Benchmarking Outcomes after Ablative Radiotherapy for Molecularly Characterized Intrahepatic Cholangiocarcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection, Workup, and Treatment
2.2. Data Collection
2.3. Mutational Profiling
2.4. Statistical Analysis
3. Results
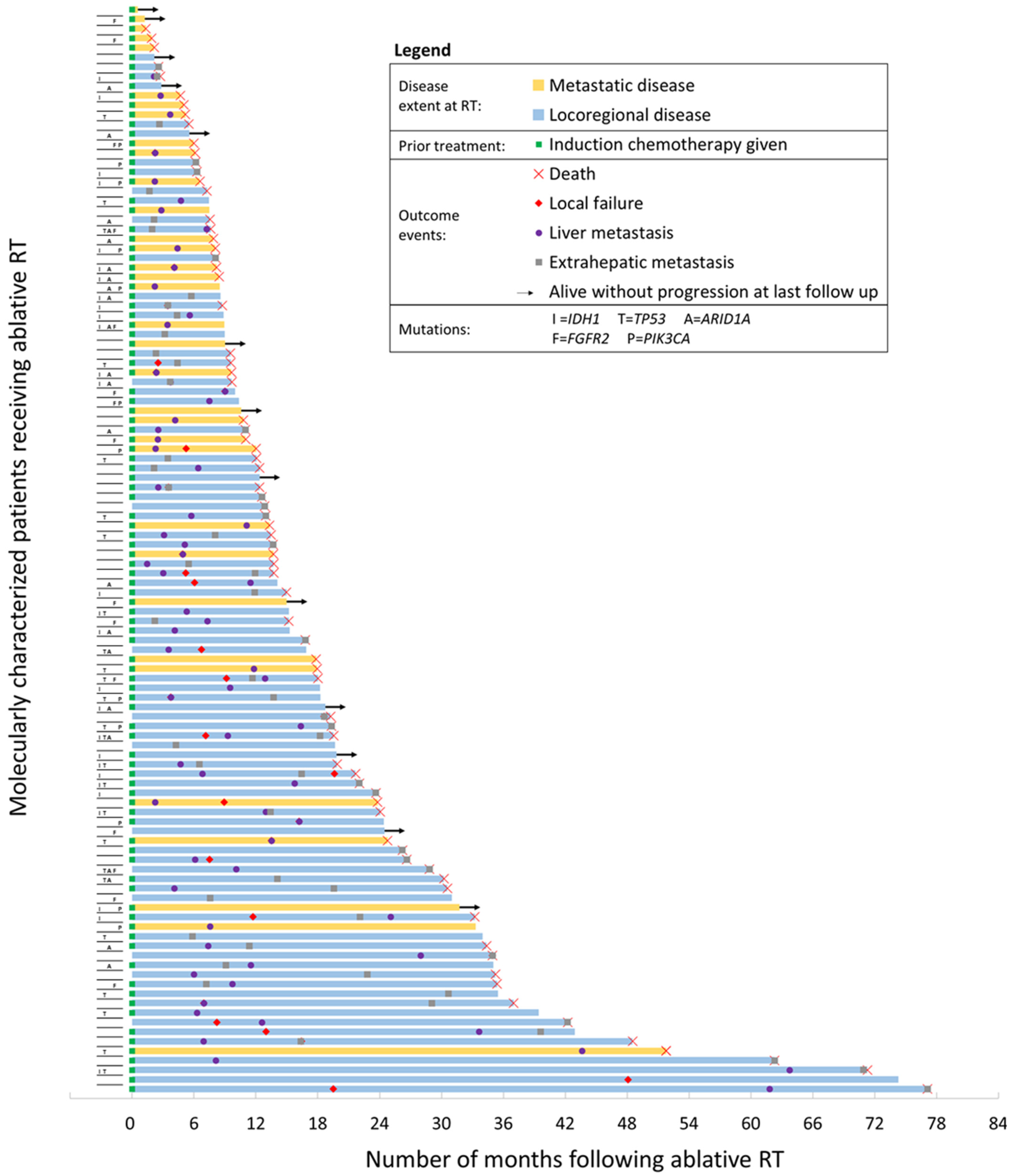
3.1. Baseline Characteristics
3.2. Mutational Profiling Results
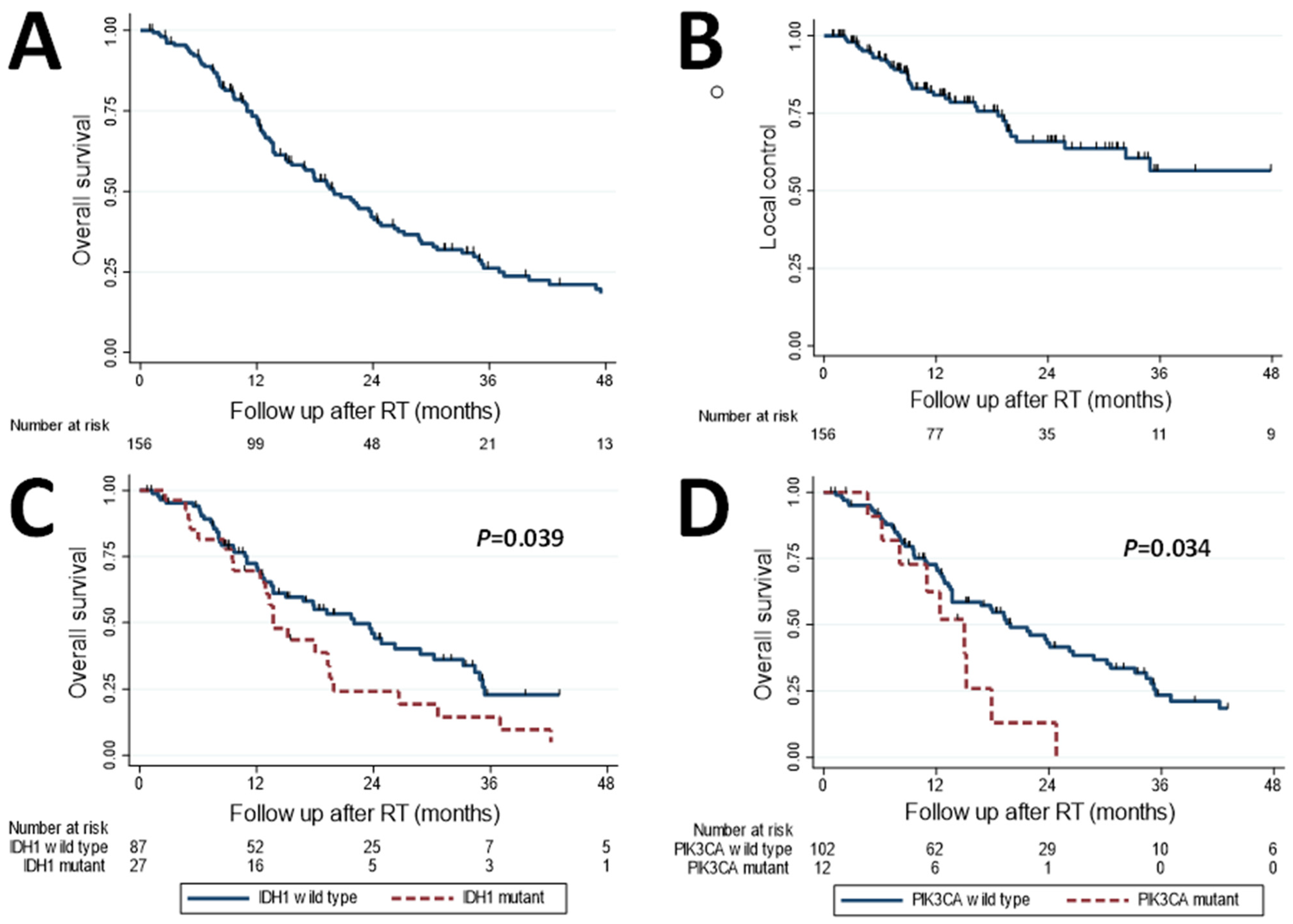
3.3. Disease Control and Survival
4. Discussion
4.1. Hypofractionated RT for ICC
4.2. Mutation Prevalence in ICC
4.3. Significance and Co-Occurrence of Mutations
4.4. Use of Novel Systemic Therapies
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001, 33, 1353–1357. [Google Scholar] [CrossRef]
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599. [Google Scholar] [CrossRef] [Green Version]
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef]
- Sebastian, N.T.; Tan, Y.; Miller, E.D.; Williams, T.M.; Diaz, D.A. Stereotactic body radiation therapy is associated with improved overall survival compared to chemoradiation or radioembolization in the treatment of unresectable intrahepatic cholangiocarcinoma. Clin. Transl. Radiat. Oncol. 2019, 19, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauterio, A.; De Carlis, R.; Centonze, L.; Buscemi, V.; Incarbone, N.; Vella, I.; De Carlis, L. Current Surgical Management of Peri-Hilar and Intra-Hepatic Cholangiocarcinoma. Cancers 2021, 13, 3657. [Google Scholar] [CrossRef] [PubMed]
