LASSO and Bioinformatics Analysis in the Identification of Key Genes for Prognostic Genes of Gynecologic Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microarray Data Mining in Gene Expression Omnibus (GEO)
2.2. Data Processing
2.3. Differentially Gene Expression Analysis
2.4. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis of DEGs
2.5. Feature Selection Using the LASSO Regression Model
2.6. Integration of Protein–Protein Interaction (PPI) Network
2.7. Validation of Hub Genes Using Survival Analysis
3. Results
3.1. Identification of the Common DEGs in Cervical and Endometrial Carcinoma
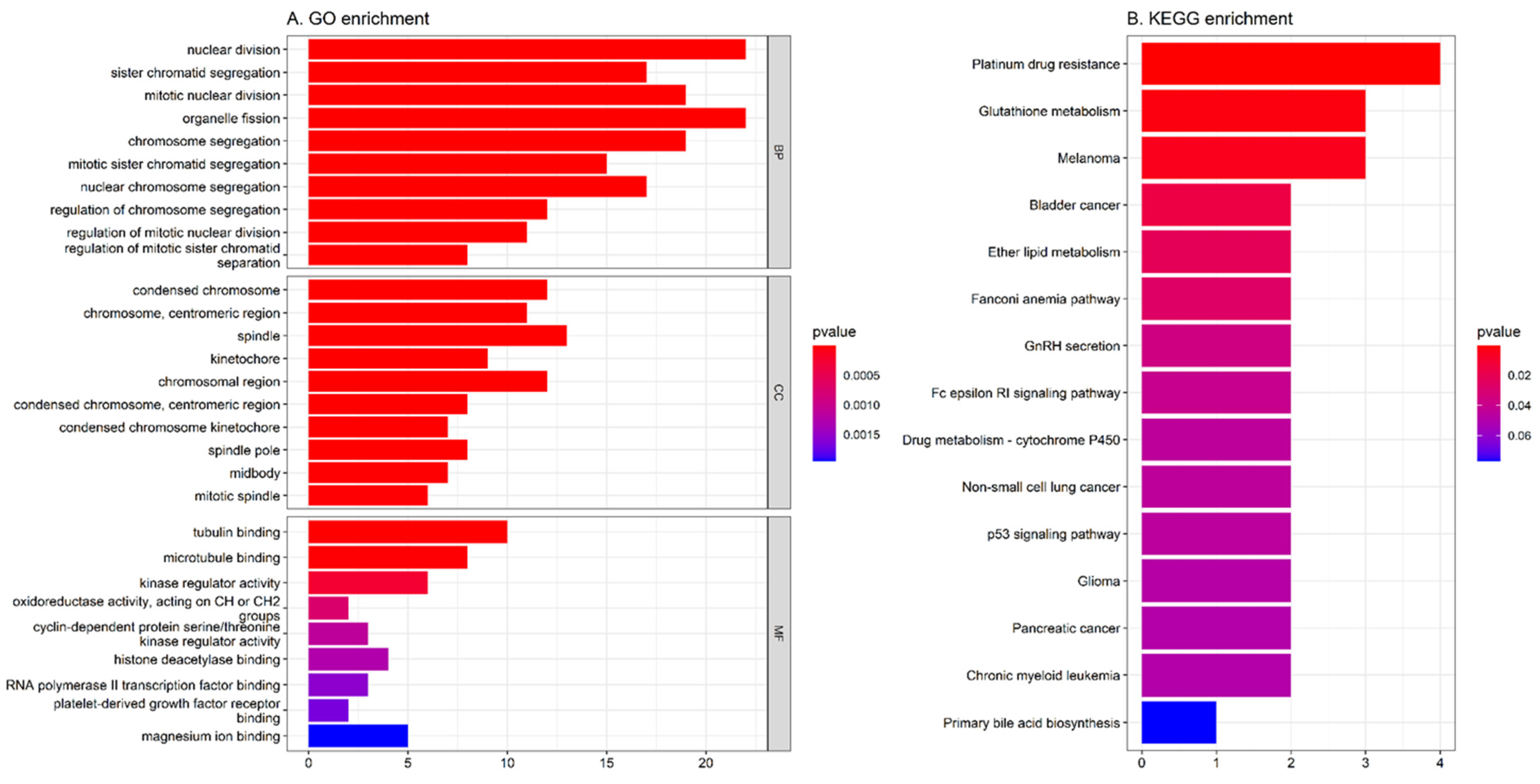
3.2. GO and KEGG Enrichment Analysis of the Common 78 DEGs
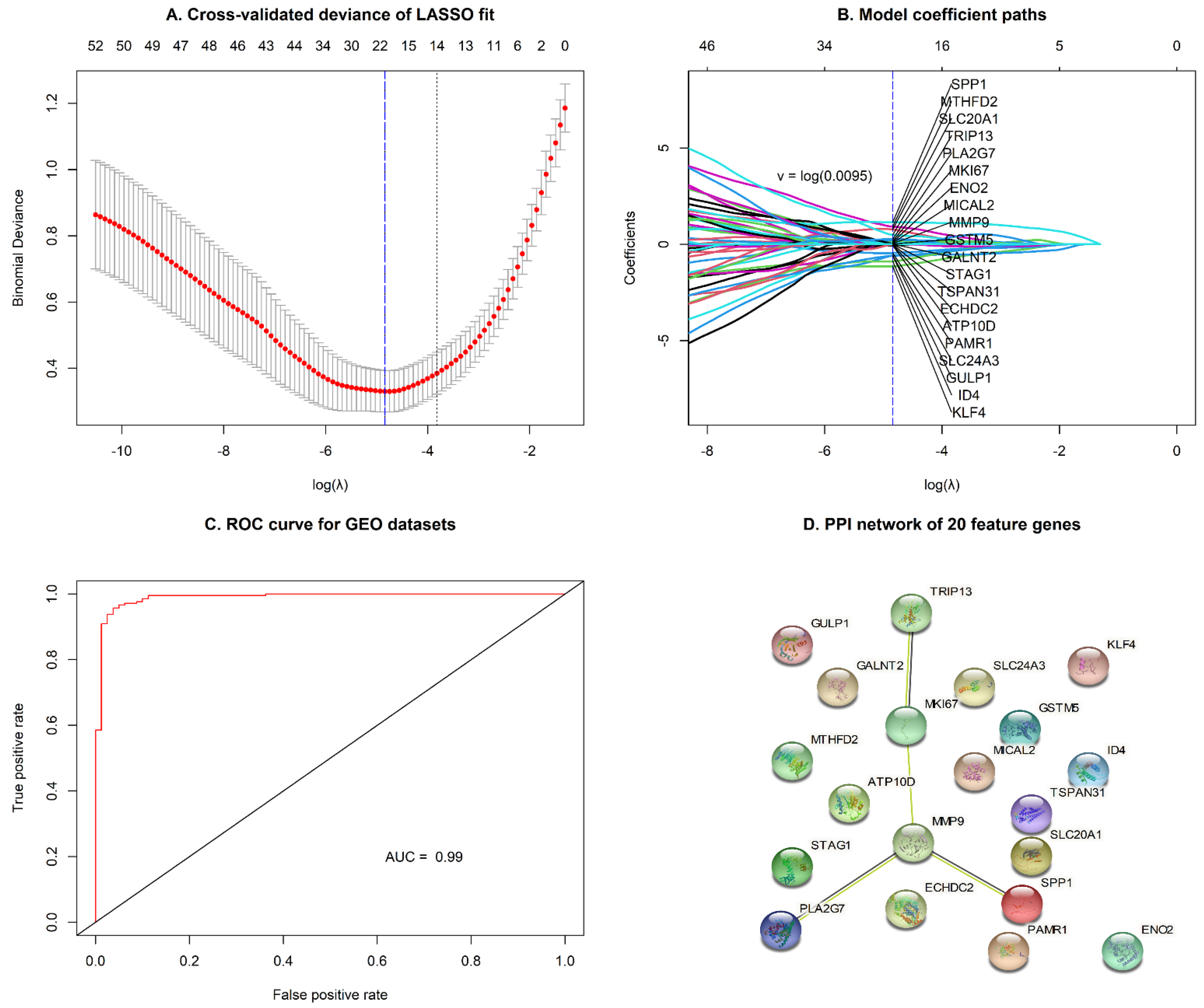
3.3. Selection of Significant Genes in Gynecologic Tumor Types Using the LASSO Regression Model
3.4. Verification of Prognostic Value for 20 Significant Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Momenimovahed, Z.; Salehiniya, H. Incidence, mortality and risk factors of cervical cancer in the world. Biomed. Res. Ther. 2017, 4, 1795–1811. [Google Scholar] [CrossRef]
- Diouf, D.; Diop, G.; Fall, C.; Sarr, S.; Diarra, C.A.T.; Ngom, A.I.; Ka, S.; Lo, S.; Faye, O.; Dem, A. The Association of Molecular Biomarkers in the Diagnosis of Cervical Pre-Cancer and Cancer and Risk Factors in Senegalese. Asian Pac. J. Cancer Prev. 2020, 21, 3221–3227. [Google Scholar] [CrossRef]
- Hernández, J.E.; González-Montiel, A.; Allos-Villalva, J.C.C.; Cantú, D.; Barquet, S.; Olivares-Mundo, A.; Herrera, L.A.; Prada, D. Prognostic molecular biomarkers in endometrial cancer: A review. J. Cancer Res. Ther. 2019, 7, 17–28. [Google Scholar]
- Bosse, T.; Lax, S.; Abu-Rustum, N.; Matias-Guiu, X. The Role of Predictive Biomarkers in Endocervical Adenocarcinoma: Recommendations From the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. 2021, 40, S102–S110. [Google Scholar] [CrossRef]
