Machine Learning Approach Using Routine Immediate Postoperative Laboratory Values for Predicting Postoperative Mortality
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data
2.2. Use of the SASA Scoring System
2.3. Machine Learning-Based Model Development
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Cohorts
3.2. Profile of Routine Immediate Postoperative Laboratory Values
3.3. ML Approach for Predicting Postoperative Mortality
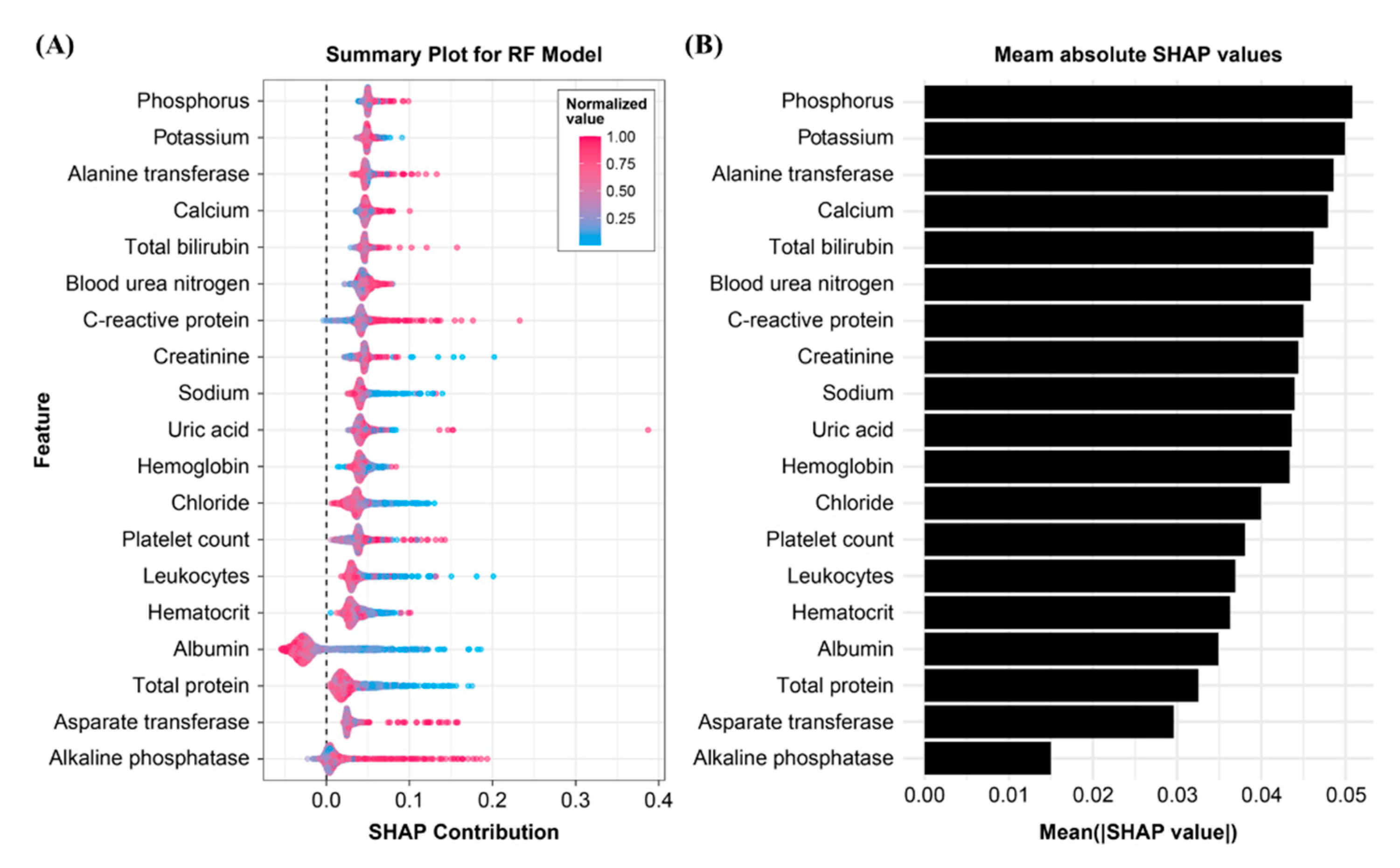
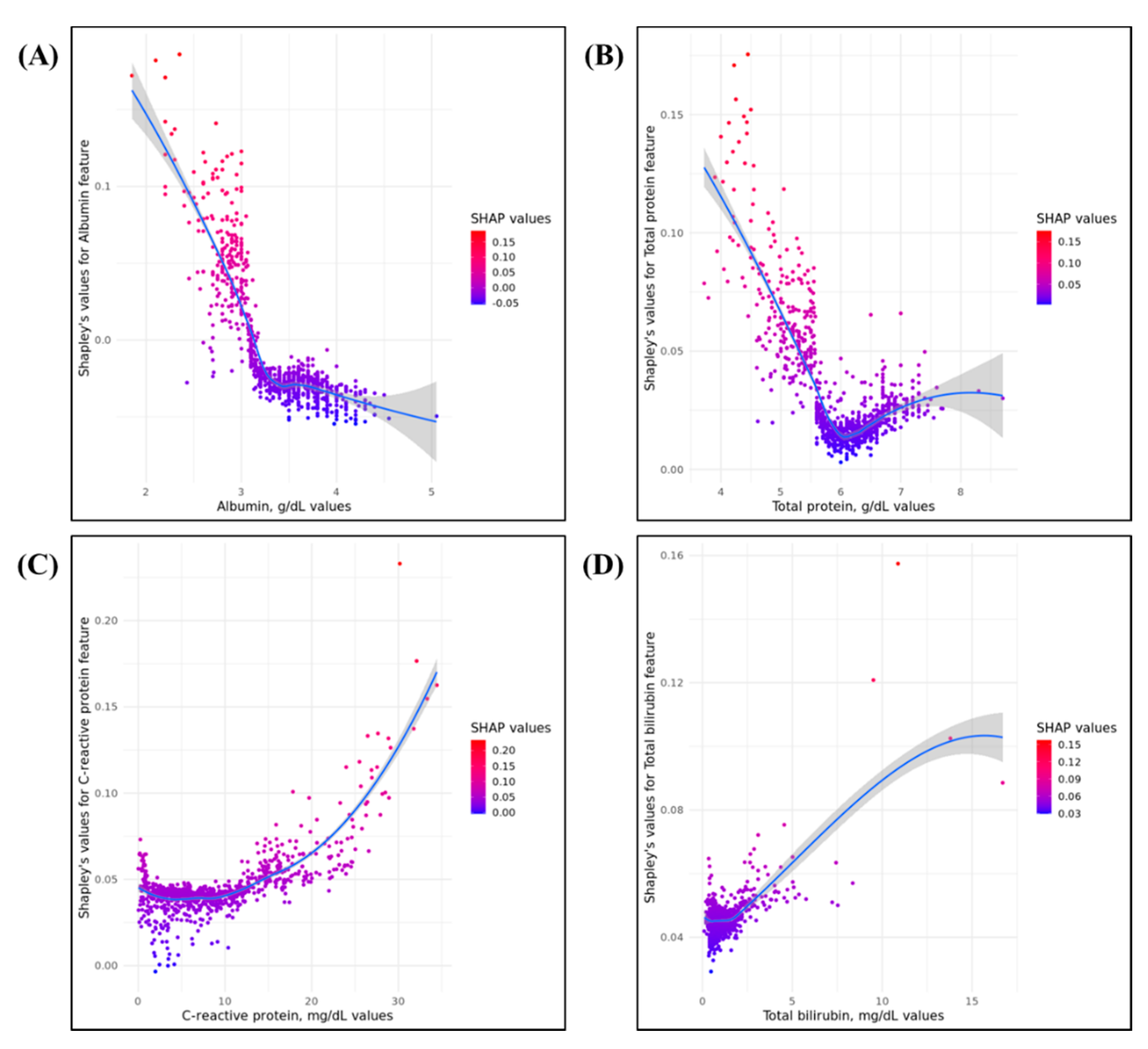
3.4. Importance of Model Feature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohiuddin, K.; Swanson, S.J. Maximizing the benefit of minimally invasive surgery. J. Surg. Oncol. 2013, 108, 315–319. [Google Scholar] [CrossRef]
- Weiser, T.G.; Regenbogen, S.E.; Thompson, K.D.; Haynes, A.B.; Lipsitz, S.R.; Berry, W.R.; Gawande, A.A. An estimation of the global volume of surgery: A modelling strategy based on available data. Lancet 2008, 372, 139–144. [Google Scholar] [CrossRef]
- Ozgediz, D.; Jamison, D.; Cherian, M.; McQueen, K. The burden of surgical conditions and access to surgical care in low- and middleincome countries. Bull. World Health Organ. 2008, 86, 646–647. [Google Scholar] [PubMed]
- Healy, M.A.; Mullard, A.J.; Campbell, D.A., Jr.; Dimick, J.B. Hospital and payer costs associated with surgical complications. JAMA Surg. 2016, 151, 823–830. [Google Scholar] [CrossRef]
- Wang, H.; Chen, T.; Wang, H.; Song, Y.; Li, X.; Wang, J. A systematic review of the Physiological 14 and Operative Severity Score for the enUmeration of Mortality and morbidity and its Portsmouth modification as predictors of post-operative morbidity and mortality in patients undergoing pancreatic surgery. Am. J. Surg. 2013, 205, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, L.A.; Fleischmann, K.E.; Auerbach, A.D.; Barnason, S.A.; Beckman, J.A.; Bozkurt, B.; Davila-Roman, V.G.; Gerhard-Herman, M.D.; Holly, T.A.; Kane, G.C.; et al. 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e77–e137. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Liu, Y.; Paruch, J.L.; Zhou, L.; Kmiecik, T.E.; Ko, C.Y.; Cohen, M.E. Development and evaluation of the universal ACS NSQIP surgical risk calculator:A decision aid and informed consent tool for patients and surgeons. J. Am. Coll. Surg. 2013, 217, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Mascha, E.J.; Yang, D.; Weiss, S.; Sessler, D.I. Intraoperative Mean Arterial Pressure Variability and 30-day Mortality in Patients Having Noncardiac Surgery. Anesthesiology 2015, 123, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Studer, P.; Raber, G.; Ott, D.; Candinas, D.; Schnuriger, B. Risk factors for fatal outcome in surgical patients with postoperative aspiration pneumonia. Int. J. Surg. 2016, 27, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Chiew, C.J.; Liu, N.; Wong, T.H.; Sim, Y.E.; Abdullah, H.R. Utilizing Machine Learning Methods for Preoperative Prediction of Postsurgical Mortality and Intensive Care Unit Admission. Ann. Surg. 2020, 272, 1133–1139. [Google Scholar] [CrossRef]
- Lee, H.C.; Jung, C.W. Vital Recorder-a free research tool for automatic recording of high-resolution time-synchronised physiological data from multiple anaesthesia devices. Sci. Rep. 2018, 8, 1527. [Google Scholar] [CrossRef]
- Sterne, J.A.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef] [PubMed]
- Gawande, A.A.; Kwaan, M.R.; Regenbogen, S.E.; Lipsitz, S.A.; Zinner, M.J. An Apgar score for surgery. J. Am. Coll. Surg. 2007, 204, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, D.; Mendonca, V.; Murthy, B.V.S. A review of ASA physical status—historical perspectives and modern developments. Anaesthesia 2019, 74, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Morioka, N.; Yabuuchi, M.; Ozaki, M. New surgical scoring system to predict postoperative mortality. J. Anesth. 2017, 312, 198–205. [Google Scholar] [CrossRef][Green Version]
- Lundberg, S.M.; Lee, S.I. A unified approach to interpreting model predictions. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017. [Google Scholar]