- Hepatobiliary Cancers (Version 5.2021). 2021 09/30/2021]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (accessed on 3 November 2021).
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Thongprasert, S. The role of chemotherapy in cholangiocarcinoma. Ann. Oncol. 2005, 16 (Suppl. 2), ii93–ii96. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Koay, E.J.; Passot, G.; Shroff, R.; Raghav, K.P.; Conrad, C.; Chun, Y.S.; Aloia, T.A.; Vauthey, J.N. Local therapy reduces the risk of liver failure and improves survival in patients with intrahepatic cholangiocarcinoma: A comprehensive analysis of 362 consecutive patients. Cancer 2017, 123, 1354–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koay, E.J.; Odisio, B.C.; Javle, M.; Vauthey, J.N.; Crane, C.H. Management of unresectable intrahepatic cholangiocarcinoma: How do we decide among the various liver-directed treatments? Hepatobiliary Surg. Nutr. 2017, 6, 105–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila, S.; Smani, D.A.; Koay, E.J. Radiation dose escalation for locally advanced unresectable intrahepatic and extrahepatic chol-angiocarcinoma. Chin. Clin. Oncol. 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Krishnan, S.; Bhosale, P.R.; Javle, M.M.; Aloia, T.A.; Shroff, R.T.; Kaseb, A.O.; Bishop, A.; Swanick, C.W.; Koay, E.J.; et al. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients with Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J. Clin. Oncol. 2016, 34, 219–226. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807, Erratum in 2021, 21, 796–807. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- DiPeri, T.P.; Javle, M.M.; Meric-Bernstam, F. Next generation sequencing for biliary tract cancers. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 471–474. [Google Scholar] [CrossRef]
- Andersen, J.B.; Spee, B.; Blechacz, B.R.; Avital, I.; Komuta, M.; Barbour, A.; Conner, E.A.; Gillen, M.C.; Roskams, T.; Roberts, L.; et al. Genomic and Genetic Characterization of Cholangiocarcinoma Identifies Therapeutic Targets for Tyrosine Kinase Inhibitors. Gastroenterology 2012, 142, 1021–1031.e15. [Google Scholar] [CrossRef] [Green Version]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Ni Huang, M.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crane, C.H.; Koay, E.J. Solutions that enable ablative radiotherapy for large liver tumors: Fractionated dose painting, simultaneous integrated protection, motion management, and computed tomography image guidance. Cancer 2016, 122, 1974–1986. [Google Scholar] [CrossRef] [Green Version]
- FoundationOne CDx: Technical Specifications. 2021 July 2021. Available online: https://assets.ctfassets.net/w98cd481qyp0/YqqKHaqQmFeqc5ueQk48w/c35460768c3a76ef738dcf88f8219524/F1CDx_Tech_Specs_072021.pdf (accessed on 24 November 2021).