- Onyango, C.G.; Ogonda, L.; Guyah, B.; Shiluli, C.; Ganda, G.; Orang’o, O.E.; Patel, K. Novel biomarkers with promising benefits for diagnosis of cervical neoplasia: A systematic review. Infect. Agents Cancer 2020, 15, 68. [Google Scholar] [CrossRef]
- Malhone, C.; Longatto-Filho, A. Cervical, Ovarian and Endometrial Tumor Markers: Potential Clinical Value. Semin. Ultrasound CT MRI 2019, 40, 350–357. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Finocchario-Kessler, S.; Wexler, C.; Maloba, M.; Mabachi, N.; Ndikum-Moffor, F.; Bukusi, E. Cervical cancer prevention and treatment research in Africa: A systematic review from a public health perspective. BMC Women’s Health 2016, 16, 29. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Byun, S.J.; Kim, Y.S.; Nam, J.H. Disease courses in patients with residual tumor following concurrent chemoradiotherapy for locally advanced cervical cancer. Gynecol. Oncol. 2017, 144, 34–39. [Google Scholar] [CrossRef]
- Charo, L.M.; Plaxe, S.C. Recent advances in endometrial cancer: A review of key clinical trials from 2015 to 2019. F1000Research 2019, 8, F1000 Faculty Rev-849. [Google Scholar] [CrossRef]
- Fontham, E.T.H.; Wolf, A.M.D.; Church, T.R.; Etzioni, R.; Flowers, C.R.; Herzig, A.; Guerra, C.E.; Oeffinger, K.C.; Shih, Y.-C.T.; Walter, L.C. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA A Cancer J. Clin. 2020, 70, 321–346. [Google Scholar] [CrossRef]
- Duska, L.R.; Scalici, J.M.; Temkin, S.M.; Schwarz, J.K.; Crane, E.K.; Moxley, K.M.; Hamilton, C.A.; Wethington, S.L.; Petroni, G.R.; Varhegyi, N.E. Results of an early safety analysis of a study of the combination of pembrolizumab and pelvic chemoradiation in locally advanced cervical cancer. Cancer 2020, 126, 4948–4956. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, L.; Tong, R.; Yang, F.; Yin, L.; Li, M.; You, L.; Xue, J.; Lu, Y. PD-1/PD-L1 Inhibitors in Cervical Cancer. Front. Pharmacol. 