Pre-Treatment Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios as Predictors of Occult Cervical Metastasis in Clinically Negative Neck Supraglottic and Glottic Cancer
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
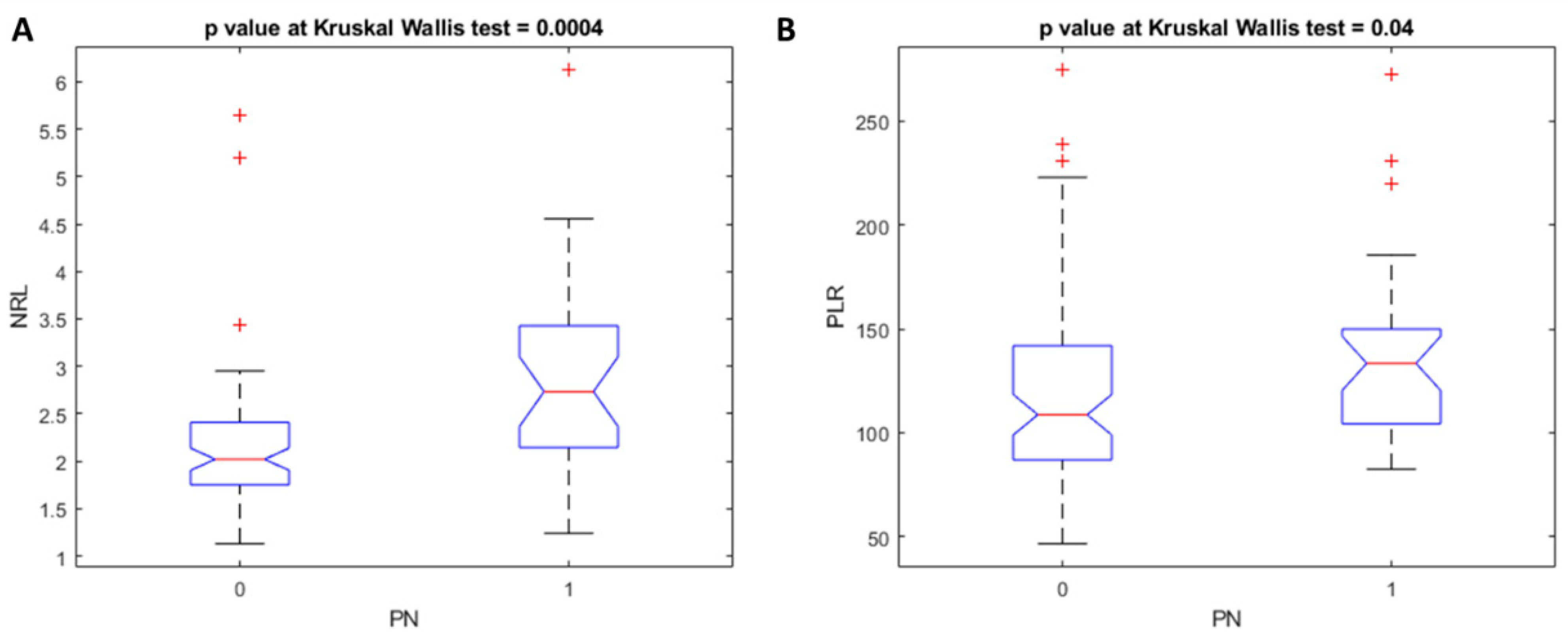
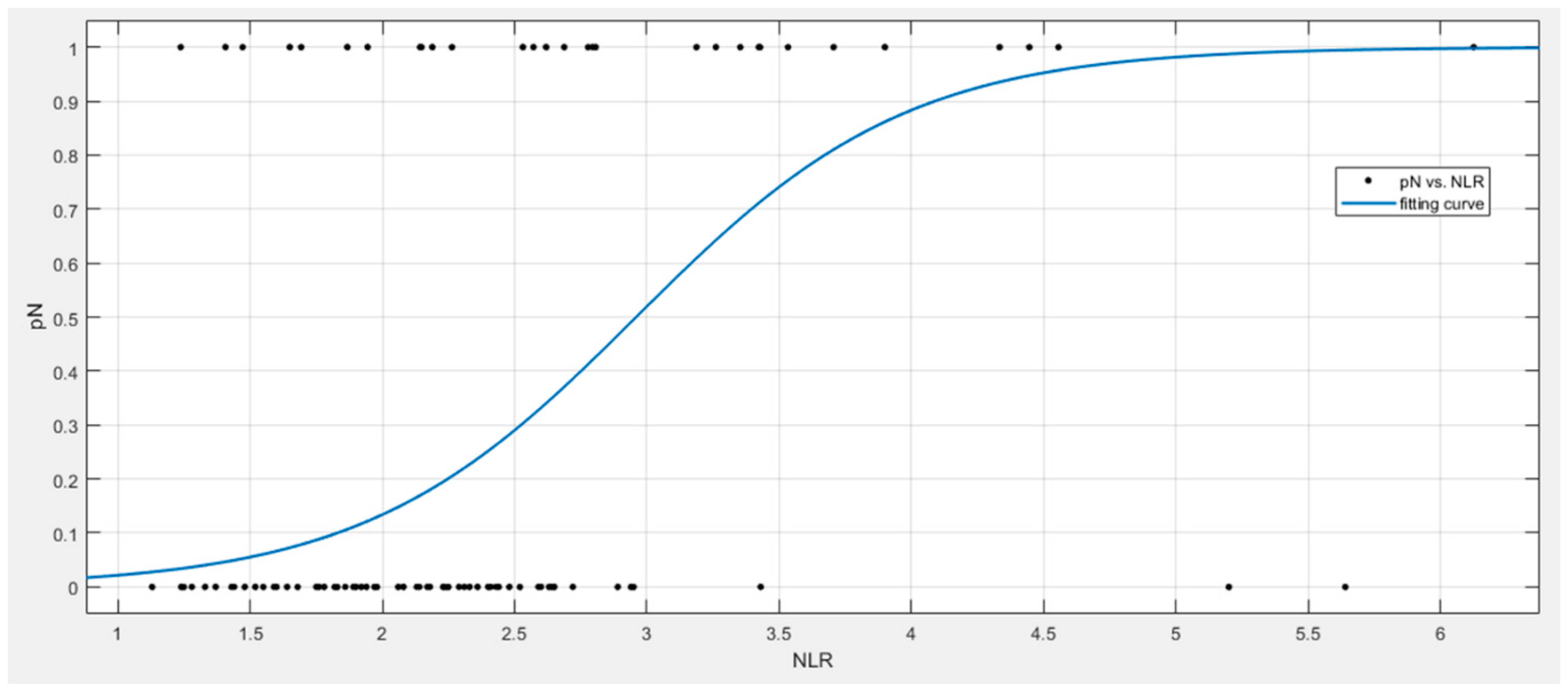
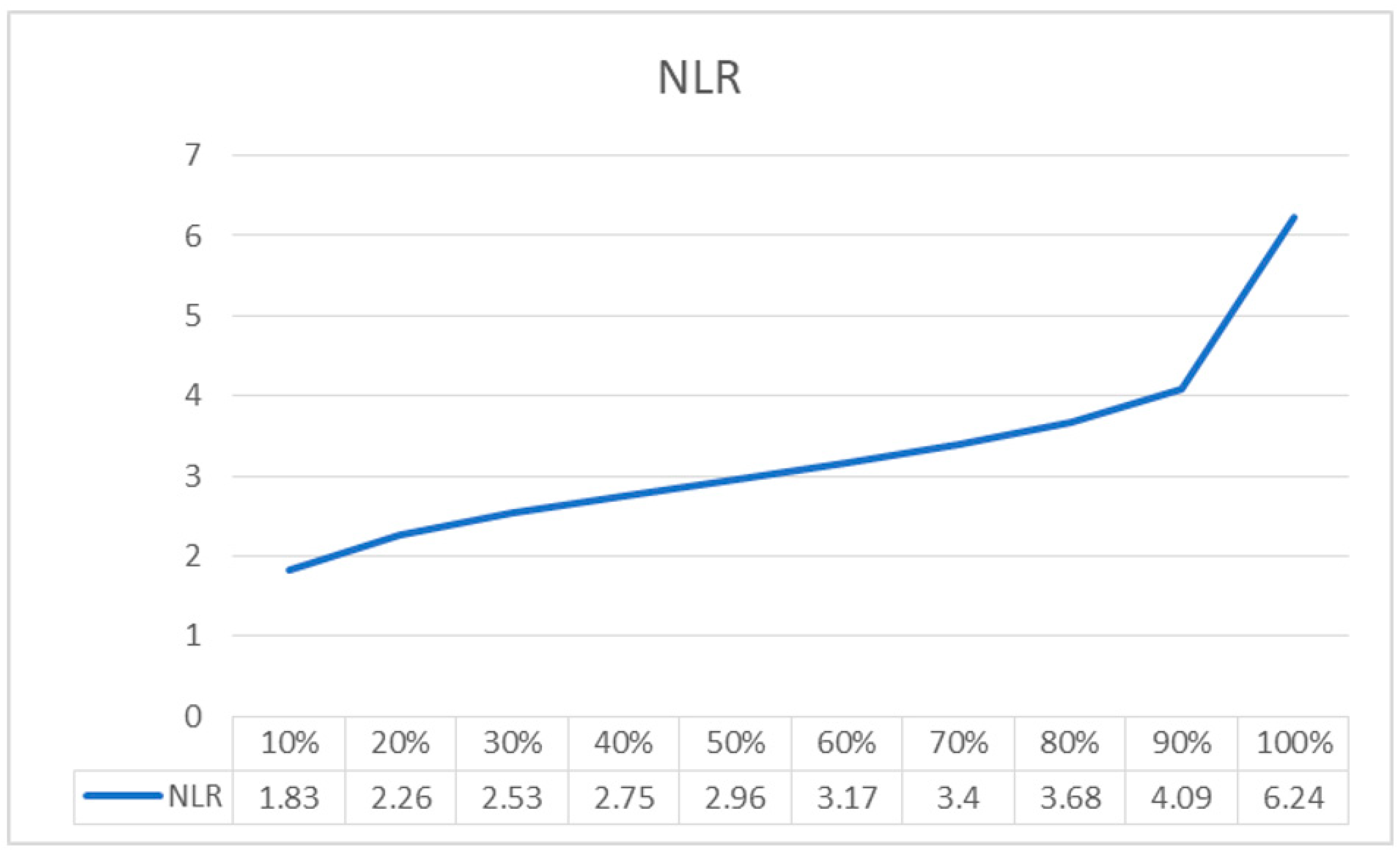
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlito, A.; Rinaldo, A. Controversies in the treatment of N(0) neck in laryngeal cancer: Neck dissection, no surgery, or sentinel lymph node biopsy? ORL J. Otorhinolaryngol. Relat. Spec. 2000, 62, 287–289. [Google Scholar] [CrossRef]
- Ferlito, A.; Rinaldo, A.; Silver, C.E.; Robbins, K.T.; Medina, J.E.; Rodrigo, J.P.; Shaha, A.R.; Takes, R.P.; Bradley, P.J. Neck dissection for laryngeal cancer. J. Am. Coll Surg. 2008, 207, 587–593. [Google Scholar] [CrossRef]
- Moe, K.; Wolf, G.T.; Fisher, S.G.; Hong, W.K. Regional metastases in patients with advanced laryngeal cancer. Department of Veterans Affairs Laryngeal Cancer Study Group. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Motiee Langroudi, M.; Amirzargar, B.; Amali, A.; Sadeghi, M.; Jafar, M.; Hoseini, M.R.; Tavakolnejad, F. Rate of occult cervical lymph node involvement in supraglottic squamous cell carcinoma. Iran. J. Otorhinolaryngol. 2017, 29, 133–136. [Google Scholar] [PubMed]
