.; Lopez, N. Endoscopic sleeve gastroplasty for obesity treatment: Two years of experience. Arq. Bras. Cir. Dig. 2017, 30, 18–20. [Google Scholar] [CrossRef]
- Lopez-Nava, G.; Bautista-Castano, I.; Sharaiha, R.Z.; Bazerbachi, F.; Manoel, G.N.; Bautista-Castaño, I.; Acosta, A.; Topazian, M.D.; Mundi, M.S.; Kumta, N.; et al. Endoscopic sleeve gastroplasty for obesity: A multicenter study of 248 patients with 24 months follow-up. Obes. Surg. 2017, 27, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Sartoretto, A.; Sui, Z.; Hill, C.; Dunlap, M.; Rivera, A.R.; Khashab, M.A.; Kalloo, A.N.; Fayad, L.; Cheskin, L.J.; Marinos, G.; et al. Endoscopic sleeve gastroplasty (ESG) is a reproducible and effective endoscopic bariatric therapy suitable for widespread clinical adoption: A large, international multicenter study. Obes. Surg. 2018, 28, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Graus Morales, J.; Crespo Pérez, L.; Marques, A.; Arribas, B.M.; Arribas, R.B.; Ramo, E.; Escalada, C.; Arribas, C.; Himpens, J. Modified endoscopic gastroplasty for the treatment of obesity. Surg. Endosc. 2018, 32, 3936–3942. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Al-Darwish, A.; Mahmoud, A.E.; Alqahtani, Y.A.; Elahmedi, M. Short-term outcomes of endoscopic sleeve gastroplasty in 1000 consecutive patients. Gastrointest. Endosc. 2019, 89, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Barrichello, S.; Hourneaux de Moura, D.T.; Hourneaux de Moura, E.G.; Jirapinyo, P.; Hoff, A.C.; Fittipaldi-Fernandez, R.J.; Baretta, G.; Lima, J.H.F.; Usuy, E.N.; Salles de Almeida, L.; et al. Endoscopic sleeve gastroplasty in the management of overweight and obesity: An international multicenter study. Gastrointest. Endosc. 2019, 90, 770–780. [Google Scholar] [CrossRef]
- Bhandari, M.; Jain, S.; Mathur, W.; Kosta, S.; Neto, M.G.; Brunaldi, V.O.; Fobi, M. Endoscopic sleeve gastroplasty is an effective and safe minimally invasive approach for treatment of obesity: First Indian experience. Dig. Endosc. 2020, 32, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Sharaiha, R.Z.; Hajifathalian, K.; Kumar, R.; Saunders, K.; Mehta, A.; Ang, B.; Skaf, D.; Shah, S.; Herr, A.; Igel, L.; et al. Five-Year Outcomes of Endoscopic Sleeve Gastroplasty for the Treatment of Obesity. Clin. Gastroenterol. Hepatol. 2020, 19, 1051–1057.e2. [Google Scholar] [CrossRef]
- Hajifathalian, K.; Mehta, A.; Ang, B.; Skaf, D.; Shah, S.L.; Saumoy, M.; Dawod, Q.; Dawod, E.; Shukla, A.; Aronne, L.; et al. Improvement in insulin resistance and estimated hepatic steatosis and fibrosis after endoscopic sleeve gastroplasty. Gastrointest. Endosc. 2021, 93, 1110–1118. [Google Scholar] [CrossRef]
- Huberty, V.; Ibrahim, M.; Hiernaux, M.; Chau, A.; Dugardeyn, S.; Devière, J. Safety and feasibility of an endoluminal-suturing device for endoscopic gastric reduction (with video). Gastrointest. Endosc. 2017, 85, 833–837. [Google Scholar] [CrossRef]
- Huberty, V.; Machytka, E.; Boškoski, I.; Barea, M.; Costamagna, G.; Deviere, J. Endoscopic gastric reduction with an endoluminal suturing device: A multicenter prospective trial with 1-year follow-up. Endoscopy 2018, 50, 1156–1162. [Google Scholar] [CrossRef]
- Huberty, V.; Boskoski, I.; Bove, V.; Ouytsel, P.V.; Costamagna, G.; Barthet, M.A.; Devière, J. Endoscopic sutured gastroplasty in addition to lifestyle modification: Short-term efficacy in a controlled randomised trial. Gut 2020, 70, 1479–1485. [Google Scholar] [CrossRef]