- Clark, T.A.; Chung, J.H.; Kennedy, M.; Hughes, J.D.; Chennagiri, N.; Lieber, D.S.; Fendler, B.; Young, L.; Zhao, M.; Coyne, M.; et al. Analytical Validation of a Hybrid Capture–Based Next-Generation Sequencing Clinical Assay for Genomic Profiling of Cell-Free Circulating Tumor DNA. J. Mol. Diagn. 2018, 20, 686–702. [Google Scholar] [CrossRef]
- FoundationOne Liquid CDx: Technical Specifications. 2021 July 2021. Available online: https://assets.ctfassets.net/w98cd481qyp0/wVEm7VtICYR0sT5C1VbU7/55f0a7f3cbfd30fae686c64c1c3d77ae/F1LCDx_Technical_Specs_072021.pdf (accessed on 24 November 2021).
- Hong, T.S.; Wo, J.Y.; Yeap, B.Y.; Ben-Josef, E.; McDonnell, E.I.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; Goyal, L.; et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients with Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J. Clin. Oncol. 2016, 34, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Smart, A.C.; Goyal, L.; Horick, N.; Bs, N.P.; Zhu, A.X.; Ferrone, C.R.; Tanabe, K.K.; Allen, J.N.; Np, L.C.D.; Qadan, M.; et al. Hypofractionated Radiation Therapy for Unresectable/Locally Recurrent Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2019, 27, 1122–1129. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, C.; Pillai, A.; Ahmed, O. Twenty Years of Radiation Therapy of Unresectable Intrahepatic Cholangiocarinoma: Internal or External? A Systematic Review and Meta-Analysis. Liver Cancer 2021, 10, 433–450. [Google Scholar] [CrossRef]
- Javle, M.M.; Murugesan, K.; Shroff, R.T.; Borad, M.J.; Abdel-Wahab, R.; Schrock, A.B.; Chung, J.; Goyal, L.; Frampton, G.M.; Kelley, R.K.; et al. Profiling of 3,634 cholangiocarcinomas (CCA) to identify genomic alterations (GA), tumor mutational burden (TMB), and genomic loss of heterozygosity (gLOH). J. Clin. Oncol. 2019, 37, 4087. [Google Scholar] [CrossRef]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Wahab, R.; Yap, T.A.; Madison, R.; Pant, S.; Cooke, M.; Wang, K.; Zhao, H.; Bekaii-Saab, T.; Karatas, E.; Kwong, L.N.; et al. Genomic profiling reveals high frequency of DNA repair genetic aberrations in gallbladder cancer. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Reitman, Z.J.; Yan, H. Isocitrate Dehydrogenase 1 and 2 Mutations in Cancer: Alterations at a Crossroads of Cellular Metabolism. J. Natl. Cancer Inst. 2010, 102, 932–941. [Google Scholar] [CrossRef] [Green Version]
- Sia, D.; Hoshida, Y.; Villanueva, A.; Roayaie, S.; Ferrer-Fabrega, J.; Tabak, B.; Peix, J.; Sole, M.; Tovar, V.; Alsinet, C.; et al. Integrative Molecular Analysis of Intrahepatic Cholangiocarcinoma Reveals 2 Classes That Have Different Outcomes. Gastroenterology 2013, 144, 829–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, A.X.; Borger, D.R.; Kim, Y.; Cosgrove, D.; Ejaz, A.; Alexandrescu, S.; Groeschl, R.T.; Deshpande, V.; Lindberg, J.M.; Ferrone, C.; et al. Genomic Profiling of Intrahepatic Cholangiocarcinoma: Refining Prognosis and Identifying Therapeutic Targets. Ann. Surg. Oncol. 2014, 21, 3827–3834. [Google Scholar] [CrossRef] [Green Version]