- Yang, C.Y.; Andersen, P.E.; Everts, E.C.; Cohen, J.I. Nodal disease in purely glottic carcinoma: Is elective neck treatment worthwhile? Laryngoscope 1998, 108, 1006–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, Z.G.; Tang, P.Z. Elective lateral neck dissection for laryngeal cancer in the clinically negative neck. J. Surg. Oncol. 2006, 93, 464–467. [Google Scholar] [CrossRef]
- Sanabria, A.; Shah, J.P.; Medina, J.E.; Olsen, K.D.; Robbins, K.T.; Silver, C.E.; Rodrigo, J.P.; Suárez, C.; Coca-Pelaz, A.; Shaha, A.R.; et al. Incidence of Occult Lymph Node Metastasis in Primary Larynx Squamous Cell Carcinoma, by Subsite, T Classification and Neck Level: A Systematic Review. Cancers 2020, 12, 1059. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; Cabanillas, R.; Franco, V.; Suárez, C. Efficacy of routine bilateral neck dissection in the management of the N0 neck in T1-T2 unilateral supraglottic cancer. Head Neck 2006, 28, 534–539. [Google Scholar] [CrossRef]
- Ferlito, A.; Silver, C.E.; Rinaldo, A. Selective neck dissection (IIA, III): A rational replacement for complete functional neck dissection in patients with N0 supraglottic and glottic squamous carcinoma. Laryngoscope 2008, 118, 676–679. [Google Scholar] [CrossRef]