- Semel, M.E.; Lipsitz, S.R.; Funk, L.M.; Bader, A.M.; Weiser, T.G.; Gawande, A.A. Rates and patterns of death after surgery in the United States, 1996 and 2006. Surgery 2012, 151, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.L.; Riall, T.S.; Coleman, J.; Belcher, K.A. One thousand consecutive pancreaticoduodenectomies. Ann. Surg. 2006, 244, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Low, D.E.; Kunz, S.; Schembre, D.; Otero, H.; Malpass, T.; Hsi, A.; Song, G.; Hinke, R.; Kozarek, R.A. Esophagectomy—It’s not just about mortality anymore: Standardized perioperative clinical pathways improve outcomes in patients with esophageal cancer. J. Gastrointest. Surg. 2007, 11, 1395–1402. [Google Scholar] [CrossRef]
- Hackett, N.J.; De Oliveira, G.S.; Jain, U.K.; Kim, J.Y. ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int. J. Surg. 2015, 18, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.Q.; Sanders, N.W.; Schildcrout, J.S.; Mercaldo, N.D.; St. Jacques, P.J. Expansion of the surgical Apgar score across all surgical subspecialties as a means to predict postoperative mortality. Anesthesiology 2011, 114, 1305–1312. [Google Scholar] [CrossRef]
- Haynes, A.B.; Regenbogen, S.E.; Weiser, T.G.; Lipsitz, S.R.; Dziekan, G.; Berry, W.R.; Gawande, A.A. Surgical outcome measurement for a global patient population: Validation of the Surgical Apgar Score in 8 countries. Surgery 2011, 149, 519–524. [Google Scholar] [CrossRef]
- Da, J.; Xie, X.; Wolf, M.; Disthabanchong, S.; Wang, J.; Zha, Y.; Lv, J.; Zhang, L.; Wang, H. Serum Phosphorus and Progression of CKD and Mortality: A Meta-analysis of Cohort Studies. Am. J. Kidney Dis. 2015, 66, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Kim, Y.C.; Park, S.; Kim, C.T.; Ha, J.; Han, D.J.; Oh, J.; Lim, C.S.; Jung, I.M.; Ahn, C.; et al. Association of Serum Phosphorus Concentration with Mortality and Graft Failure among Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2017, 12, 653–662. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, C.; Chen, L.; Zhang, X.; Kou, Q. Impact of hypophosphatemia on outcome of patients in intensive care unit: A retrospective cohort study. BMC Anesthesiol. 2019, 19, 86. [Google Scholar] [CrossRef]
- Burra, V.; Nagaraja, P.S.; Singh, N.G.; Prabhakar, V.; Manjunatha, N. Early Prediction of Acute Kidney Injury using Serum Phosphorus as a Biomarker in Pediatric Cardiac Surgical Patients. Ann. Card. Anaesth. 2018, 21, 455–459. [Google Scholar] [PubMed]