- Boscoe, A.N.; Rolland, C.; Kelley, R.K. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: A systematic literature review. J. Gastrointest. Oncol. 2019, 10, 751–765. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Su, F.; Kalyana-Sundaram, S.; Khazanov, N.; Ateeq, B.; Cao, X.; Lonigro, R.J.; Vats, P.; Wang, R.; Lin, S.-F.; et al. Identification of Targetable FGFR Gene Fusions in Diverse Cancers. Cancer Discov. 2013, 3, 636–647. [Google Scholar] [CrossRef] [Green Version]
- De Luca, A.; Abate, R.E.; Rachiglio, A.M.; Maiello, M.R.; Esposito, C.; Schettino, C.; Izzo, F.; Nasti, G.; Normanno, N. FGFR Fusions in Cancer: From Diagnostic Approaches to Therapeutic Intervention. Int. J. Mol. Sci. 2020, 21, 6856. [Google Scholar] [CrossRef]
- Jain, A.; Borad, M.J.; Kelley, R.K.; Wang, Y.; Abdel-Wahab, R.; Meric-Bernstam, F.; Baggerly, K.A.; Kaseb, A.O.; Al-Shamsi, H.O.; Ahn, D.H.; et al. Cholangiocarcinoma With FGFR Genetic Aberrations: A Unique Clinical Phenotype. JCO Precis. Oncol. 2018, 2, 1–12. [Google Scholar] [CrossRef]
- Arafeh, R.; Samuels, Y. PIK3CA in cancer: The past 30 years. Semin. Cancer Biol. 2019, 59, 36–49. [Google Scholar] [CrossRef]
- Bian, J.-L.; Wang, M.-M.; Tong, E.-J.; Sun, J.; Li, M.; Miao, Z.-B.; Li, Y.-L.; Zhu, B.-H.; Xu, J.-J. Benefit of everolimus in treatment of an intrahepatic cholangiocarcinoma patient with a PIK3CA mutation. World J. Gastroenterol. 2017, 23, 4311–4316. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Vicentini, C.; Ruzzenente, A.; Brunelli, M.; Conci, S.; Fassan, M.; Mafficini, A.; Rusev, B.; Corbo, V.; Capelli, P.; et al. Genetic alterations analysis in prognostic stratified groups identified TP53 and ARID1A as poor clinical performance markers in intrahepatic cholangiocarcinoma. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Hill, M.A.; Alexander, W.B.; Guo, B.; Kato, Y.; Patra, K.C.; O’Dell, M.R.; McCall, M.N.; Whitney-Miller, C.L.; Bardeesy, N.; Hezel, A.F. Kras and Tp53 Mutations Cause Cholangiocyte- and Hepatocyte-Derived Cholangiocarcinoma. Cancer Res. 2018, 78, 4445–4451. [Google Scholar] [CrossRef] [Green Version]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Demetri, G.D. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburd, C.M.; Nagasubramanian, N.; Berlin, J.D.; Drilon, A. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
| Attribute | Value |
|---|---|
| Female sex | 54% |
| Median age at start of radiotherapy (range) | 66 (31–89) |
| Median number of tumors (range) | 1 (1–5) |
| Satellitosis | 51% |
| Median dominant tumor size (range, cm) | 7.3 (2.2–18.2) |
