Very Long-Term Follow-Up in Cardiac Resynchronization Therapy: Wider Paced QRS Equals Worse Prognosis
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design and Data Collection
2.2. Initial ECG Recordings
2.3. Definition of CRT Responders
2.4. Definition of Ischemic Cardiomyopathy vs. Non-Ischemic Cardiomyopathy
2.5. Long-Term Follow-Up Collection
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Follow-Up Duration
3.3. Adverse Events
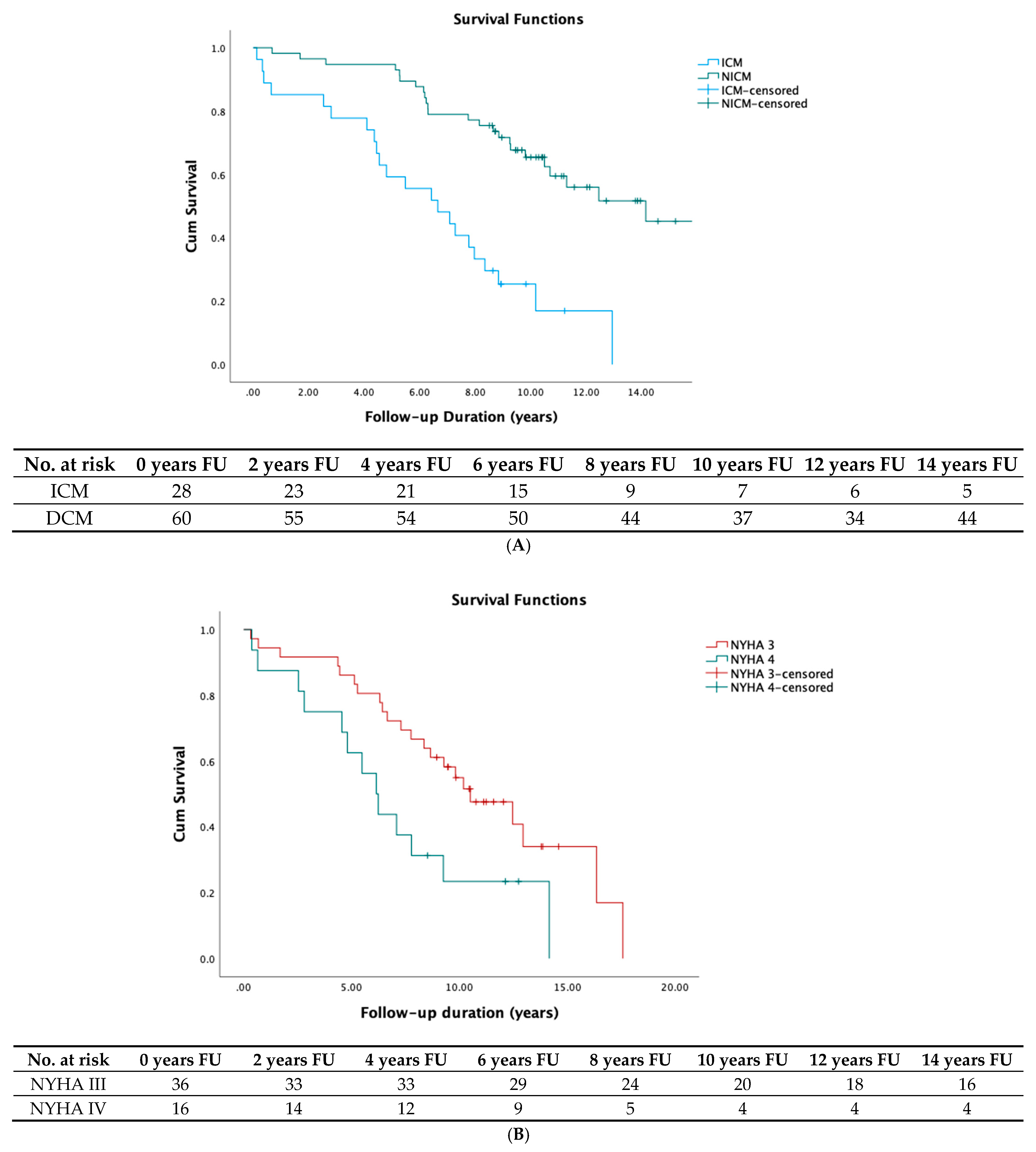
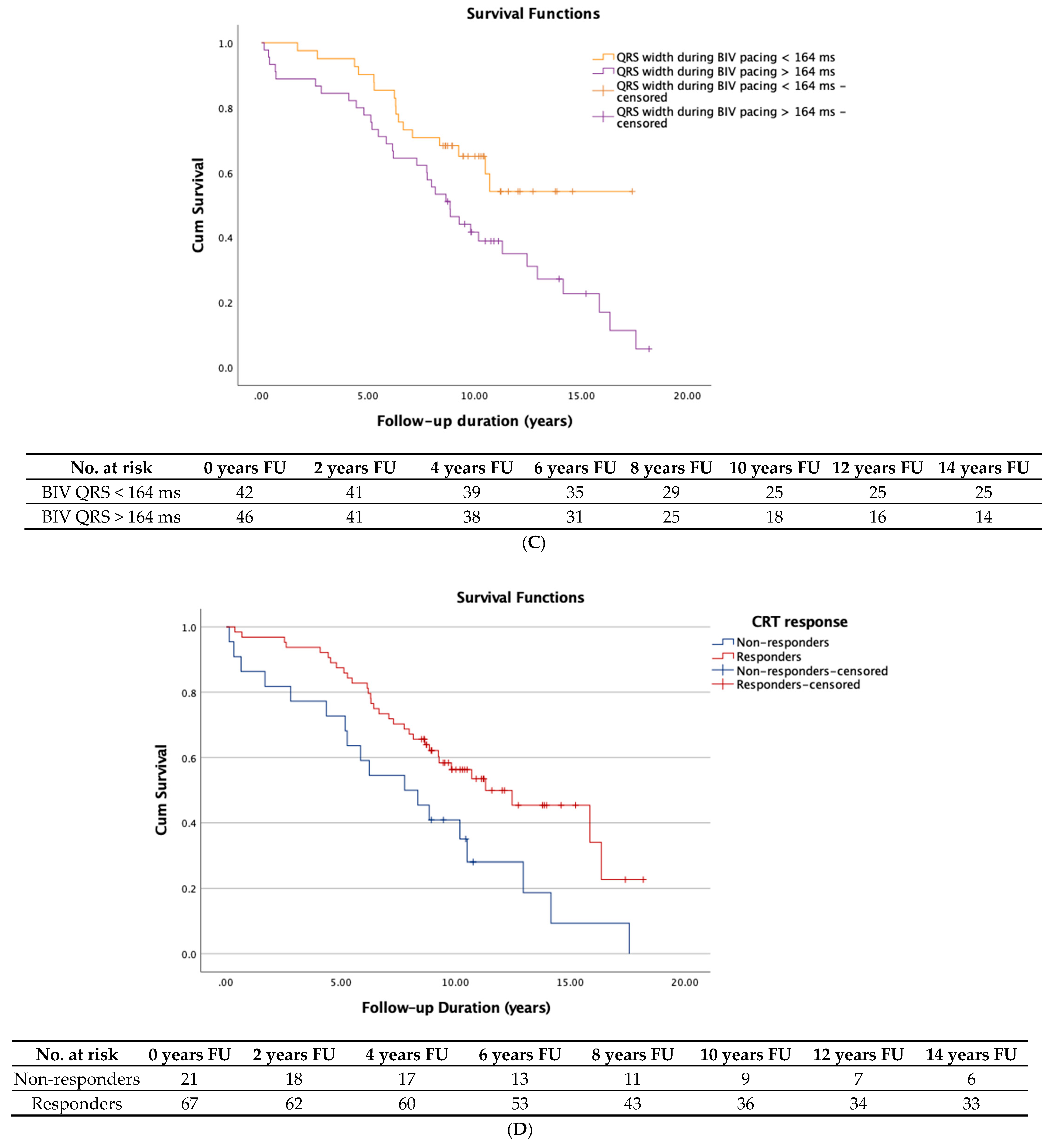
3.4. Survival
3.5. Possible Predictors of Long-Term Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef]
- Bristow, M.R.; Krueger, S.; Carson, P.; White, B.G. Cardiac-Resynchronization Therapy with or without an Implantable Defibrillator in Advanced Chronic Heart Failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Erdmann, E.; Kappenberger, L. The Effect of Cardiac Resynchronization on Morbidity and Mortality in Heart Failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef] [Green Version]
- Köbe, J.; Dechering, D.G.; Rath, B.; Reinke, F.; Mönnig, G.; Wasmer, K.; Eckardt, L. Prospective evaluation of electrocardiographic parameters in cardiac resynchronization therapy: Detecting nonresponders by left ventricular pacing. Heart Rhythm 2012, 9, 499–504. [Google Scholar] [CrossRef]
- Appert, L.; Menet, A.; Altes, A.; Ennezat, P.V.; Bardet-Bouchery, H.; Binda, C.; Guyomar, Y.; Delelis, F.; Castel, A.-L.; Goffic, C.L.; et al. Clinical Significance of Electromechanical Dyssynchrony and QRS Narrowing in Patients With Heart Failure Receiving Cardiac Resynchronization Therapy. Can. J. Cardiol. 2019, 35, 27–34. [Google Scholar] [CrossRef]
- Dickstein, K.; Vardas, P.E.; Auricchio, A.; Daubert, J.C.; Linde, C.; McMurray, J.; Ponikowski, P.; Priori, S.G.; Sutton, R.; van Veldhuisen, D.J.; et al. 2010 Focused Update of ESC Guidelines on device therapy in heart failure: An update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Eur. Heart J. 2010, 12, 1526–1536. [Google Scholar]
