Monocyte/Lymphocyte Ratio and MCHC as Predictors of Collateral Carotid Artery Disease—Preliminary Report
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dettori, P.; Paliogiannis, P.; Pascale, R.M.; Zinellu, A.; Mangoni, A.A.; Pintus, G. Blood Cell Count Indexes of Systemic Inflammation in Carotid Artery Disease: Current Evidence and Future Perspectives. Curr. Pharm. Des. 2021, 27, 2170–2179. [Google Scholar] [CrossRef]
- Kamtchum-Tatuene, J.; Jickling, G.C. Blood Biomarkers for Stroke Diagnosis and Management. Neuromolecular Med. 2019, 21, 344–368. [Google Scholar] [CrossRef]
- Montellano, F.A.; Ungethüm, K.; Ramiro, L.; Nacu, A.; Hellwig, S.; Fluri, F.; Whiteley, W.N.; Bustamante, A.; Montaner, J.; Heuschmann, P.U. Role of Blood-Based Biomarkers in Ischemic Stroke Prognosis: A Systematic Review. Stroke 2021, 52, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Wu, M.Y.; Li, C.J.; Hou, M.F.; Chu, P.Y. New Insights into the Role of Inflammation in the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2017, 18, 2034. [Google Scholar] [CrossRef]
- Kose, N.; Akin, F.; Yildirim, T.; Ergun, G.; Altun, I. The association between the lymphocyte-to-monocyte ratio and coronary artery disease severity in patients with stable coronary artery disease. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2570–2575. [Google Scholar] [PubMed]
- Selvaggio, S.; Abate, A.; Brugaletta, G.; Musso, C.; Di Guardo, M.; Di Guardo, C.; Vicari, E.S.D.; Romano, M.; Luca, S.; Signorelli, S.S. Platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio and monocyte-to-HDL cholesterol ratio as markers of peripheral artery disease in elderly patients. Int. J. Mol. Med. 2020, 46, 1210–1216. [Google Scholar] [CrossRef]
- Luke, K.; Purwanto, B.; Herawati, L.; Al-Farabi, M.J.; Oktaviono, Y.H. Predictive Value of Hematologic Indices in the Diagnosis of Acute Coronary Syndrome. Open Access Maced. J. Med. Sci. 2019, 7, 2428–2433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornal, M.; Wizner, B.; Cwynar, M.; Królczyk, J.; Kwater, A.; Korbut, R.A.; Grodzicki, T. Association of red blood cell distribution width, inflammation markers and morphological as well as rheological erythrocyte parameters with target organ damage in hypertension. Clin. Hemorheol. Microcirc. 2014, 56, 325–335. [Google Scholar] [CrossRef]
- Serfözö, G.; Horváth, T.; Földesi, I.; Rafael, B.; von Känel, R.; Keresztes, M. The monocyte-to-lymphocyte ratio correlates with psycho-neuro-inflammatory factors in patients with stable coronary artery disease. Neuroimmunomodulation 2016, 23, 67–74. [Google Scholar] [CrossRef]