| ECOG Performance Status | |
| 0 | 42 (27%) |
| 1 | 103 (66%) |
| 2 | 11 (7%) |
| AJCC 8th Edition Stage * | |
| I | 12% |
| II | 22% |
| III | 38% |
| IV | 29% |
| Portal vein thrombus | 10% |
| Lymphovascular invasion (n = 143) | 5 (4%) |
| Perineural invasion (n = 143) | 2 (1%) |
| Median CA 19-9 level (IQR, units/mL) | 54 (22–197) |
| Systemic therapy | |
| Before RT | 81% |
| During RT | 63% |
| After RT | 58% |
| RT technique | |
| Photon | 73% |
| Proton | 27% |
| Median gross tumor volume (cm3, IQR) | 168 (70–350) |
| Planning target volume (cm3, IQR) | 350 (157–662) |
| Median RT dose (range, Gy) | 67.5 (58–100) |
| Median RT fractions (range) | 15 (10–28) |
| Median RT BED10 (range, Gy) | 98 (81–144) |
| Outcomes (95% CI) at 1 Year Following RT | ||||
|---|---|---|---|---|
| Mutation Status | OS | LC | Intrahepatic DMFS | Extrahepatic DMFS |
| IDH1 mutant (n = 28) | 70% (48–84%) | 64% (40–80%) | 16% (5–34%) | 50% (27–69%) |
| TP53 mutant (n = 25) | 58% (35–76%) | 66% (41–82%) | 10% (2–26%) | 44% (19–67%) |
| ARID1A mutant (n = 22) | 77% (53–90%) | 74% (48–88%) | 32% (14–51%) | 61% (33–80%) |
| FGFR2 mutant/fusion (n = 15) | 92% (57–99%) | 93% (59–99%) | 15% (2–37%) | 50% (18–75%) |
| All patients (n = 156) | 73% (65–80%) | 81% (73–87%) | 34% (26–42%) | 60% (50–68%) |
| Attribute | OS | LC | Intrahepatic DMFS | Extrahepatic DMFS | ||||
|---|---|---|---|---|---|---|---|---|
| HR | P-Value | HR | P-Value | HR | P-Value | HR | P-Value | |
| Female sex | 0.75 | 0.146 | 1.61 | 0.173 | 0.87 | 0.437 | 0.86 | 0.493 |
| Performance status | 1.47 | 0.021 * | 1.08 | 0.774 | 1.19 | 0.224 | 1.27 | 0.168 |
| Tumor size | 1.06 | 0.043 * | 0.99 | 0.861 | 1.08 | 0.003 * | 1.03 | 0.444 |
| T-stage | 1.12 | 0.300 | 1.32 | 0.110 | 1.17 | 0.118 | 1.29 | 0.031 * |
| N-stage | 1.37 | 0.102 | 0.92 | 0.800 | 1.29 | 0.144 | 1.21 | 0.375 |
| M1 disease at RT | 2.15 | <0.001 * | 1.65 | 0.183 | 1.80 | 0.003 * | - | - |
| CA 19-9 | 1.0001 | <0.001 * | 1.0001 | 0.517 | 1.0001 | 0.001 * | 1.0004 | 0.005 * |
| PVT | 2.30 | 0.008 * | 1.15 | 0.821 | 1.93 | 0.018 * | 2.46 | 0.041 * |
| Satellitosis | 1.63 | 0.013 * | 2.57 | 0.006 * | 1.61 | 0.008 * | 1.50 | 0.060 |
| Lymphovascular invasion | 1.31 | 0.602 | 3.44 | 0.044 * | 1.08 | 0.886 | 1.13 | 0.815 |
| Proton RT technique | 0.76 | 0.201 | 0.45 | 0.060 | 0.92 | 0.661 | 0.88 | 0.556 |
| D90% to GTV | 0.97 | 0.005 * | 1.00 | 0.996 | 0.98 | 0.060 | 0.96 | 0.006 * |
| IDH1 mutation | 1.68 | 0.041 * | 2.07 | 0.079 | 1.71 | 0.028 * | 1.68 | 0.063 |
| TP53 mutation | 1.53 | 0.136 | 2.35 | 0.035 * | 1.72 | 0.031 * | 1.31 | 0.422 |
| ARID1A mutation | 1.53 | 0.109 | 1.46 | 0.386 | 1.42 | 0.153 | 1.27 | 0.426 |
| FGFR2 mutation/fusion | 0.60 | 0.154 | 0.32 | 0.118 | 1.19 | 0.544 | 0.87 | 0.719 |
| BAP1 mutation | 0.62 | 0.232 | 1.04 | 0.948 | 1.10 | 0.748 | 0.69 | 0.367 |