- Lopez-Nava, G.; Asokkumar, R.; Turró Arau, R.; Neto, M.G.; Dayyeh, B.A. Modified primary obesity surgery endoluminal (POSE-2) procedure for the treatment of obesity. VideoGIE 2020, 5, 91–93. [Google Scholar] [CrossRef] [Green Version]
- Jirapinyo, P.; Thompson, C.C. Endoscopic gastric body plication for the treatment of obesity: Technicall success and safety of a novel technique (with video). Gastrointest. Endosc. 2020, 91, 1388–1394. [Google Scholar] [CrossRef]
- Lopez-Nava, G.; Asokkumar, R.; Rull, A.; Bautista, I.; Dayyeh, B.A. Dayyeh Safety and Feasibility of a Novel Endoscopic Suturing Device (EndoZip TM) for Treatment of Obesity: First-in-Human Study. Obes. Surg. 2020, 30, 1696–1703. [Google Scholar] [CrossRef]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H.; Obesity Management Task Force of the European Association for the Study of Obesity. European guidelines for obesity management in adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Neto, M.G.; Silva, L.B.; de Quadros, L.G.; Grecco, E.; Filho, A.C.; Barbosa de Amorim, A.M.; Falcao de Santana, M.; Santos, N.T.D.; Felicio de Lima, J.H.; Ferreira de Souza, T.; et al. Brazilian Consensus on Endoscopic Sleeve Gastroplasty. Obes. Surg. 2021, 31, 70–78. [Google Scholar] [CrossRef]
- Sauerland, S.; Angrisani, L.; Belachew, M.; Chevallier, J.M.; Favretti, F.; Finer, N.; Fingerhut, A.; Caballero, M.G.; Macias, J.A.G.; Mittermair, R.; et al. Obesity surgery: Evidence-based guidelines of the European Association for Endoscopic Surgery (EAES). Surg. Endosc. 2005, 19, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Due-Petersson, R.; Poulsen, I.M.; Hedbäck, N.; Karstensen, J.G. Effect and safety of endoscopic sleeve gastroplasty for treating obesity—A systematic review. Dan. Med. J. 2020, 67, A05200359. [Google Scholar] [PubMed]
- Novikov, A.A.; Afaneh, C.; Saumoy, M.; Parra, V.; Shukla, A.; Dakin, G.F.; Pomp, A.; Dawod, E.; Shah, S.; Aronne, L.J.; et al. Endoscopic sleeve gastroplasty, laparoscopic sleeve gastrectomy, and laparoscopic band for weight loss: How do they compare? J. Gastrointest. Surg. 2018, 22, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nava, G.; Asokkumar, R.; Bautista-Castaño, I.; Laster, J.; Negi, A.; Fook-Chong, S.; Nebreda Duran, J.; Espinett Coll, E.; Gebelli, J.P.; de Gordejuela, A.G.R. Endoscopic sleeve gastroplasty, laparoscopic sleeve gastrectomy, and laparoscopic greater curve plication: Do they differ at 2 years? Endoscopy 2021, 53, 235–243. [Google Scholar] [CrossRef]
- Marincola, G.; Gallo, C.; Hassan, C.; Raffaelli, M.; Costamagna, G.; Bove, V.; Pontecorvi, V.; Orlandini, B.; Boškoski, I. Laparoscopic sleeve gastrectomy versus endoscopic sleeve gastroplasty: A systematic review and meta-analysis. Endosc. Int. Open 2021, 9, E87–E95. [Google Scholar] [CrossRef]
- Storm, A.C.; Abu Dayyeh, B.K. Endoscopic sleeve gastroplasty for obesity: Defining the risk and reward after more than 1600 procedures. Gastrointest. Endosc. 2019, 89, 1139–1140. [Google Scholar] [CrossRef] [Green Version]
- Karmali, A.; Brar, B.; Shi, X.; Sharma, A.M.; de Gara, C.; Birch, D.W. Weight Recidivism Post-Bariatric Surgery: A Systematic Review. Obes. Surg. 2013, 23, 1922–1933. [Google Scholar] [CrossRef]
