Association between Lipid Levels and Risk for Different Types of Aneurysms: A Mendelian Randomization Study
Abstract
:1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Study Design
2.3. Instrument Identification
2.4. Summary-Level Genetic Data on Aneurysms
2.5. Two-Sample MR Analyses
3. Results
3.1. Genetic Instruments for Lipid Traits
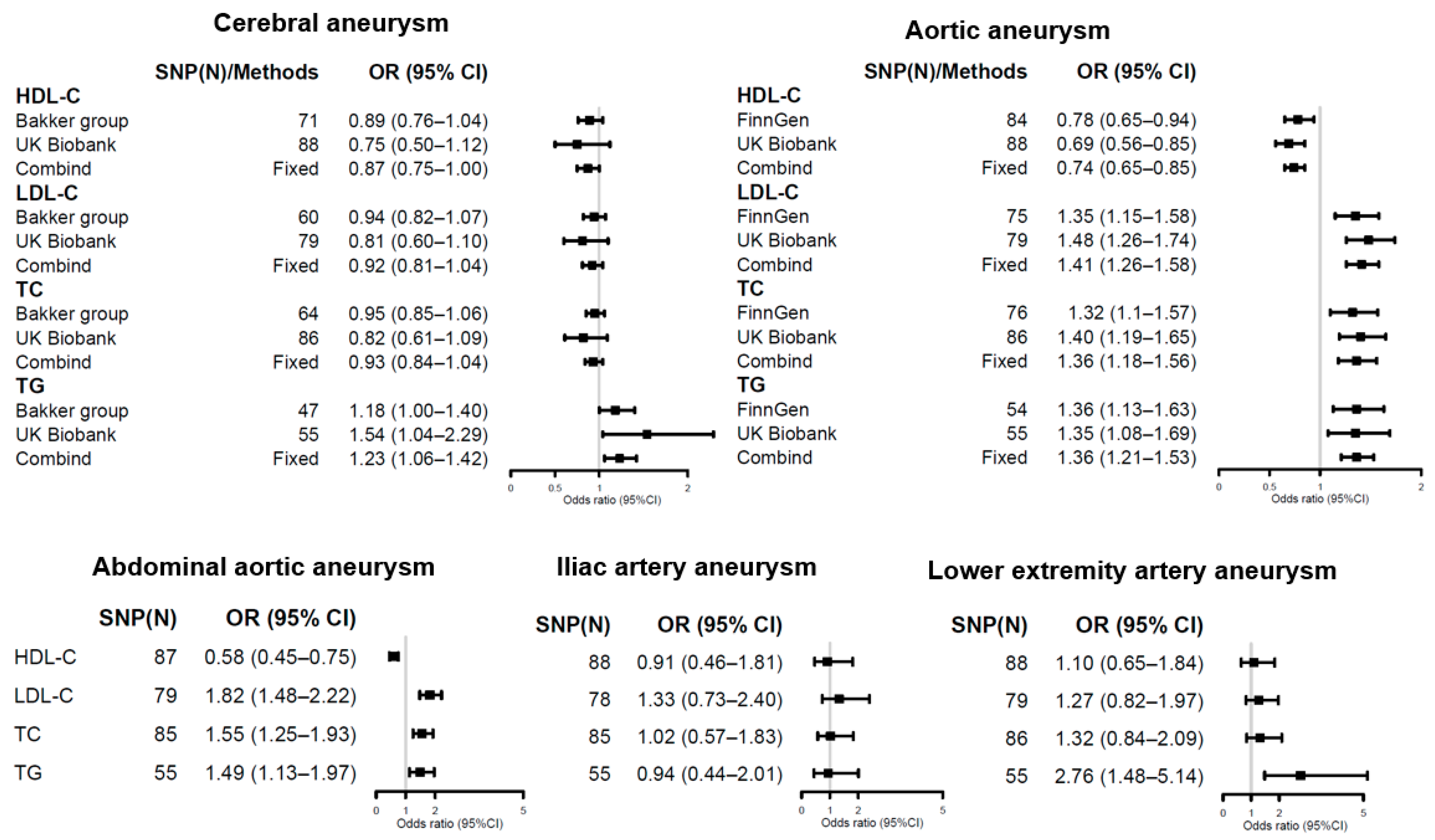
3.2. Causal Estimates for Lipid Traits on Aneurysm Risks
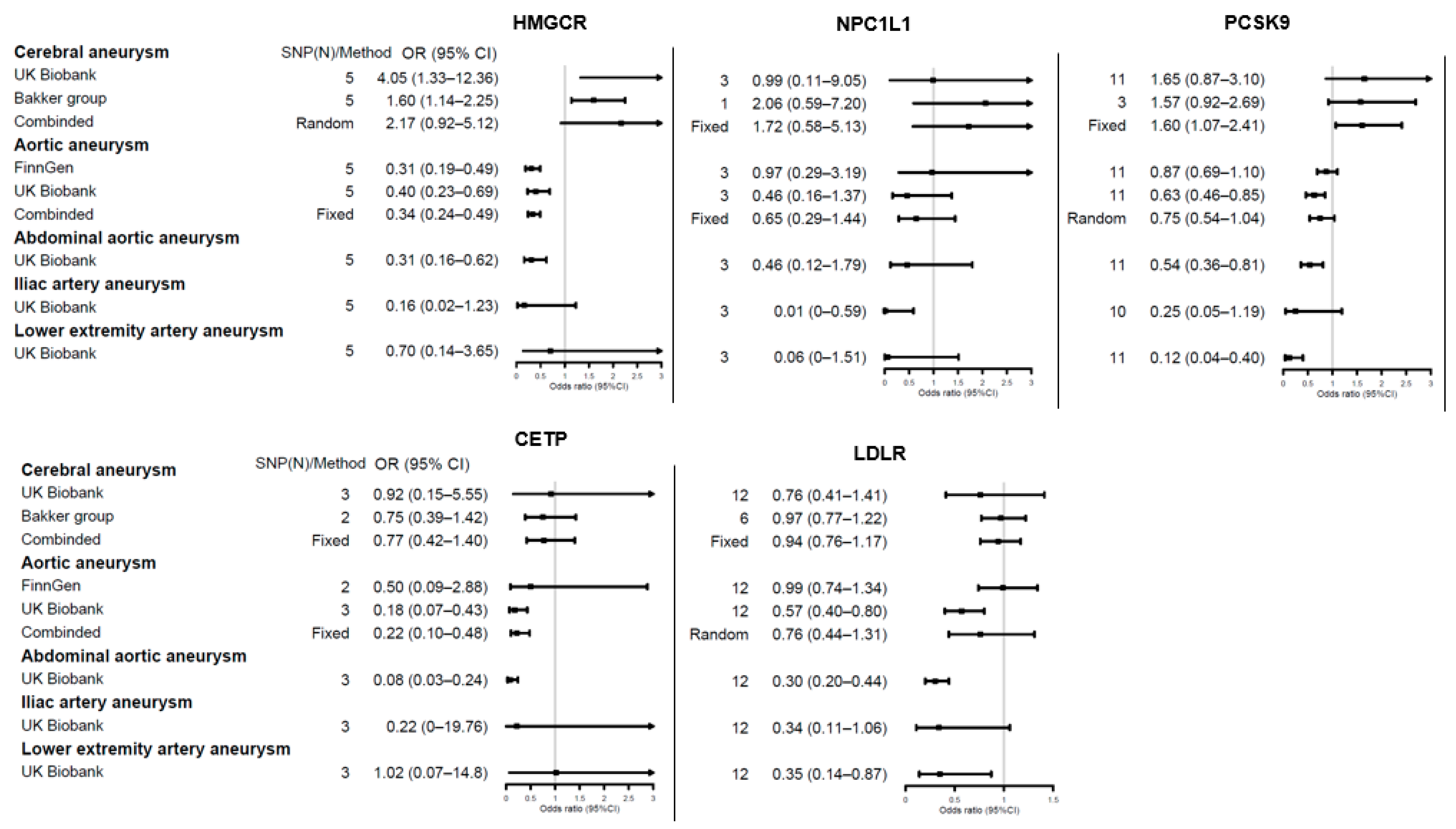
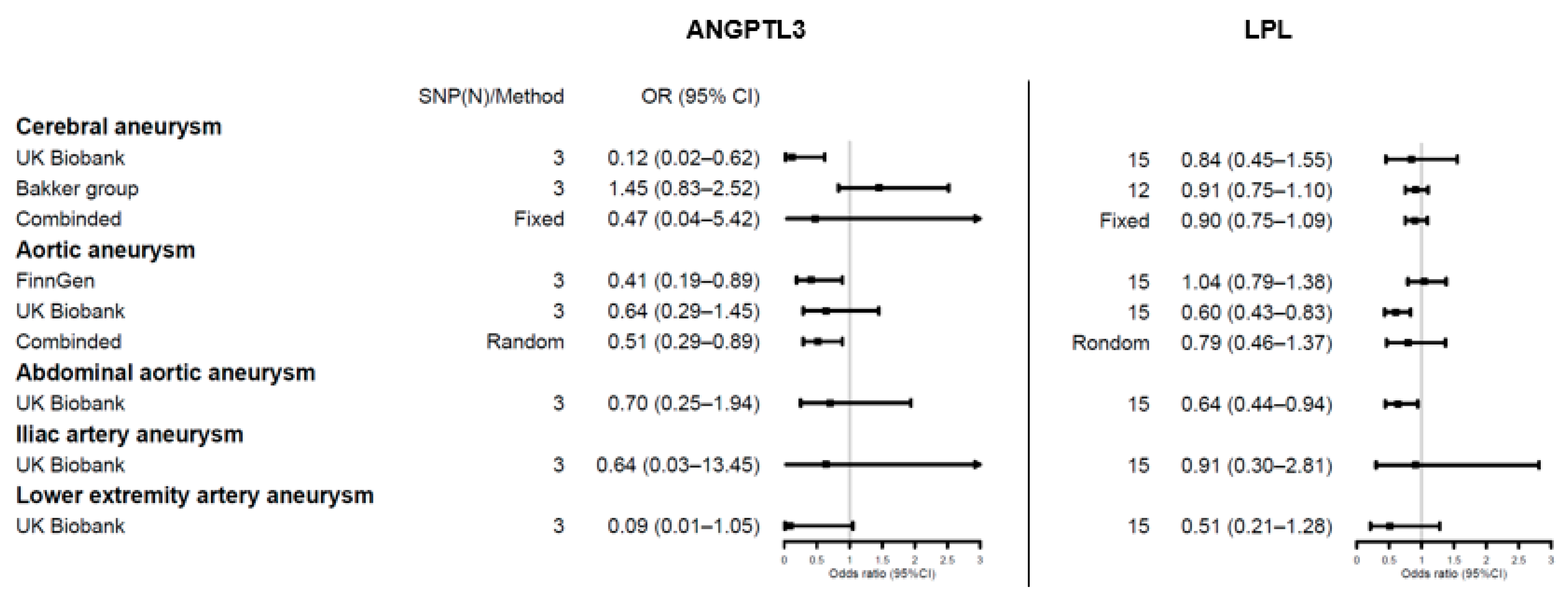
3.3. Genetic Proxies for Lipid Drug Targets and Aneurysm Risk
4. Discussion
4.1. Controversial Role of Lipid TRAITS in Cerebral Aneurysms
4.2. Lipid Dysfunction in Aortic Aneurysms: From Etiology to Drug Therapy
4.3. Limited Evidence on Other Aneurysms
5. Limitations
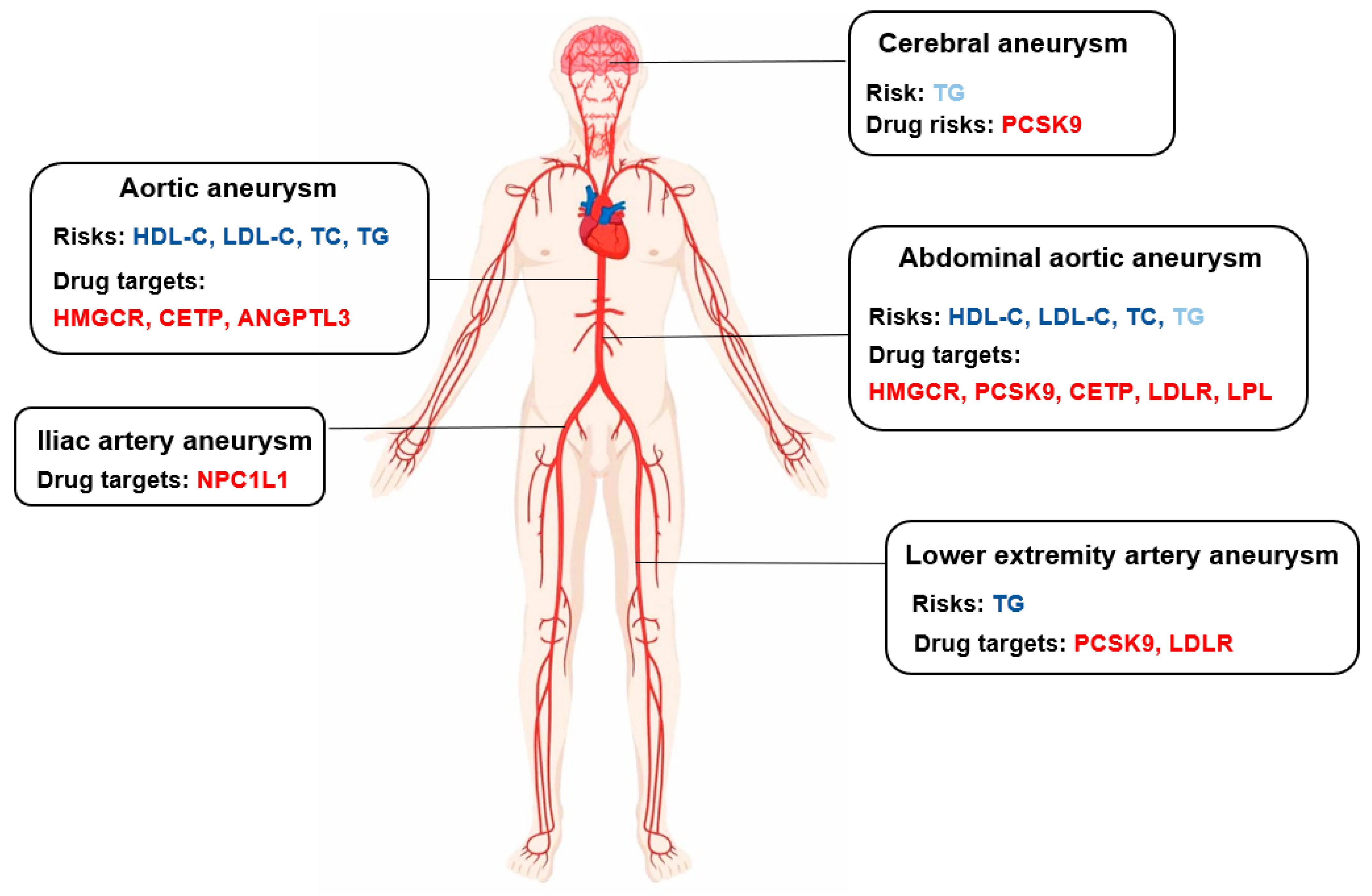
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [Green Version]
- Di Angelantonio, E.; Gao, P.; Pennells, L.; Kaptoge, S.; Caslake, M.; Thompson, A.; Butterworth, A.S.; Sarwar, N.; Wormser, D.; Emerging Risk Factors Collaboration; et al. Lipid-related markers and cardiovascular disease prediction. JAMA 2012, 307, 2499–2506. [Google Scholar] [CrossRef] [Green Version]
- Holmes, M.V.; Millwood, I.Y.; Kartsonaki, C.; Hill, M.R.; Bennett, D.A.; Boxall, R.; Guo, Y.; Xu, X.; Bian, Z.; Hu, R.; et al. Lipids, Lipoproteins, and Metabolites and Risk of Myocardial Infarction and Stroke. J. Am. Coll. Cardiol. 