- Lim, Y.C.; Choi, E.C.; Lee, J.S.; Koo, B.S.; Song, M.H.; Shin, H.A. Is dissection of level IV absolutely necessary in elective lateral neck dissection for clinically N0 laryngeal carcinoma? Oral Oncol. 2006, 42, 102–107. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Abbate, V.; Dell’Aversana Orabona, G.; Salzano, G.; Bonavolontà, P.; Maglitto, F.; Romano, A.; Tarabbia, F.; Turri-Zanoni, M.; Attanasi, F.; Di Lauro, A.E.; et al. Pre-treatment Neutrophil-to-Lymphocyte Ratio as a predictor for occult cervical metastasis in early stage (T1-T2 cN0) squamous cell carcinoma of the oral tongue. Surg. Oncol. 2018, 27, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Turri-Zanoni, M.; Salzano, G.; Lambertoni, A.; Giovannardi, M.; Karligkiotis, A.; Battaglia, P.; Castelnuovo, P. Prognostic value of pretreatment peripheral blood markers in paranasal sinus cancer: Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio. Head Neck 2017, 39, 730–736. [Google Scholar] [CrossRef]
- Kara, M.; Uysal, S.; Altinişik, U.; Cevizci, S.; Güçlü, O.; Dereköy, F.S. The pre-treatment neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and red cell distribution width predict prognosis in patients with laryngeal carcinoma. Eur. Arch. Otorhinolaryngol. 2017, 274, 535–542. [Google Scholar] [CrossRef]