- Kutyifa, V.; Breithardt, O.A. How to Assess the Nonresponder to Cardiac Resynchronization Therapy—A Comprehensive Stepwise Approach. Rev. Esp. Cardiol. Engl. Ed. 2012, 65, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Rickard, J.; Popovic, Z.; Verhaert, D.; Sraow, D.; Baranowski, B.; Martin, D.O.; Lindsay, B.D.; Varma, N.; Tchou, P.; Grimm, R.A.; et al. The QRS Narrowing Index Predicts Reverse Left Ventricular Remodeling Following Cardiac Resynchronization Therapy. Pacing Clin. Electrophysiol. 2011, 34, 604–611. [Google Scholar] [CrossRef]
- Bryant, A.R.; Wilton, S.B.; Lai, M.P.; Exner, D.V. Association between QRS duration and outcome with cardiac resynchronization therapy: A systematic review and meta-analysis. J. Electrocardiol. 2013, 46, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Bazoukis, G.; Naka, K.K.; Alsheikh-Ali, A.; Tse, G.; Letsas, K.P.; Korantzopoulos, P.; Liu, T.; Yeung, C.; Efremidis, M.; Tsioufis, K.; et al. Association of QRS narrowing with response to cardiac resynchronization therapy—A systematic review and meta-analysis of observational studies. Heart Fail. Rev. 2020, 25, 745–756. [Google Scholar] [CrossRef]
- Ter Horst, I.A.; Bogaard, M.D.; Tuinenburg, A.E.; Mast, T.P.; de Boer, T.P.; Doevendans, P.A.; Meine, M. The concept of triple wavefront fusion during biventricular pacing: Using the EGM to produce the best acute hemodynamic improvement in CRT. Pacing Clin. Electrophysiol. 2017, 40, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Varma, N.; O’Donnell, D.; Bassiouny, M.; Ritter, P.; Pappone, C.; Mangual, J.; Cantillon, D.; Mangual, J.; Cantillon, D.; Badie, N. Programming Cardiac Resynchronization Therapy for Electrical Synchrony: Reaching Beyond Left Bundle Branch Block and Left Ventricular Activation Delay. J. Am. Heart Assoc. 2018, 7, e007489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jastrzębski, M.; Baranchuk, A.; Fijorek, K.; Kisiel, R.; Kukla, P.; Sondej, T.; Czarnecka, D. Cardiac resynchronization therapy-induced acute shortening of QRS duration predicts long-term mortality only in patients with left bundle branch block. EP Eur. 2019, 21, 281–289. [Google Scholar] [CrossRef]