- Van der Laan, A.M.; Hirsch, A.; Robbers, L.F.; Nijveldt, R.; Lommerse, I.; Delewi, R.; van der Vleuten, P.A.; Biemond, B.J.; Zwaginga, J.J.; van der Giessen, W.J.; et al. A proinflammatory monocyte response is associated with myocardial injury and impaired functional outcome in patients with ST-segment elevation myocardial infarction: Monocytes and myocardial infarction. Am. Heart J. 2012, 163, 57–65. [Google Scholar] [CrossRef]
- Davignon, J.; Cohn, J.S.; Mabile, L.; Bernier, L. Apolipoprotein E and atherosclerosis: Insight from animal and human studies. Clin. Chim. Acta. 1999, 286, 115–143. [Google Scholar] [CrossRef]
- Zuo, B.; Zhu, S.; Meng, X.; Zhao, D.; Zhang, J. Monocyte/lymphocyte ratio is associated with carotid stenosis in ischemic stroke: A retrospective analysis. Brain Behav. 2019, 9, e01429. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, R.; Nachiappan, S.; Howlett, D.C. Carotid artery stenosis screening: Where are we now? Br. J. Radiol. 2018, 91, 20170380. [Google Scholar] [CrossRef]
- Juega, J.; Palacio-Garcia, C.; Rodriguez, M.; Deck, M.; Rodriguez-Luna, D.; Requena, M.; García-Tornel, Á.; Rodriguez-Villatoro, N.; Rubiera, M.; Boned, S.; et al. Monocyte-to-Lymphocyte Ratio in Clot Analysis as a Marker of Cardioembolic Stroke Etiology. Transl Stroke Res. 2021. [Google Scholar] [CrossRef]
- Liu, H.; Zhan, F.; Wang, Y. Evaluation of monocyte-to-high-density lipoprotein cholesterol ratio and monocyte-to-lymphocyte ratio in ischemic stroke. J. Int. Med. Res. 2020, 48, 0300060520933806. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, E.; Guzel, M.; Akpinar, C.K.; Yucel, M.; Demir, M.T.; Baydin, A. The relationship between neutrophil/lymphocyte, monocyte/ /lymphocyte, platelet/lymphocyte ratios and clinical outcomes after ninety days in patients who were diagnosed as having acute ischemic stroke in the emergency room and underwent a mechanical thro. Bratisl. Lek. Listy. 2020, 121, 634–639. [Google Scholar] [CrossRef]
- Naess, A.; Nilssen, S.S.; Mo, R.; Eide, G.E.; Sjursen, H. Role of neutrophil to lymphocyte and monocyte to lymphocyte ratios in the diagnosis of bacterial infection in patients with fever. Infection 2017, 45, 299–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djordjevic, D.; Rondovic, G.; Surbatovic, M.; Stanojevic, I.; Udovicic, I.; Andjelic, T.; Zeba, S.; Milosavljevic, S.; Stankovic, N.; Abazovic, D.; et al. Neutrophil-to-Lymphocyte Ratio, Monocyte-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Mean Platelet Volume-to-Platelet Count Ratio as Biomarkers in Critically Ill and Injured Patients, Which Ratio to Choose to Predict Outcome and Nature of Bacteremia? Mediators Inflamm. 2018, 2018, 3758068. [Google Scholar]