- Ozturk, K.; Akyildiz, N.S.; Uslu, M.; Gode, S.; Uluoz, U. The effect of preoperative neutrophil, platelet and lymphocyte counts on local recurrence and survival in early-stage tongue cancer. Eur. Arch. Otorhinolaryngol. 2016, 273, 4425–4429. [Google Scholar] [CrossRef]
- Rachidi, S.; Wallace, K.; Wrangle, J.M.; Day, T.A.; Alberg, A.J.; Li, Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck 2015, 38, E1068–E1074. [Google Scholar] [CrossRef] [PubMed]
- Rassouli, A.; Saliba, J.; Castano, R.; Hier, M.; Zeitouni, A.G. Systemic inflammatory markers as independent prognosticators of head and neck squamous cell carcinoma. Head Neck 2015, 37, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. (Eds.) American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- El-Naggar, A.K.; Chan, J.K.C.; Takata, T.; Grandis, J.R.; Slootweg, P. WHO Classification of Tumors Pathology and Genetics of Head and Neck Tumors, 4th ed.; IARC Press: Lyon, France, 2017. [Google Scholar]
- Chaturvedi, A.K.; Anderson, W.F.; Lortet-Tieulent, J.; Curado, M.P.; Ferlay, J.; Franceschi, S.; Gillison, M.L. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J. Clin. Oncol. 2013, 31, 4550. [Google Scholar] [CrossRef]
- National Cancer Institute. SEER: Cancer Stat Facts: Larynx Cancer. 2018. Available online: http://seer.cancer.gov/statfacts/html/laryn.html (accessed on 10 February 2018).