- Rudasill, S.E.; Morales, R.R.; Sanaiha, Y.; Sareh, S.; Antonios, J.W.; Khoury, H.; Mardock, A.L.; Benharash, P. Predicting morbidity and mortality in laparoscopic cholecystectomy: Preoperative serum albumin still matters. Am. J. Surg. 2020, 220, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.; Cull, W.; Henderson, W.; Daley, J.; Hur, K.; Khuri, S.F. Preoperative serum albumin level as a predictor of operative mortality and morbidity: Results from the National VA Surgical Risk Study. Arch. Surg. 1999, 134, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Labgaa, I.; Joliat, G.; Kefleyesus, A.; Mantziari, S.; Schäfer, M.; Demartines, N.; Hübner, M. Is postoperative decrease of serum albumin an early predictor of complications after major abdominal surgery? A prospective cohort study in a European centre. BMJ Open 2017, 7, e013966. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, U.; Morelli, D.; Leuzzi, G.; Gisabella, M.; Suatoni, P.; Taverna, F.; Bertocchi, E.; Boeri, M.; Sozzi, G.; Cantarutti, A.; et al. Baseline and postoperative C-reactive protein levels predict mortality in operable lung cancer. Eur. J. Cancer 2017, 79, 90–97. [Google Scholar] [CrossRef] [PubMed]
| Postoperative Mortality | p Value | ||
|---|---|---|---|
| No (n = 5538) | Yes (n = 402) | ||
| Mean age, year | 58.0 ± 14.1 | 64.1 ± 13.4 | <0.001 |
| Gender, no. (%) | <0.001 | ||
| Female | 2808 (50.7%) | 139 (34.6%) | |
| Male | 2730 (49.3%) | 263 (65.4%) | |
| Mean body mass index, kg/m2 | 23.5 ± 3.5 | 21.8 ± 3.7 | <0.001 |
| ASA physical status classification | <0.001 | ||
| 1 | 1622 (29.3%) | 33 (8.2%) | |
| 2 | 3364 (60.7%) | 230 (57.2%) | |
| 3 | 434 (7.8%) | 118 (29.4%) | |
| 4 | 15 (0.3%) | 12 (3.0%) | |
| N/A | 103 (1.9%) | 9 (2.2%) | |
| Type of Surgery, no. (%) | <0.001 | ||
| Elective | 4977 (89.9%) | 300 (74.6%) | |
| Emergency | 561 (10.1%) | 102 (25.4%) | |
| Department | |||
| General surgery | 4189 (75.6%) | 320 (79.6%) | |
| Gynecology | 223 (4.0%) | 5 (1.2%) | |
| Thoracic surgery | 1011 (18.3%) | 76 (18.9%) | |
| Urology | 115 (2.1%) | 1 (0.2%) | |
| Type of Anesthesia, no. (%) | |||
| General | 5220 (94.3%) | 379 (94.3%) | |