- Chen, H.; Li, M.; Liu, L.; Dang, X.; Zhu, D.; Tian, G. Monocyte/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients with non-ST-elevation myocardial infarction. Medicine 2019, 98, e16267. [Google Scholar] [CrossRef]
- Mirna, M.; Schmutzler, L.; Topf, A.; Hoppe, U.C.; Lichtenauer, M. Neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio predict length of hospital stay in myocarditis. Sci. Rep. 2021, 11, 18101–18110. [Google Scholar] [CrossRef] [PubMed]
- Metta, S.; Uppala, S.; Basalingappa, D.; Gunti, S.; Badeti, S. Impact of smoking on erythrocyte indices and oxidative stress in acute myocardial infarction. J. Dr. NTR Univ. Health Sci. 2015, 4, 159–164. [Google Scholar] [CrossRef]
- Zhan, Y.L.; Zou, B.; Kang, T.; Xiong, L.B.; Zou, J.; Wei, Y.F. Multiplicative interaction between mean corpuscular volume and red cell distribution width with target organ damage in hypertensive patients. J. Clin. Lab. Anal. 2017, 31, e22082. [Google Scholar] [CrossRef] [PubMed]

| One Vessel Disease No = 115 (85.2%) | Collateral Disease No = 20 (14.8%) | p | |
|---|---|---|---|
| Demographical: | |||
| 1. Age (median (Q1–Q3)) | 71 (66–75) | 68 (64–72) | p = 0.1860 |
| 2. Gender (M(%)/F(%)) | 73(63.5%)/42(36.5%) | 14 (70%)/6 (30%) | p = 0.5753 |
| 2. BMI (median (Q1–Q3)) | 26 (24.1–29.6) | 26.82(24.5–29.3) | p = 0.8811 |
| Carotid artery disease | |||
| 1. L(%)/R(%) | 57 (49.6%)/58 (50.4%) | ||
| 2. Artery occlusion | 10 (8.7%) | 8 (40.0%) | p < 0.0001 * |
| 3. Symptoms | 70 (60.9%) | 16 (80%) | p = 0.41 |
| Concomitant disease | |||
| 1. Hypertension | 90 (78.3%) | 14 (70%) | p = 0.4176 |
| 2. Hypercholesterolemia | 71 (65%) | 23 (65%) | p = 0.9746 |
| 3. stroke | 53 (46.1%) | 9 (45%) | p = 0.7933 |
| 4. DM | 39 (33.9%) | 9 (45%) | p = 0.3391 |
| 5. CCS | 37 (35%) | 12 (33%) | p = 0.9849 |
| 6. COPD | 6 (5.22%) | 1 (5%) | p = 0.9677 |
| 7. Smoking | 18 (19.8%) | 2 (11.8%) | p= 0.4348 |
| 8.Atrial fibrillation | 18 (15.7%) | 3 (15.0%) | p= 0.9408 |
| One Vessel Disease No = 104,115 (85.2%) | Collateral Disease No = 20 (14.8%) | p-Value | |
|---|---|---|---|
| Whole blood count: | |||
| 1. WBC × 109/L (median (Q1–Q3)) | 8.5 (6.7–9.8) | 7.5 (6.4–11.3) | p = 0.8489 |