- Candela, F.C.; Shah, J.; Jaques, D.P.; Shah, J.P. Patterns of cervical node metastases from squamous carcinoma of the larynx. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 432–435. [Google Scholar] [CrossRef]
- Chiu, R.J.; Myers, E.N.; Johnson, J.T. Efficacy of routine bilateral neck dissection in the management of supraglottic cancer. Otolaryngol. Head Neck Surg. 2004, 131, 485–488. [Google Scholar] [CrossRef]
- Ma, H.; Lian, M.; Feng, L.; Li, P.; Hou, L.; Liu, H.; Fang, J. Management of cervical lymph nodes for cN0 advanced glottic laryngeal carcinoma and its long-term results. Acta Otolaryngol. 2014, 134, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.H.; Harrison, L.B.; Isaacs, R.S. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch. Otolaryngol. Head Neck Surg. 1994, 120, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Sharbel, D.D.; Abkemeier, M.; Groves, M.W.; Albergotti, W.G.; Byrd, J.K.; Reyes-Gelves, C. Occult Metastasis in Laryngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Ann Otol. Rhinol. Laryngol. 2021, 130, 67–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Liu, W.; Wang, X.; Wang, K.; Liu, S.; Xu, Z.; Liu, J. Rational choice of neck dissection in clinically N0 patients with supraglottic cancer. Head Neck 2020, 42, 365–373. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2018; Volume 1, Available online: https://www.nccn.org/professionals/physician_glsf_guidelines.asp (accessed on 15 February 2018).
- Ferlito, A.; Rinaldo, A. Is radical neck dissection a current option for neck disease? Laryngoscope 2008, 118, 1717–1718. [Google Scholar] [CrossRef]
- Ozdek, A.; Sarac, S.; Akyol, M.U.; Unal, O.F.; Sungur, A. Histopathological predictors of occult lymph node metastases in supraglottic squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2000, 257, 389–392. [Google Scholar] [CrossRef]
- Mnejja, M.; Hammami, B.; Bougacha, L.; Chakroun, A.; Charfeddine, I.; Khabir, A.; Ghorbel, A. Occult lymph node metastasis in laryngeal squamous cell carcinoma: Therapeutic and prognostic impact. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2010, 127, 173–176. [Google Scholar] [CrossRef]
- Dias, F.L.; Lima, R.A.; Manfro, G.; Barbosa, M.M.; Salviano, S.; Rocha, R.M.; Kligerman, J. Management of the N0 neck in moderately advanced squamous cell carcinoma of the larynx. Otolaryngol. Head Neck Surg. 2009, 141, 59–65. [Google Scholar] [CrossRef]
- Yuce, I.; Cagli, S.; Bayram, A.; Guney, E. Occult metastases from T1-T2 supraglottic carcinoma: Role of primary tumor localization. Eur. Arch. Otorhinolaryngol. 2009, 266, 1301–1304. [Google Scholar] [CrossRef]
- Jia, S.; Wang, Y.; He, H.; Xiang, C. Incidence of level IIB lymph node metastasis in supraglottic laryngeal squamous cell carcinoma with clinically negative neck–a prospective study. Head Neck 2013, 35, 987–991. [Google Scholar] [CrossRef]
- Spriano, G.; Piantanida, R.; Pellini, R.; Muscatello, L. Elective treatment of the neck in squamous cell carcinoma of the larynx: Clinical experience. Head Neck 2003, 25, 97–102. [Google Scholar] [CrossRef] [PubMed]