- Schuchert, A.; Muto, C.; Maounis, T.; Frank, R.; Ella, R.O.; Polauck, A.; Padeletti, L. One-year outcome after CRT implantation in NYHA class IV in comparison to NYHA class III patients. Clin. Res. Cardiol. 2013, 102, 505–511. [Google Scholar] [CrossRef] [PubMed]
- McLeod, C.J.; Shen, W.K.; Rea, R.F.; Friedman, P.A.; Hayes, D.L.; Wokhlu, A.; Webster, T.L.; Wiste, H.J.; Hodge, D.O.; Bradley, D.J.; et al. Differential outcome of cardiac resynchronization therapy in ischemic cardiomyopathy and idiopathic dilated cardiomyopathy. Heart Rhythm 2011, 8, 377–382. [Google Scholar] [CrossRef]
- Mangiavacchi, M.; Gasparini, M.; Faletra, F.; Klersy, C.; Morenghi, E.; Galimberti, P.; Genovese, L.; Regoli, F.; De Chiara, F.; Bragato, R.; et al. Clinical predictors of marked improvement in left ventricular performance after cardiac resynchronization therapy in patients with chronic heart failure. Am. Heart J. 2006, 151, 477.e1–477.e6. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Abraham, W.T.; Linde, C.; Gold, M.R.; Young, J.B.; Claude Daubert, J.; Sherfesee, L.; Wells, G.A.; Tang, A.S.L. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur. Heart J. 2013, 34, 3547–3556. [Google Scholar] [CrossRef] [Green Version]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Mark Estes, N.A., III; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-Resynchronization Therapy for the Prevention of Heart-Failure Events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef] [Green Version]
- Barsheshet, A.; Goldenberg, I.; Moss, A.J.; Eldar, M.; Huang, D.T.; McNitt, S.; Klein, H.U.; Hall, W.J.; Brown, M.W.; Goldberger, J.J.; et al. Response to preventive cardiac resynchronization therapy in patients with ischaemic and nonischaemic cardiomyopathy in MADIT-CRT. Eur. Heart J. 2011, 32, 1622–1630. [Google Scholar] [CrossRef]