| 2. Neutrophils × 109/L (median (Q1–Q3)) | 5.3 (4.1–6.6) | 5.3 (4.2–7.6) | p = 0.5001 |
| 3. Lymphocyte × 109/L (median (Q1–Q3)) | 2.0 (1.6–2.5) | 1.7 (1.3–2.1) | p = 0.0367 * |
| 4. Monocyte × 109/L (median (Q1–Q3)) | 0.5 (0.4–0.7) | 0.5 (0.4–0.7) | p = 0.8465 |
| 4. Eosinophils × 109/L (median (Q1–Q3)) | 0.13 (0.08–0.18) | 0.2 (0.07–0.21) | p = 0.8907 |
| 5. Basophils × 109/L (median (Q1–Q3)) | 0.04 (0.03–0.06) | 0.05 (0.03–0.07) | p = 0.5242 |
| 6. Luc × 109/L (median (Q1–Q3)) | 0.16 (0.12–0.2) | 0.15 (0.1–0.26) | p = 0.3425 |
| 7. Hemoglobin (mmol/L) (median (Q1–Q3)) | 8.7 (8.1–9.2) | 9 (8.8–9.6) | p = 0.0139 * |
| 5. Rbc × 109/L (median (Q1–Q3)) | 4.45 (4.2–4.6) | 4.56 (4.2–4.92) | p = 0.1381 |
| 6. Hematocrit (%) (median (Q1–Q3)) | 41.0 (39.0–43.0) | 42.5 (40.0–43.0) | p = 0.3549 |
| 7. MCV (fL) (median (Q1–Q3)) | 92 (90–95) | 91 (88–95) | p = 0.2084 |
| 8. MCH (pg) (median (Q1–Q3)) | 1.96 (1.89–2.02) | 2 (1.94–2.05) | p = 0.1564 |
| 9. MCHC (mmol/dL) (median (Q1–Q3)) | 21 (20.7–21.5) | 22 (21–22.6) | p = 0.0116 * |
| 10. RDW (%) (median (Q1–Q3)) | 13.4 (13–14.1) | 13.4 (12.9–13.8) | p = 0.5806 |
| 11. Plt × 103/uL (median (Q1–Q3)) | 228 (182–292) | 228 (190–267) | p = 0.4518 |
| 12. MPV (fL) (median (Q1–Q3)) | 7.9 (7.3–8.9) | 8 (7.3–8.5) | p = 0.9776 |
| 13. MLR (median (Q1–Q3)) | 0.25 (0.21–031) | 0.35 (0.26–0.51) | p = 0.0288 * |
| 14. PDW (fL) (median (Q1–Q3)) | 58 (52–65) | 59 (54–65) | p = 0.8489 |
| Lipidogram: | |||
| 1. total cholesterol mmol/L (median (Q1–Q3)) | 3.9 (3.2–4.5) | 4.1 (3.4–4.7) | p = 0.3571 |
| 2. HDL mmol/L (median (Q1–Q3)) | 1.1 (0.9–1.5) | 1.2 (1–1.4) | p = 0.4098 |
| 3. LDL mmol/L (median (Q1–Q3)) | 2.2 (1.8–2.7) | 2.4 (1.8–3.1) | p = 0.3619 |
| Kidney function test: | |||
| 1. Creatinine mmol/L (median (Q1–Q3)) | 86 (69–106) | 81 (69–116) | p = 0.7242 |
| 2. GFR (median (Q1–Q3)) | 76 (54–90) | 80 (56–90) | p = 0.5208 |
| Fibrinogen (mg/dL) (median (Q1–Q3)) | 364 (311–426) | 355 (300–418) | p = 0.6257 |
| Odds | Std. Err. | z | p > z | 95% Conf. Interval | |
|---|---|---|---|---|---|
| Age | 0.9713 | 0.0292 | −0.97 | 0.334 | 0.9157–1.0303 |
| BMI | 0.9975 | 0.061 | −0.04 | 0.967 | 0.8847–1.1246 |
| Concomitant diseases: | |||||
| 1. ischemic heart disease | 1.0096 | 0.5129 | 0.02 | 0.985 | 0.3729–2.7328 |
| 2. Stroke | 0.8799 | 0.4297 | −0.26 | 0.793 | 0.3379–2.2913 |
| 3. Hypertension | 0.6481 | 0.3485 | −0.81 | 0.420 | 0.2258–1.859 |
| 4. DM | 1.5944 | 0.7824 | 0.95 | 0.342 | 0.6094–4.1717 |
| Whole blood count: | |||||
| 1. WBC | 1.1118 | 0.0967 | 1.22 | 0.223 | 0.9374–1.319 |