- He, J.R.; Shen, G.P.; Ren, Z.F.; Qin, H.; Cui, C.; Zhang, Y.; Jia, W.H. Pretreatment levels of peripheral neutrophils and lymphocytes as independent prognostic factors in patients with nasopharyngeal carcinoma. Head Neck 2012, 34, 1769–1776. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, L.; Luo, M.; Hu, G.; Mei, Q.; Liu, D.; Hu, G. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma: Neutrophillymphocyte ratio and platelet-lymphocyte ratio. Head Neck 2016, 38 (Suppl. S1), E1332–E1340. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Chen, C.; Mai, Z.Y.; Gao, J.; Shen, L.J.; Zhao, B.C.; Xia, Y.F. Pretreatment platelet count as a predictor for survival and distant metastasis in nasopharyngeal carcinoma patients. Oncol. Lett. 2015, 9, 1458–1466. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donskov, F.; von der Maase, H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J. Clin. Oncol. 2006, 24, 1997–2005. [Google Scholar] [CrossRef]
- Krenn–Pilko, S.; Langsenlehner, U.; Stojakovic, T.; Pichler, M.; Gerger, A.; Kapp, K.S.; Langsenlehner, T. The elevated preoperative derived neutrophil-to-lymphocyte ratio predicts poor clinical outcome in breast cancer patients. Tumour Biol. 2016, 37, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Teramukai, S.; Kitano, T.; Kishida, Y.; Kawahara, M.; Kubota, K.; Komuta, K.; Fukushima, M. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: An analysis of Japan Multinational Trial Organisation LC00-03. Eur. J. Cancer 2009, 45, 1950–1958. [Google Scholar] [CrossRef]
- Templeton, A.J.; Pezaro, C.; Omlin, A.; McNamara, M.G.; Leibowitz-Amit, R.; Vera-Badillo, F.E.; Amir, E. Simple prognostic score for metastatic castration-resistant prostate cancer with incorporation of neutrophilto-lymphocyte ratio. Cancer 2014, 120, 3346–3352. [Google Scholar] [CrossRef] [PubMed]
- Halazun, K.J.; Hardy, M.A.; Rana, A.A.; Woodland, D.C., IV; Luyten, E.J.; Mahadev, S.; Emond, J.C. Negative impact of neutrophillymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann. Surg. 2009, 250, 141–151. [Google Scholar] [CrossRef]
- Clark, E.J.; Connor, S.; Taylor, M.A.; Madhavan, K.K.; Garden, O.J.; Parks, R.W. Preoperative lymphocytes count as a prognostic factor in resected pancreatic ductal adenocarcinoma. HPB 2007, 9, 456–460. [Google Scholar] [CrossRef]
- Wu, W.C.; Sun, H.W.; Chen, H.T.; Liang, J.; Yu, X.J.; Wu, C.; Zheng, L. Circulating hematopoietic stem and progenitor cell are myeloid-biased in cancer patients. Proc. Natl. Acad. Sci. USA 2014, 111, 4221–4226. [Google Scholar] [CrossRef] [PubMed]
- El-Hag, A.; Clark, R.A. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J. Immunol. 1987, 139, 2406–2413. [Google Scholar] [PubMed]
- Buergy, D.; Wenz, F.; Groden, C.; Brockmann, M.A. Tumor-platelet interaction in solid tumors. Int. J. Cancer 2012, 130, 2747–2760. [Google Scholar] [CrossRef] [PubMed]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Kum, R.O.; Ozcan, M.; Baklacı, D. Elevated neutrophil-tolymphocyte ratio in squamous cell carcinoma of larynx compared to benign and precancerous laryngeal lesions. Asian Pac. J. Cancer Prev. 2014, 15, 7351–7355. [Google Scholar] [CrossRef]

| N0 | N1 | N2a | N2b | N2c | |