- Cooper, T.C.; Simmons, E.B.; Webb, K.; Burns, J.L.; Kushner, R.F. Trends in weight regain following Roux-en-Y gastric bypass (RYGB) bariatric surgery. Obes. Surg. 2015, 25, 1474–1481. [Google Scholar] [CrossRef]
- Jirapinyo, P.; de Moura, D.T.H.; Thompson, C.C. Sleeve in sleeve: Endoscopic revision for weight regain after sleeve gastrectomy. Video GIE 2019, 4, 454–457. [Google Scholar] [CrossRef] [Green Version]
- Westerveld, D.; Yang, D. Through Thick and Thin: Identifying Barriers to Bariatric Surgery, Weight Loss Maintenance, and Tailoring Obesity Treatment for the Future. Surg. Res. Pract. 2016, 2016, 8616581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cambi, M.P.C.; Baretta, G.A.P.; De Oliveira Magro, D.; Boguszewski, C.L.; Ribeiro, I.B.; Jirapinyo, P.; de Moura, D.T.H. Multidisciplinary Approach for Weight Regain—how to Manage this Challenging Condition: An Expert Review. Obes. Surg. 2021, 31, 1290–1303. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Bray, G.A.; Carey, V.J.; Smith, S.R.; Ryan, D.H.; Anton, S.D.; McManus, K.; Champagne, C.M.; Bishop, L.M.; Laranjo, N.; et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 2009, 360, 859–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorron, R.; Veltzke-Schlieker, W.; Adler, A.; Denecke, C.; Dziodzio, T.; Pratschke, J.; Benzing, C. Endoscopic sleeve gastroplasty using Apollo Overstitch as a bridging procedure for superobese and high risk patients. Endoscopy 2018, 50, 81–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Veltzke-Schlieker, W.; Adler, A.; Specht, M.; Eskander, W.; Ismail, M.; Badakhshi, H.; Galvao, M.P.; Zorron, R. Endoscopic Sleeve Gastroplasty (ESG) for High-Risk Patients, High Body Mass Index (>50 kg/m2), and Contraindication to Abdominal Surgery. Obes. Surg. 2021, 31, 3400–3409. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines: Liver transplantation. J. Hepatol. 2016, 64, 433–485. [Google Scholar] [CrossRef]
- Chadban, S.J.; Ahn, C.; Axelrod, D.A.; Foster, B.J.; Kasiske, B.L.; Kher, V.; Kumar, D.; Oberbauer, R.; Pascual, J.; Pilmore, H.L.; et al. KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplant. 2020, 104, S11–S103. [Google Scholar] [CrossRef]
- Lee, Y.; Raveendran, L.; Lovrics, O.; Tian, C.; Khondker, A.; Koyle, M.A.; Farcas, M.; Doumouras, A.G.; Hong, D. The role of bariatric surgery on kidney transplantation: A systematic review and meta-analysis. Can. Urol. Assoc. J. 2021, 15, E553–E562. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Valdes, D.; Watt, K.D.; Kellogg, T.A.; Poterucha, J.J.; Cecco, S.R.D.; Francisco-Ziller, N.M.; Taner, T.; Rosen, C.B.; Heimbach, J.K. Long-term outcomes of patients undergoing simultaneous liver transplantation and sleeve gastrectomy. Hepatology 2018, 68, 485. [Google Scholar] [CrossRef] [Green Version]
- Boškoski, I.; Pontecorvi, V.; Gallo, C.; Bove, V.; Laterza, L.; Costamagna, G. Redo endoscopic sleeve gastroplasty: Technical aspects and short-term outcomes. Ther. Adv. Gastroenterol. 2020, 13, 1756284819896179. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nava, G.; Asokkumar, R.; Rull, A.; Corbelle, F.; Beltran, L.; Bautista, I. Bariatric endoscopy procedure type or follow-up: What predicted success at 1 year in 962 obese patients? Endosc. Int. Open 2019, 7, E1691–E1698. [Google Scholar] [CrossRef] [Green Version]