- Goldenberg, I.; Kutyifa, V.; Klein, H.U.; Cannom, D.S.; Brown, M.W.; Dan, A.; Daubert, J.P.; Mark Estes, N.A.; Foster, E.; Greenberg, H.; et al. Survival with Cardiac-Resynchronization Therapy in Mild Heart Failure. N. Engl. J. Med. 2014, 370, 1694–1701. [Google Scholar] [CrossRef] [Green Version]
- McMurray, J.J.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [Green Version]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornwalt, B.K.; Sprague, W.W.; BeDell, P.; Suever, J.D.; Gerritse, B.; Merlino, J.D.; Fyfe, D.A.; León, A.R.; Oshinski, J.N. Agreement Is Poor Among Current Criteria Used to Define Response to Cardiac Resynchronization Therapy. Circulation 2010, 121, 1985–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boidol, J.; Średniawa, B.; Kowalski, O.; Szulik, M.; Mazurek, M.; Sokal, A.; Pruszkowska-Skrzep, P.; Kukulski, T.; Kalarus, Z.; Lenarczyk, R. Triple-Site Versus Standard Cardiac Resynchronisation Trial (TRUST CRT) Investigators. Many response criteria are poor predictors of outcomes after cardiac resynchronization therapy: Validation using data from the randomized trial. Europace 2013, 15, 835–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landolina, M.; Gasparini, M.; Lunati, M.; Iacopino, S.; Boriani, G.; Bonanno, C.; Vado, A.; Roclemer, A.; Capucci, A.; Zucchiatti, C.; et al. Long-Term Complications Related to Biventricular Defibrillator Implantation. Circulation 2011, 123, 2526–2535. [Google Scholar] [CrossRef] [PubMed]
 : in person physician contact;
: in person physician contact;  : telephone physician contact; ECHO: trans-thoracic echocardiogram;
: telephone physician contact; ECHO: trans-thoracic echocardiogram;  : electrocardiogram;
: electrocardiogram;  : Minnesota Living with Heart Failure Questionnaire;
: Minnesota Living with Heart Failure Questionnaire;  : Device interrogation;
: Device interrogation;  : Interrogation on current NYHA class, hospitalizations;
: Interrogation on current NYHA class, hospitalizations;  : Deceased;
: Deceased;  : End of data collection and start statistical analysis).