|---|---|---|---|---|---|
| T1 | 8 | ||||
| T2 | 40 | 4 | 5 | 1 | 1 |
| T3 | 27 | 7 | 3 | 6 | 1 |
| T4 | 3 | 1 | 1 |
| N0 | N1 | N2a | N2b | N2c | |
|---|---|---|---|---|---|
| G1 | 1 | ||||
| G2 | 29 | 3 | 4 | 1 | |
| G3 | 48 | 9 | 5 | 6 | 2 |
| Factor | Estimate | 95% Confident Interval |
|---|---|---|
| InterceptNLR | −5.76 | −8.63; −2.89 |
| βNLR | 1.95 | 0.85; 3.04 |
| Coefficients | p Value | p Value at Kruskal–Wallis Test | |
|---|---|---|---|
| Intercept | −0.27 | 0.30 | 0.01 |
| NLR | 0.05 | 0.04 | |
| PLR | 0.00 | 0.93 | |
| T stage | 0.14 | 0.02 | |
| Grading | 0.01 | 0.90 | |
| Perineural invasion | 0.14 | 0.19 | |
| Lymphovascular invasion | −0.09 | 0.31 |
| NRL Linear Regression Model | Coefficients | p Value | AUC | Accuracy | Sensitivity | Specificity | PPV | NPV | Cut-Off |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 0.06 | 0.482 | 0.67 | 0.60 | 0.76 | 0.49 | 0.83 | 0.71 | 0.27 |
| NRL | 0.07 | 0.002 | |||||||
| PLR Linear Regression Model | Coefficients | p value | AUC | Accuracy | Sensitivity | Specificity | PPV | NPV | Cut-off |
| Intercept | 153.58 | 2.4 × 10−20 | 0.61 | 0.70 | 0.54 | 0.37 | 0.82 | 0.58 | 4378.12 |
| PLR | 34.22 | 0.18 | |||||||
| Linear regression model of all clinico-pathologic variables | 0.73 | 0.80 | 0.64 | 0.46 | 0.89 | 0.69 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salzano, G.; Perri, F.; Maglitto, F.; Togo, G.; De Fazio, G.R.; Apolito, M.; Calabria, F.; Laface, C.; Vaira, L.A.; Committeri, U.; et al. Pre-Treatment Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios as Predictors of Occult Cervical Metastasis in Clinically Negative Neck Supraglottic and Glottic Cancer. J. Pers. Med. 2021, 11, 1252. https://doi.org/10.3390/jpm11121252
Salzano G, Perri F, Maglitto F, Togo G, De Fazio GR, Apolito M, Calabria F, Laface C, Vaira LA, Committeri U, et al. Pre-Treatment Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios as Predictors of Occult Cervical Metastasis in Clinically Negative Neck Supraglottic and Glottic Cancer. Journal of Personalized Medicine. 2021; 11(12):1252. https://doi.org/10.3390/jpm11121252
Chicago/Turabian StyleSalzano, Giovanni, Francesco Perri, Fabio Maglitto, Giulia Togo, Gianluca Renato De Fazio, Michela Apolito, Federica Calabria, Claudia Laface, Luigi Angelo Vaira, Umberto Committeri, and et al. 2021. "Pre-Treatment Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios as Predictors of Occult Cervical Metastasis in Clinically Negative Neck Supraglottic and Glottic Cancer" Journal of Personalized Medicine 11, no. 12: 1252. https://doi.org/10.3390/jpm11121252
APA StyleSalzano, G., Perri, F., Maglitto, F., Togo, G., De Fazio, G. R., Apolito, M., Calabria, F., Laface, C., Vaira, L. A., Committeri, U., Balia, M., Pavone, E., Aversa, C., Salzano, F. A., Abbate, V., Ottaiano, A., Cascella, M., Santorsola, M., Fusco, R., ... Ionna, F. (2021). Pre-Treatment Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios as Predictors of Occult Cervical Metastasis in Clinically Negative Neck Supraglottic and Glottic Cancer. Journal of Personalized Medicine, 11(12), 1252. https://doi.org/10.3390/jpm11121252