2019, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Kim, M.; Lee, S.; Jung, W.; Kim, B. Therapeutic Potential of Natural Products in Treatment of Cervical Cancer: A Review. Nutrients 2021, 13, 154. [Google Scholar] [CrossRef]
- San Segundo-Val, I.; Sanz-Lozano, C.S. Introduction to the gene expression analysis. Mol. Genet. Asthma 2016, 29–43. [Google Scholar]
- Sonoda, K. Molecular biology of gynecological cancer (Review). Oncol. Lett. 2016, 11, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, S.G.; Capriglione, S.; Zito, G.; Lopez, S.; Gulino, F.A.; Di Guardo, F.; Vitagliano, A.; Noventa, M.; La Rosa, V.L.; Sapia, F. Management of endometrial, ovarian and cervical cancer in the elderly: Current approach to a challenging condition. Arch. Gynecol. Obstet. 2019, 299, 299–315. [Google Scholar] [CrossRef]
- Patel, S.; Liyanage, S.H.; Sahdev, A.; Rockall, A.G.; Reznek, R.H. Imaging of endometrial and cervical cancer. Insights Into Imaging 2010, 1, 309–328. [Google Scholar] [CrossRef] [Green Version]
- Kidd, A.C.; McGettrick, M.; Tsim, S.; Halligan, D.L.; Bylesjo, M.; Blyth, K.G. Survival prediction in mesothelioma using a scalable Lasso regression model: Instructions for use and initial performance using clinical predictors. BMJ Open Respir. Res. 2018, 5, e000240. [Google Scholar] [CrossRef]
- Muthukrishnan, R.; Rohini, R. LASSO: A feature selection technique in predictive modeling for machine learning. In Proceedings of the 2016 IEEE International Conference on Advances in Computer Applications (ICACA), Coimbatore, India, 24 October 2016; pp. 18–20. [Google Scholar]
- Gautier, L.; Irizarry, R.; Cope, L.; Bolstad, B. Description of Affy. Bioconductor Vignettes. 2012. Available online: https://www.bioconductor.org/packages/devel/bioc/vignettes/affy/inst/doc/affy.pdf (accessed on 3 July 2021).