| Sedation/Analgesia | 52 (0.9%) | 19 (4.7%) | |
| Spinal | 266 (4.8%) | 4 (1.0%) | |
| Duration of operation, min. | 126.1 ± 93.2 | 145.5 ± 106.8 | <0.001 |
| Duration of anesthesia, min. | 166.0 ± 101.4 | 187.0 ± 114.0 | <0.001 |
| Intraoperative monitoring | |||
| Minimal heart rate, beats per min. | 46.7 ± 18.7 | 49.1 ± 24.5 | 0.142 |
| Minimal mean BP, mmHg | 64.7 ± 13.4 | 63.4 ± 14.6 | 0.137 |
| Estimated blood loss, mL | 279.6 ± 674.9 | 686.3 ± 2160.7 | 0.004 |
| SASA score | 16.0 ± 2.3 | 14.1 ± 2.6 | <0.001 |
| VitalDB Cohort | AUSOM Cohort | |||||
|---|---|---|---|---|---|---|
| Postoperative Mortality | p Value | Postoperative Mortality | p Value | |||
| No (n = 3523) | Yes (n = 294) | No (n = 20,954) | Yes (n = 686) | |||
| White blood cell count, ×1000/mcL | 10.2 ± 3.4 | 10.2 ± 4.3 | 0.997 | 10.4 ± 3.8 | 11.9 ± 5.6 | <0.001 |
| Hemoglobin, g/dL | 11.6 ± 1.8 | 10.5 ± 1.7 | <0.001 | 11.5 ± 1.8 | 10.4 ± 1.6 | <0.001 |
| Hematocrit, % | 35.5 ± 5.2 | 31.8 ± 5.1 | <0.001 | 34.2 ± 5.2 | 30.8 ± 4.7 | <0.001 |
| Platelet count, ×1000/mcL | 202.7 ± 74.4 | 210.3 ± 112.2 | 0.255 | 208.4 ± 87.8 | 155.2 ± 90.4 | <0.001 |
| Blood urea nitrogen, mg/dL | 13.5 ± 8.4 | 16.3 ± 10.8 | <0.001 | 12.5 ± 7.2 | 20.5 ± 14.1 | <0.001 |
| Creatinine, mg/dL | 0.9 ± 1.0 | 1.0 ± 0.9 | 0.434 | 0.9 ± 0.8 | 1.3 ± 1.3 | <0.001 |
| Sodium, mmol/L | 138.1 ± 2.6 | 137.1 ± 3.5 | <0.001 | 138.9 ± 2.9 | 141.4 ± 6.4 | <0.001 |
| Potassium, mmol/L | 4.0 ± 0.4 | 4.1 ± 0.4 | 0.602 | 4.0 ± 0.4 | 3.8 ± 0.5 | <0.001 |
| Chloride, mmol/L | 102.7 ± 3.0 | 102.2 ± 3.9 | 0.014 | 103.3 ± 3.7 | 106.1 ± 6.7 | <0.001 |
| Calcium, mg/dL | 8.4 ± 0.5 | 8.2 ± 0.5 | <0.001 | 8.3 ± 0.7 | 7.8 ± 0.7 | <0.001 |
| Phosphorus, mg/dL | 2.9 ± 0.8 | 3.0 ± 0.8 | 0.289 | 3.2 ± 0.8 | 3.2 ± 1.1 | 0.781 |
| Uric acid, mg/dL | 3.4 ± 1.5 | 3.4 ± 1.6 | 0.717 | 3.7 ± 1.5 | 3.9 ± 2.1 | 0.004 |
| Total bilirubin, mg/dL | 1.1 ± 1.1 | 1.6 ± 3.0 | 0.007 | 0.9 ± 1.1 | 1.9 ± 3.2 | <0.001 |
| Asparate transferase, IU/L | 52.8 ± 204.4 | 129.8 ± 542.6 | 0.016 | 53.2 ± 134.0 | 161.0 ± 460.5 | <0.001 |
| Alanine transferase, IU/L | 51.5 ± 166.2 | 92.0 ± 264.8 | 0.01 | 43.1 ± 90.9 | 85.2 ± 219.8 | <0.001 |