2018, 71, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Rist, P.M.; Buring, J.E.; Ridker, P.M.; Kase, C.S.; Kurth, T.; Rexrode, K.M. Lipid levels and the risk of hemorrhagic stroke among women. Neurology 2019, 92, e2286–e2294. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.C.; Holmes, M.V.; Burgess, S.; Asselbergs, F.W.; Jones, G.T.; Baas, A.F.; van ‘t Hof, F.N.; de Bakker, P.I.W.; Blankensteijn, J.D.; Powell, J.T.; et al. Genetic Association of Lipids and Lipid Drug Targets With Abdominal Aortic Aneurysm: A Meta-analysis. JAMA Cardiol. 2018, 3, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Allara, E.; Morani, G.; Carter, P.; Gkatzionis, A.; Zuber, V.; Foley, C.N.; Rees, J.M.B.; Mason, A.M.; Bell, S.; Gill, D.; et al. Genetic Determinants of Lipids and Cardiovascular Disease Outcomes: A Wide-Angled Mendelian Randomization Investigation. Circ. Genom. Precis. Med. 2019, 12, e002711. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; van Bockxmeer, F.; Jamrozik, K.; McCann, M.; Norman, P.E. Association between serum lipoproteins and abdominal aortic aneurysm. Am. J. Cardiol. 2010, 105, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tuomilehto, J.; Jousilahti, P.; Wang, Y.; Antikainen, R.; Hu, G. Total and high-density lipoprotein cholesterol and stroke risk. Stroke 2012, 43, 1768–1774. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Shang-Guan, H.C.; Wu, S.Y.; Yao, P.S.; Sun, Y.; Zeng, Y.L.; Zheng, S.F.; Chen, G.R.; Lin, Y.X.; Kang, D.Z. High-Density Lipoprotein Is Associated with Progression of Intracranial Aneurysms. World Neurosurg. 2018, 120, e234–e240. [Google Scholar] [CrossRef]