- Gautier, L.; Cope, L.; Bolstad, B.M.; Irizarry, R.A. affy—Analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 2004, 20, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Xiong, D.; Ye, L.; Yang, H.; Mei, S.; Wu, J.; Chen, S.; Mi, R. SPP1 inhibition improves the cisplatin chemo-sensitivity of cervical cancer cell lines. Cancer Chemother. Pharmacol. 2019, 83, 603–613. [Google Scholar] [CrossRef]
- Annapurna, S.D.A.; Pasumarthi, D.; Pasha, A.; Doneti, R.; Sheela, B.; Botlagunta, M.; Vijaya, L.B.; Pawar, S.C. Identification of Differentially Expressed Genes in Cervical Cancer Patients by Comparative Transcriptome Analysis. BioMed Res. Int. 2021, 2021, 8810074. [Google Scholar]
- Nilsson, R.; Jain, M.; Madhusudhan, N.; Sheppard, N.G.; Strittmatter, L.; Kampf, C.; Huang, J.; Asplund, A.; Mootha, V.K. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat. Commun. 2014, 5, 3128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Huang, L.; Deng, L.; Lin, H.; Pan, W.; Pang, L. Identification of Candidate Biomarkers Associated with the Diagnosis and Prognosis of Endometrial Cancer: A Bioinformatics Analysis. 2021. Research Square. Available online: https://www.researchsquare.com/article/rs-318398/v1 (accessed on 3 July 2021).
- Liu, X.; Shen, X.; Zhang, J. TRIP13 exerts a cancer-promoting role in cervical cancer by enhancing Wnt/β-catenin signaling via ACTN4. Environ. Toxicol. 2021, 36, 1829–1840. [Google Scholar] [CrossRef]
- Lu, S.; Qian, J.; Guo, M.; Gu, C.; Yang, Y. Insights into a Crucial Role of TRIP13 in Human Cancer. Comput. Struct. Biotechnol. J. 2019, 17, 854–861. [Google Scholar] [CrossRef]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer (Review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef] [Green Version]
- Kitson, S.; Sivalingam, V.N.; Bolton, J.; McVey, R.; Nickkho-Amiry, M.; Powell, M.E.; Leary, A.; Nijman, H.W.; Nout, R.A.; Bosse, T. Ki-67 in endometrial cancer: Scoring optimization and prognostic relevance for window studies. Mod. Pathol. 2017, 30, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, T.; Zhang, B.; Yao, Y.; Yin, G. Matrix metalloproteinase-9 is a prognostic marker for patients with cervical cancer. Med. Oncol. 2012, 29, 3394–3399. [Google Scholar] [CrossRef]
- Tanaka, T.; Terai, Y.; Ohmichi, M. Association of matrix metalloproteinase-9 and decorin expression with the infiltration of cervical cancer. Oncol. Lett. 2019, 17, 1306–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.T.; Zheng, P.S. Krüppel-like factor 4 functions as a tumor suppressor in cervical carcinoma. Cancer 2012, 118, 3691–3702. [Google Scholar] [CrossRef]
- Simmons, C.D.; Pabona, J.M.P.; Heard, M.E.; Friedman, T.M.; Spataro, M.T.; Godley, A.L.; Simmen, F.A.; Burnett, A.F.; Simmen, R.C.M. Krüppel-Like Factor 9 Loss-of-Expression in Human Endometrial Carcinoma Links Altered Expression of Growth-Regulatory Genes with Aberrant Proliferative Response to Estrogen1. Biol. Reprod. 2011, 85, 378–385. [Google Scholar] [CrossRef]
- Lo, P.H.Y.; Tanikawa, C.; Katagiri, T.; Nakamura, Y.; Matsuda, K. Identification of novel epigenetically inactivated gene PAMR1 in breast carcinoma. Oncol. Rep. 2015, 33, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Ma, M.; Yu, S.; Li, X.; Zhang, J.; Wu, S. High Expression of PAMR1 Predicts Favorable Prognosis and Inhibits Proliferation, Invasion, and Migration in Cervical Cancer. Front. Oncol. 2021, 11, 742017. [Google Scholar] [CrossRef] [PubMed]