| Alkaline phosphatase, IU/L | 61.0 ± 29.0 | 82.1 ± 56.9 | <0.001 | 84.0 ± 101.2 | 93.9 ± 95.9 | 0.012 |
| Albumin, g/dL | 3.4 ± 0.4 | 3.0 ± 0.4 | <0.001 | 3.5 ± 0.5 | 3.0 ± 0.5 | <0.001 |
| Total protein, g/dL | 6.0 ± 0.7 | 5.5 ± 0.7 | <0.001 | 5.8 ± 0.8 | 5.1 ± 0.9 | <0.001 |
| C-reactive protein, mg/dL | 8.3 ± 6.1 | 11.9 ± 7.6 | <0.001 | 5.9 ± 6.2 | 12.6 ± 9.7 | <0.001 |
| Candidate Models | |||||
|---|---|---|---|---|---|
| SASA | LASSO | DNN | RF | XGB | |
| AUROC | 0.73 | 0.73 | 0.84 | 0.74 | 0.82 |
| AUPRC | 0.06 | 0.26 | 0.35 | 0.24 | 0.30 |
| Machine Learning Models | |||||
|---|---|---|---|---|---|
| LASSO | DNN | RF | XGB | ||
| AUROC | Train | 0.81 | 0.82 | 0.77 | 0.90 |
| Test | 0.77 | 0.79 | 0.75 | 0.80 | |
| External validation * | 0.70 | 0.72 | 0.82 | 0.75 | |
| AUPRC | Train | 0.35 | 0.31 | 0.31 | 0.53 |
| Test | 0.26 | 0.27 | 0.29 | 0.30 | |
| External validation * | 0.09 | 0.08 | 0.13 | 0.09 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.; Park, J.; Jeong, E.; Shin, J.; Ahn, S.; Park, M.G.; Park, R.W.; Park, Y. Machine Learning Approach Using Routine Immediate Postoperative Laboratory Values for Predicting Postoperative Mortality. J. Pers. Med. 2021, 11, 1271. https://doi.org/10.3390/jpm11121271
Cho J, Park J, Jeong E, Shin J, Ahn S, Park MG, Park RW, Park Y. Machine Learning Approach Using Routine Immediate Postoperative Laboratory Values for Predicting Postoperative Mortality. Journal of Personalized Medicine. 2021; 11(12):1271. https://doi.org/10.3390/jpm11121271
Chicago/Turabian StyleCho, Jaehyeong, Jimyung Park, Eugene Jeong, Jihye Shin, Sangjeong Ahn, Min Geun Park, Rae Woong Park, and Yongkeun Park. 2021. "Machine Learning Approach Using Routine Immediate Postoperative Laboratory Values for Predicting Postoperative Mortality" Journal of Personalized Medicine 11, no. 12: 1271. https://doi.org/10.3390/jpm11121271
APA StyleCho, J., Park, J., Jeong, E., Shin, J., Ahn, S., Park, M. G., Park, R. W., & Park, Y. (2021). Machine Learning Approach Using Routine Immediate Postoperative Laboratory Values for Predicting Postoperative Mortality. Journal of Personalized Medicine, 11(12), 1271. https://doi.org/10.3390/jpm11121271