- Murphy, R.; Tsai, P.; Jüllig, M.; Liu, A.; Plank, L.; Booth, M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes. Surg. 2017, 27, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Belda, E.; Prifti, E.; Everard, A.; Kayser, B.D.; Bouillot, J.-L.; Chevallier, J.-M.; Pons, N.; Chatelier, E.L.; Ehrlich, S.D.; et al. Akkermansia muciniphila abundance is lower in severe obesity, but its increased level after bariatric surgery is not associated with metabolic health improvement. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E446–E459. [Google Scholar] [CrossRef]
- De Jonge, C.; Fuentes, S.; Zoetendal, E.G.; Bouvy, N.D.; Nelissen, R.; Buurman, W.A.; Greve, J.W.; Vos, W.M.de.; Rensen, S.S. Metabolic improvement in obese patients after duodenal-jejunal exclusion is associated with intestinal microbiota composition changes. Int. J. Obes. 2019, 43, 2509–2517. [Google Scholar] [CrossRef]
- Wang, F.G.; Bai, R.X.; Yan, W.M.; Yan, M.; Dong, L.Y.; Song, M.M. Differential composition of gut microbiota among healthy volunteers, morbidly obese patients and post-bariatric surgery patients. Exp. Ther. Med. 2019, 17, 2268–2278. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Repiso, C.; Moreno-Indias, I.; de Hollanda, A.; Martín-Núñez, G.M.; Vidal, J.; Tinahones, F.J. Gut microbiota specific signatures are related to the successful rate of bariatric surgery. Am. J. Transl. Res. 2019, 11, 942–952. [Google Scholar]
| Search engine | PubMed |
| Date | 28/10/2021 |
| Query | “Endoscopic sleeve gastroplasty” OR “endoscopic gastric reduction” or “endoscopic gastric plication” or “endosleeve” or “apollo overstitch” or “endomina” or “primary obesity surgery” |
| Field | Title/Abstract |
| Text availability | Full text |
| Publication date | 10 years (2011–2021) |
| Language | English |
| Article type | Original articles; clinical studies; randomized controlled-trial. |
| Total results | 209 |
| Exclusion criteria |
|
| Article selected | 21 |
| Study | Stapling Device | N. of Patients | Post-Procedural Ancillary Program | TBWL 6 Months (%) | EWL 6 Months (%) | TBWL 12 Months (%) | EWL 12 Months (%) | TBWL 24 Months (%) | EWL 24 Months (%) | TBWL 5 Years (%) | EWL 5 Years (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lopez-Nava et al. [13] | Apollo Overstitch | 20 |
| 17.8 ± 7.5 | 53.9 ± 26.3 | NA | NA | NA | NA | NA | NA |
| Sharaiha et al. [14] | Apollo Overstitch | 10 | NA | NA | 30 | NA | NA | NA | NA | NA | NA |
| Lopez-Nava et al. [15] | Apollo Overstitch | 50 |
| 17.2 ± 7.5 | 53.5 ± 26.2 | 19.0 ± 10.8 | 57.0 ± 33.9 | NA | NA | NA | NA |
| Lopez-Nava et al. [16] | Apollo Overstitch | 25 |
| 17.8 ± 7.5 | 53.9 ± 24.8 | 18.7 ± 10.7 | 54.6 ± 31.9 | NA | NA | NA | NA |
| Kumar et al. [17] | Apollo Overstitch | 77 | NA | 16.2 ± 0.7 | NA | 17.4 ± 1.1 | NA | NA | NA | NA | NA |
| Sharaiha et al. [18] | Apollo Overstitch | 91 |
| 14.4 | NA | 17.6 | NA | 20.9 | NA | NA | NA |
| Abu Dayyeh et al. [19] | Apollo Overstitch | 25 | NA | NA | 53 ± 17 | NA | 54 ± 40 | NA | 45 ± 41 † | NA | NA |
| Lopez-Nava et al. [20] | Apollo Overstitch | 154 |
| 15.8 ± 7.1 | 47.8 ± 29.4 | 18.2 ± 10.1 | 52.6 ± 31.3 | 19.5 ± 10.5 | 60.4 ± 31.1 | NA | NA |
| Lopez-Nava et al. [21] | Apollo Overstitch | 248 | NA | 15.2 | NA | NA | NA | 18.6 | NA | NA | NA |
| Sartoretto et al. [22] | Apollo Overstitch | 112 |
| 14.9 ± 6.1 | 50.3 ± 22.4 | NA | NA | NA | NA | NA | NA |
| Graus-Morales et al. [23] | Apollo Overstitch | 148 |