- Makoukji, J.; Makhoul, N.; Khalil, M.; El-Sitt, S.; Aldin, E.; Jabbour, M.; Boulos, F.; Gadaleta, E.; Sangaralingam, A.; Chelala, C. Gene expression profiling of breast cancer in Lebanese women. Sci. Rep. 2016, 6, 36639. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xu, X.; Li, P.; Zhou, F.; Kong, L.; Qiu, J.; Yuan, Z.; Tan, J. TMT Based Proteomic Analysis of Human Follicular Fluid from Overweight/Obese and Normal-Weight Patients With Polycystic Ovary Syndrome. Front. Endocrinol. 2019, 10, 821. [Google Scholar] [CrossRef]
- Tible, M.; Loupy, A.; Vernerey, D.; Suberbielle, C.; Beuscart, T.; Cazes, A.; Guillemain, R.; Amrein, C.; Pezzella, V.; Fabiani, J.-N. Pathologic classification of antibody-mediated rejection correlates with donor-specific antibodies and endothelial cell activation. J. Heart Lung Transplant. 2013, 32, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.N.; Go, S.M.; Park, S.M.; Jung, E.M.; Jeung, E.B. Loss of Nckx3 Exacerbates Experimental DSS-Induced Colitis in Mice through p53/NF-κB Pathway. Int. J. Mol. Sci. 2021, 22, 2645. [Google Scholar] [CrossRef]
- Jalloul, A.H.; Szerencsei, R.T.; Schnetkamp, P.P.M. Cation dependencies and turnover rates of the human K+-dependent Na+-Ca2+ exchangers NCKX1, NCKX2, NCKX3 and NCKX4. Cell Calcium 2016, 59, 1–11. [Google Scholar] [CrossRef]
- Jeung, E.-B. 189 the Calcium Exchangers Nckx3 and Ncx1 Are Distinctly Expressed and Regulated by Steroids in the Human Endometrium during the Menstrual Cycle. Reprod. Fertil. Dev. 2010, 23, 195–196. [Google Scholar] [CrossRef]
- Yang, H.; Kim, T.H.; Lee, H.H.; Choi, K.C.; Jeung, E.B. Distinct expression of the calcium exchangers, NCKX3 and NCX1, and their regulation by steroid in the human endometrium during the menstrual cycle. Reprod. Sci. 2011, 18, 577–585. [Google Scholar] [CrossRef]






| Datasets | Tissues | Tumor | Normal | Platform |
|---|---|---|---|---|
| GSE9750 | cervix | 33 | 24 | GPL96 |
| GSE7803 | 21 | 10 | GPL96 | |
| GSE63514 | 28 | 24 | GPL570 | |
| GSE17025 | endometrium | 91 | 12 | GPL570 |
| GSE115810 | 24 | 3 | GPL96 | |
| GSE36389 | 13 | 7 | GPL96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.-H.; Cai, J.-H.; Chen, D.-L.; Liao, S.-H.; Lin, Y.-Z.; Chung, Y.-T.; Tsai, J.J.P.; Wang, C.C.N. LASSO and Bioinformatics Analysis in the Identification of Key Genes for Prognostic Genes of Gynecologic Cancer. J. Pers. Med. 2021, 11, 1177. https://doi.org/10.3390/jpm11111177
Yu S-H, Cai J-H, Chen D-L, Liao S-H, Lin Y-Z, Chung Y-T, Tsai JJP, Wang CCN. LASSO and Bioinformatics Analysis in the Identification of Key Genes for Prognostic Genes of Gynecologic Cancer. Journal of Personalized Medicine. 2021; 11(11):1177. https://doi.org/10.3390/jpm11111177
Chicago/Turabian StyleYu, Shao-Hua, Jia-Hua Cai, De-Lun Chen, Szu-Han Liao, Yi-Zhen Lin, Yu-Ting Chung, Jeffrey J. P. Tsai, and Charles C. N. Wang. 2021. "LASSO and Bioinformatics Analysis in the Identification of Key Genes for Prognostic Genes of Gynecologic Cancer" Journal of Personalized Medicine 11, no. 11: 1177. https://doi.org/10.3390/jpm11111177
APA StyleYu, S.-H., Cai, J.-H., Chen, D.-L., Liao, S.-H., Lin, Y.-Z., Chung, Y.-T., Tsai, J. J. P., & Wang, C. C. N. (2021). LASSO and Bioinformatics Analysis in the Identification of Key Genes for Prognostic Genes of Gynecologic Cancer. Journal of Personalized Medicine, 11(11), 1177. https://doi.org/10.3390/jpm11111177