| IDH2 mutation | 1.00 | 0.990 | 1.73 | 0.235 | 0.74 | 0.348 | 1.12 | 0.777 |
| NRAS mutation | 0.73 | 0.596 | 2.87 | 0.097 | 3.23 | 0.004 * | 0.64 | 0.536 |
| CDKN2A mutation | 1.11 | 0.783 | 1.90 | 0.235 | 1.25 | 0.529 | 0.86 | 0.730 |
| MLL2 mutation | 0.99 | 0.967 | 1.81 | 0.229 | 1.02 | 0.948 | 0.86 | 0.743 |
| PIK3CA mutation | 2.13 | 0.039 * | 1.50 | 0.514 | 1.49 | 0.219 | 2.56 | 0.035 * |
| No mutations | 0.72 | 0.370 | 0.62 | 0.429 | 0.65 | 0.168 | 0.474 | 0.086 |
| Attribute | OS | LC | ||
|---|---|---|---|---|
| HR | P-Value | HR | P-Value | |
| Female sex | ||||
| Performance status | 1.81 | 0.021 * | ||
| Tumor size | 1.02 | 0.578 | ||
| T-stage | ||||
| N-stage | ||||
| M1 disease at RT | 2.00 | 0.012 * | ||
| CA 19-9 | 1.0001 | <0.001 * | ||
| PVT | 2.11 | 0.069 | ||
| Satellitosis | 1.56 | 0.110 | 2.63 | 0.054 |
| Lymphovascular invasion | 3.96 | 0.091 | ||
| Proton RT technique | 0.63 | 0.374 | ||
| D90% to GTV | 0.96 | 0.018 * | ||
| IDH1 mutation | 1.80 | 0.042 * | 1.28 | 0.601 |
| TP53 mutation | 2.41 | 0.041 * | ||
| ARID1A mutation | ||||
| FGFR2 mutation/fusion | ||||
| NRAS mutation | 2.21 | 0.232 | ||
| PIK3CA mutation | 2.29 | 0.034 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De, B.; Abu-Gheida, I.; Patel, A.; Ng, S.S.W.; Zaid, M.; Thunshelle, C.P.; Elganainy, D.; Corrigan, K.L.; Rooney, M.K.; Javle, M.; et al. Benchmarking Outcomes after Ablative Radiotherapy for Molecularly Characterized Intrahepatic Cholangiocarcinoma. J. Pers. Med. 2021, 11, 1270. https://doi.org/10.3390/jpm11121270
De B, Abu-Gheida I, Patel A, Ng SSW, Zaid M, Thunshelle CP, Elganainy D, Corrigan KL, Rooney MK, Javle M, et al. Benchmarking Outcomes after Ablative Radiotherapy for Molecularly Characterized Intrahepatic Cholangiocarcinoma. Journal of Personalized Medicine. 2021; 11(12):1270. https://doi.org/10.3390/jpm11121270
Chicago/Turabian StyleDe, Brian, Ibrahim Abu-Gheida, Aashini Patel, Sylvia S. W. Ng, Mohamed Zaid, Connor P. Thunshelle, Dalia Elganainy, Kelsey L. Corrigan, Michael K. Rooney, Milind Javle, and et al. 2021. "Benchmarking Outcomes after Ablative Radiotherapy for Molecularly Characterized Intrahepatic Cholangiocarcinoma" Journal of Personalized Medicine 11, no. 12: 1270. https://doi.org/10.3390/jpm11121270
APA StyleDe, B., Abu-Gheida, I., Patel, A., Ng, S. S. W., Zaid, M., Thunshelle, C. P., Elganainy, D., Corrigan, K. L., Rooney, M. K., Javle, M., Raghav, K., Lee, S. S., Vauthey, J.-N., Tzeng, C.-W. D., Tran Cao, H. S., Ludmir, E. B., Minsky, B. D., Smith, G. L., Holliday, E. B., ... Koay, E. J. (2021). Benchmarking Outcomes after Ablative Radiotherapy for Molecularly Characterized Intrahepatic Cholangiocarcinoma. Journal of Personalized Medicine, 11(12), 1270. https://doi.org/10.3390/jpm11121270