: End of data collection and start statistical analysis).
 : in person physician contact;
: in person physician contact;  : telephone physician contact; ECHO: trans-thoracic echocardiogram;
: telephone physician contact; ECHO: trans-thoracic echocardiogram;  : electrocardiogram;
: electrocardiogram;  : Minnesota Living with Heart Failure Questionnaire;
: Minnesota Living with Heart Failure Questionnaire;  : Device interrogation;
: Device interrogation;  : Interrogation on current NYHA class, hospitalizations;
: Interrogation on current NYHA class, hospitalizations;  : Deceased;
: Deceased;  : End of data collection and start statistical analysis).
: End of data collection and start statistical analysis).
| Survivors n = 36 | Deceased n = 52 | Entire Population n = 102 | p-Value | |
|---|---|---|---|---|
| Age (Years) | 70 ± 10 | 76 ± 9 | 74 ± 10 | 0.03 |
| Weight (kg) | 86 ± 16 | 86 ± 19 | 85 ± 19 | 0.56 |
| NYHA Class | 2.7 ± 1 | 3.17 ± 1 | 3.4 ± 1.2 | <0.001 |
| CHA2DS2-Vasc | 3.4 ± 2.7 | 5.5 ± 2.5 | 4.7 ± 2.7 | <0.001 |
| GFR (mL/min) | 56.7 ± 6.7 | 48.3 ± 12.8 | 51.4 ± 11.6 | 0.007 |
| Atrial Fibrillation (%) | 47.4 | 49.1 | 47 | 0.94 |
| Arterial Hypertension (%) | 50 | 61.8 | 62 | 0.116 |
| Type II Diabetes (%) | 26.3 | 29.1 | 28 | 0.818 |
| Coronary artery disease (%) | 23.7 | 60 | 45 | 0.01 |
| NICM (%) | 86.5 | 51.9 | 67.3 | 0.01 |
| Male Gender (%) | 65.8 | 85.5 | 75 | 0.042 |
| Clinical Responder (%) | 89.5 | 67.3 | 77.5 | 0.014 |
| LVEF (%) | 30.7 ± 12.3 | 28.8 ±7.7 | 29.4 ± 9.7 | 0.77 |
| NT-proBNP (ng/L) | 1534 ± 1781 | 5339 ± 7676 | 3658 ± 6086 | 0.02 |
| No pacing (ms) | 162.6 ± 33 | 170.3 ± 34.8 | 166.6 ± 33.3 | 0.173 |
| BIV pacing (ms) | 149.3 ± 27.8 | 173.7 ± 30.2 | 162.0 ± 31.4 | <0.001 |
| LV pacing (ms) | 177.5 ± 33.8 | 202 ± 40.4 | 191.5 ± 38.4 | <0.001 |
| RV pacing (ms) | 199.9 ± 28.3 | 210.7 ± 34.7 | 205.7 ± 32.4 | 0.035 |
| Δ LV paced–RV paced (ms) | −22.4 ± 37.2 | −8.7 ± 30.8 | −14.5 ± 33.6 | 0.078 |
| Primary Endpoint | Primary Endpoint | Secondary Endpoint | Secondary Endpoint | |
|---|---|---|---|---|
| p-Value Univariate Cox Regression {HR (95% CI)} | p-Value Multivariate Cox Regression {HR (95% CI)} | p-Value Univariate Cox Regression {HR (95% CI)} | p-Value Multivariate Cox Regression {HR (95% CI)} | |
| QRS width with no pacing | 0.09 {1.01(0.99–1.02)} | 0.3 {1.00(1.00–1.01)} | ||
| QRS width during BIV pacing | 0.02 {2.51(1.39–4.57)} | 0.01 {3.89(1.36–11.14)} | 0.05 {1.57(1.00–2.49)} | |
| QRS width during LV pacing | <0.001 {1.01(1.01–1.02)} | 0.006 {1.01(1.00–1.02)} | ||
| QRS width during RV pacing | 0.09 {1.01(1.00–1.02)} | 0.12 {1.01(0.99–1.01)} | ||