| 15.1 ± 4.9 | 66.0 ± 39 | 18.2 ± 6.8 | 77.6 ± 42 | 17.5 ± 7.6 ‡ | 75.4 ± 85 ‡ | NA | NA |
| Alqahtani et al. [24] | Apollo Overstitch | 1000 |
| 13.7 ± 6.8 | 64.3 ± 56.2 | 15.0 ± 7.7 | 67.5 ± 52.3 | 14.8 ± 8.5 ‡ | 64.7 ± 55.4 ‡ | NA | NA |
| Barrichello et al. [25] | Apollo Overstitch | 193 |
| 14.2 ± 5.3 | 56.2 ± 22.9 | 15.1 ± 5.2 | 59.4 ± 25.7 | NA | NA | NA | NA |
| Bhandari et al. [26] | Apollo Overstitch | 53 |
| 14.3 ± 6.2 | NA | 19.9 ± 4.9 | NA | NA | NA | NA | NA |
| Sharaiha et al. [27] | Apollo Overstitch | 216 |
| NA | NA | 15.6 (95% CI, 14.1–17.1) | 47.9 (95% CI, 42.4–53.3) | 14.9 (95% CI, 12.1–17.7) ** | 45.1 (95% CI, 34.9–55.2) ** | 15.9 95% CI, 11.7–20.5) | 45.3 (95% CI, 32.9–57.7) |
| Hajifathalian et al. [28] | Apollo Overstitch | 118 |
| 14.6 (13.3–15.9) | 45.3 (39.9–50.7) | 15.6 (13.9–17.4) | 47.8 (41.4–54.2) | 15.5 (13.3–17.8) | 45.5 (38.1–52.8) | NA | NA |
| Huberty et al. [29] | Endomina | 11 |
| NA | 41 | NA | NA | NA | NA | NA | NA |
| Huberty et al. [30] | Endomina | 51 |
| 8.0 (SD 5.0) | 31.0 (SD 20.0) | 7.0 (SD 7.0) | 29.0 (SD 28.0) | NA | NA | NA | NA |
| Huberty et al. [31] | Endomina | 71 |
| 11.0 (95% CI: 8.9–13.2) | 38.6 (95% CI, 31.1–46.0) | 11.9 (95% IC, 9.3–14.5) | 42.7 (95% CI, 33.1–52.3) | NA | NA | NA | NA |
| Lopez-Nava et al. [32] | POSE 2.0 | 73 | NA | 15.7 | NA | NA | NA | NA | NA | NA | NA |
| Jirapinyo et al. [33] | POSE 2.0 | 10 |
| 15.0 ± 7.1 | 37.9 ± 20.0 | NA | NA | NA | NA | NA | NA |
| Lopez-Nava et al. [34] | EndoZip | 11 |
| 16.2 ± 6.0 | 46.5 ± 28.6 | NA | NA | NA | NA | NA | NA |
| Weight Loss Outcomes (Mean Value) | TBWL at 6 Months (%) | EWL at 6 Months (%) | TBWL at 12 Months (%) | EWL at 12 Months (%) | TBWL at 18–24 Months (%) | EWL at 18–24 Months (%) |
|---|---|---|---|---|---|---|
| All studies | 15.7 | 54.4 | 17.3 | 57.6 | 17.4 | 58.2 |
| Studies with only nutritional or non-specified follow-up | 15.1 | 51.7 | 16.6 | 59.0 | 17.0 | 57.6 |
| Studies with multidisciplinary follow-up | 16.0 | 52.6 | 18.2 | 55.9 | 19.5 | 60.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matteo, M.V.; D’Oria, M.; Bove, V.; Carlino, G.; Pontecorvi, V.; Raffaelli, M.; Chieffo, D.; Cesario, A.; Scambia, G.; Costamagna, G.; et al. Personalized Approach for Obese Patients Undergoing Endoscopic Sleeve Gastroplasty. J. Pers. Med. 2021, 11, 1298. https://doi.org/10.3390/jpm11121298
Matteo MV, D’Oria M, Bove V, Carlino G, Pontecorvi V, Raffaelli M, Chieffo D, Cesario A, Scambia G, Costamagna G, et al. Personalized Approach for Obese Patients Undergoing Endoscopic Sleeve Gastroplasty. Journal of Personalized Medicine. 2021; 11(12):1298. https://doi.org/10.3390/jpm11121298
Chicago/Turabian StyleMatteo, Maria Valeria, Marika D’Oria, Vincenzo Bove, Giorgio Carlino, Valerio Pontecorvi, Marco Raffaelli, Daniela Chieffo, Alfredo Cesario, Giovanni Scambia, Guido Costamagna, and et al. 2021. "Personalized Approach for Obese Patients Undergoing Endoscopic Sleeve Gastroplasty" Journal of Personalized Medicine 11, no. 12: 1298. https://doi.org/10.3390/jpm11121298
APA StyleMatteo, M. V., D’Oria, M., Bove, V., Carlino, G., Pontecorvi, V., Raffaelli, M., Chieffo, D., Cesario, A., Scambia, G., Costamagna, G., & Boškoski, I. (2021). Personalized Approach for Obese Patients Undergoing Endoscopic Sleeve Gastroplasty. Journal of Personalized Medicine, 11(12), 1298. https://doi.org/10.3390/jpm11121298