| 2. Neutrophils | 1.1879 | 0.1178 | 1.74 | 0.082 | 0.9781–1.4427 |
| 3. Monocytes | 2.7299 | 3.608 | 0.76 | 0.447 | 0.2047–36.4072 |
| 4. MLR | 11.5519 | 15.9476 | 1.77 | 0.076 | 0.7719–172.8806 |
| 5. MLR > 0.3 | 5.9792 | 3.1726 | 3.37 | 0.001 * | 2.1134–16.9158 |
| 6. Hb | 2.1207 | 0.7078 | 2.25 | 0.024 * | 1.1025–4.0792 |
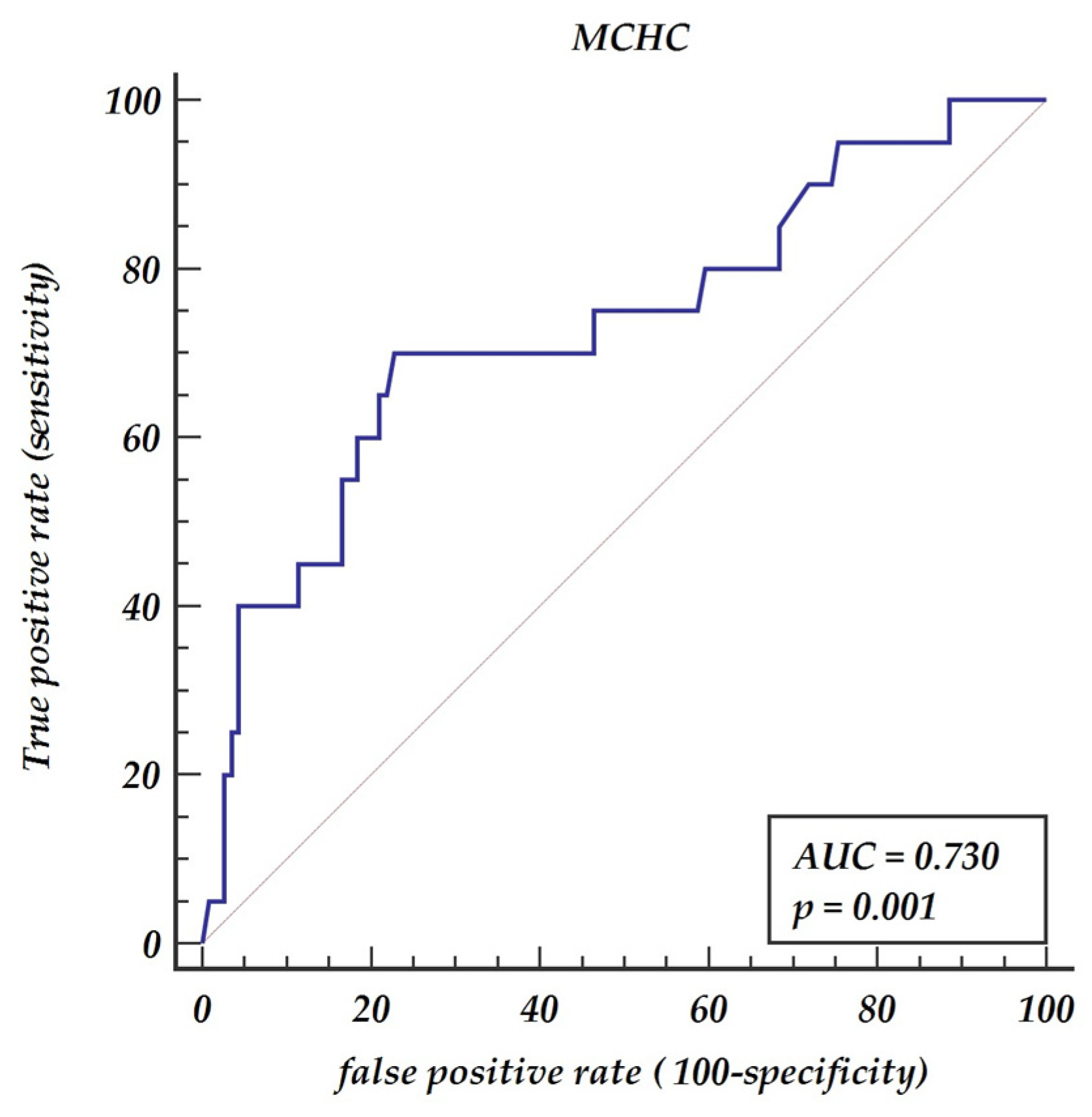
| 7. MCHC | 3.1288 | 1.01596 | 3.51 | <0.001 * | 1.6553–5.9122 |
| 8. MCHC > 21.6 | 7.52 | 4.02 | 3.77 | <0.001 * | 2.63–21.47 |
| 9. MCV | 0.9512 | 0.0434 | −1.1 | 0.273 | 0.8698–1.0403 |
| 10.Plt | 0.9959 | 0.0035 | −1.18 | 0.239 | 0.9891–1.0027 |
| Serum cholesterol: | |||||
| 1. Total | 1.1743 | 0.2531 | 0.75 | 0.456 | 0.7698–1.7915 |
| 2. HDL | 1.0955 | 0.7037 | 0.14 | 0.887 | 0.3111–3.858 |
| 3. LDL | 1.2282 | 0.2752 | 0.92 | 0.359 | 0.7917–1.9053 |
| GFR | 1.0079 | 0.0129 | 0.61 | 0.541 | 0.9828–1.0336 |
| Fibrinogen | 0.9988 | 0.0025 | −0.45 | 0.651 | 0.9941–1.0037 |
| Odds | Std. Err. | z | p > z | 95% Conf. Interval | |
|---|---|---|---|---|---|
| MLR > 0.3 | 6.20 | 3.54 | 3.19 | 0.001 | 2.02–19.01 |
| MCHC > 21.6 | 7.76 | 4.42 | 3.60 | <0.001 | 2.54–23.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanowicz, T.; Michalak, M.; Olasińska-Wiśniewska, A.; Rodzki, M.; Krasińska, A.; Perek, B.; Krasiński, Z.; Jemielity, M. Monocyte/Lymphocyte Ratio and MCHC as Predictors of Collateral Carotid Artery Disease—Preliminary Report. J. Pers. Med. 2021, 11, 1266. https://doi.org/10.3390/jpm11121266
Urbanowicz T, Michalak M, Olasińska-Wiśniewska A, Rodzki M, Krasińska A, Perek B, Krasiński Z, Jemielity M. Monocyte/Lymphocyte Ratio and MCHC as Predictors of Collateral Carotid Artery Disease—Preliminary Report. Journal of Personalized Medicine. 2021; 11(12):1266. https://doi.org/10.3390/jpm11121266
Chicago/Turabian StyleUrbanowicz, Tomasz, Michał Michalak, Anna Olasińska-Wiśniewska, Michał Rodzki, Aleksandra Krasińska, Bartłomiej Perek, Zbigniew Krasiński, and Marek Jemielity. 2021. "Monocyte/Lymphocyte Ratio and MCHC as Predictors of Collateral Carotid Artery Disease—Preliminary Report" Journal of Personalized Medicine 11, no. 12: 1266. https://doi.org/10.3390/jpm11121266
APA StyleUrbanowicz, T., Michalak, M., Olasińska-Wiśniewska, A., Rodzki, M., Krasińska, A., Perek, B., Krasiński, Z., & Jemielity, M. (2021). Monocyte/Lymphocyte Ratio and MCHC as Predictors of Collateral Carotid Artery Disease—Preliminary Report. Journal of Personalized Medicine, 11(12), 1266. https://doi.org/10.3390/jpm11121266