| ΔQRS width during LV paced– QRS width during RV paced | 0.03 {1.01(1.00–1.02)} | 0.19 {1.00(0.99–1.01)} | ||
| Age (Years) | 0.03 {1.04 (1.00–1.07} | 0.07 {1.02(0.99–1.04)} | ||
| Male Gender (%) | 0.074 {0.48(0.21–1.07)} | 0.1 {0.63(0.36–1.09)} | ||
| Weight (kg) | 0.52 {0.99 (0.98–1.01)} | 0.56 {1.00(0.99–1.02)} | ||
| NYHA Class | <0.001 {2.40 (1.40–4.13)} | 0.03 {2.46(1.08–5.58)} | 0.01 {1.68(1.31–2.48)} | 0.04 {1.71(1.02–2.88)} |
| CHA2DS2-Vasc | <0.001 {1.24(1.10–1.40)} | 0.07 {1.13(1.03–1.23)} | ||
| GFR (ml/min) | 0.007 {0.96(0.93–0.98)} | 0.19 {0.98(0.96–1.01)} | ||
| Atrial Fibrillation (%) | 0.94 {0.75(−0.53–0.69)} | 0.97{1.01(0.64–1.59)} | ||
| Arterial Hypertension (%) | 0.004 {1.62(1.17–2.25)} | 0.11 {1.31(0.94–1.81)} | ||
| Type II Diabetes (%) | 0.818 {0.94(−0.70–0.63)} | 0.922 {1.03(0.62–1.70)} | ||
| Coronary artery disease (%) | 0.001 {2.81(1.56–5.05)} | 0.002 {2.04(1.29–3.23)} | ||
| NICM (%) | 0.01 {0.31(0.17–0.56)} | 0.003 {0.23(0.09–0.60)} | 0.004 {0.49(0.30–0.80)} | |
| Clinical Responder (%) | 0.008 {0.44(0.24–0.81)} | 0.77 {0.85(0.27–2.63)} | 0.03 {0.56(0.32–0.95)} | |
| LVEF (%) | 0.56 {0.99(0.95–1.03)} | 0.30 {0.99(0.96–1.01)} | ||
| NT-proBNP (ng/L) | <0.001 {1.0(1.0–1.0)} | <0.001 {1.0(1.0–1.0)} |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitz, P.; Köbe, J.; Rath, B.; Reinke, F.; Frommeyer, G.; Andresen, C.; Güner, F.; Wolfes, J.; Lange, P.S.; Ellermann, C.; et al. Very Long-Term Follow-Up in Cardiac Resynchronization Therapy: Wider Paced QRS Equals Worse Prognosis. J. Pers. Med. 2021, 11, 1176. https://doi.org/10.3390/jpm11111176
Leitz P, Köbe J, Rath B, Reinke F, Frommeyer G, Andresen C, Güner F, Wolfes J, Lange PS, Ellermann C, et al. Very Long-Term Follow-Up in Cardiac Resynchronization Therapy: Wider Paced QRS Equals Worse Prognosis. Journal of Personalized Medicine. 2021; 11(11):1176. https://doi.org/10.3390/jpm11111176
Chicago/Turabian StyleLeitz, Patrick, Julia Köbe, Benjamin Rath, Florian Reinke, Gerrit Frommeyer, Christian Andresen, Fatih Güner, Julian Wolfes, Philipp S. Lange, Christian Ellermann, and et al. 2021. "Very Long-Term Follow-Up in Cardiac Resynchronization Therapy: Wider Paced QRS Equals Worse Prognosis" Journal of Personalized Medicine 11, no. 11: 1176. https://doi.org/10.3390/jpm11111176
APA StyleLeitz, P., Köbe, J., Rath, B., Reinke, F., Frommeyer, G., Andresen, C., Güner, F., Wolfes, J., Lange, P. S., Ellermann, C., Eckardt, L., & Dechering, D. G. (2021). Very Long-Term Follow-Up in Cardiac Resynchronization Therapy: Wider Paced QRS Equals Worse Prognosis. Journal of Personalized Medicine, 11(11), 1176. https://doi.org/10.3390/jpm11111176