- Can, A.; Castro, V.M.; Dligach, D.; Finan, S.; Yu, S.; Gainer, V.; Shadick, N.A.; Savova, G.; Murphy, S.; Cai, T.; et al. Lipid-Lowering Agents and High HDL (High-Density Lipoprotein) Are Inversely Associated With Intracranial Aneurysm Rupture. Stroke 2018, 49, 1148–1154. [Google Scholar] [CrossRef]
- Smith, G.D.; Ebrahim, S. Mendelian randomization: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Smith, G.D. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Leong, A.; Cole, J.B.; Brenner, L.N.; Meigs, J.B.; Florez, J.C.; Mercader, J.M. Cardiometabolic risk factors for COVID-19 susceptibility and severity: A Mendelian randomization analysis. PLoS Med. 2021, 18, e1003553. [Google Scholar] [CrossRef]
- Ponsford, M.J.; Gkatzionis, A.; Walker, V.M.; Grant, A.J.; Wootton, R.E.; Moore, L.S.P.; Fatumo, S.; Mason, A.M.; Zuber, V.; Willer, C.; et al. Cardiometabolic Traits, Sepsis, and Severe COVID-19: A Mendelian Randomization Investigation. Circulation 2020, 142, 1791–1793. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, J.; Bull, C.J.; Vincent, E.E.; Robinson, J.; Walther, A.; Smith, G.D.; Lewis, S.J.; Relton, C.L.; Martin, R.M. Association Between Genetically Proxied Inhibition of HMG-CoA Reductase and Epithelial Ovarian Cancer. JAMA 2020, 323, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, L.; Giambartolomei, C.; Pereira, A.C.; Gaulton, A.; Posner, D.C.; Swanson, S.A.; Ho, Y.L.; Iyengar, S.K.; Kosik, N.M.; Vujkovic, M.; et al. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat. Med. 2021, 27, 668–676. [Google Scholar] [CrossRef]
- Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; Buchkovich, M.L.; Mora, S.; et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 2013, 45, 1274–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, M.K.; van der Spek, R.A.A.; van Rheenen, W.; Morel, S.; Bourcier, R.; Hostettler, I.C.; Alg, V.S.; van Eijk, K.R.; Koido, M.; Akiyama, M.; et al. Genome-wide association study of intracranial aneurysms identifies 17 risk loci and genetic overlap with clinical risk factors. Nat. Genet. 2020, 52, 1303–1313. [Google Scholar] [CrossRef]
- Yavorska, O.O.; Burgess, S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017, 46, 1734–1739. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Dudbridge, F.; Thompson, S.G. Combining information on multiple instrumental variables in Mendelian randomization: Comparison of allele score and summarized data methods. Stat. Med. 2016, 35, 1880–1906. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Smith, G.D.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowden, J.; Smith, G.D.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Hartwig, F.P.; Smith, G.D.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, Y.; Murakami, Y.; Saitoh, M.; Yokoi, T.; Aoki, T.; Miura, K.; Ueshima, H.; Nozaki, K.; SSS Research Group. Statin use and risk of cerebral aneurysm rupture: A hospital-based case-control study in Japan. J. Stroke Cereb. Dis. 2014, 23, 343–348. [Google Scholar] [CrossRef]
- Naraoka, M.; Matsuda, N.; Shimamura, N.; Asano, K.; Akasaka, K.; Takemura, A.; Hasegawa, S.; Ohkuma, H. Long-acting statin for aneurysmal subarachnoid hemorrhage: A randomized, double-blind, placebo-controlled trial. J. Cereb. Blood Flow Metab. 2018, 38, 1190–1198. [Google Scholar] [CrossRef]
- Wong, G.K.; Chan, D.Y.; Siu, D.Y.; Zee, B.C.; Poon, W.S.; Chan, M.T.; Gin, T.; Leung, M.; Investigators, H.-S. High-dose simvastatin for aneurysmal subarachnoid hemorrhage: Multicenter randomized controlled double-blinded clinical trial. Stroke 2015, 46, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Oyama, K.; Giugliano, R.P.; Tang, M.; Bonaca, M.P.; Saver, J.L.; Murphy, S.A.; Ruzza, A.; Keech, A.C.; Sever, P.S.; Sabatine, M.S.; et al. Effect of evolocumab on acute arterial events across all vascular territories: Results from the FOURIER trial. Eur. Heart J. 2021, ehab604. [Google Scholar] [CrossRef]
- McPhee, J.T.; Hill, J.S.; Eslami, M.H. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001–2004. J. Vasc. Surg. 2007, 45, 891–899. [Google Scholar] [CrossRef] [Green Version]
- Ashton, H.A.; Buxton, M.J.; Day, N.E.; Kim, L.G.; Marteau, T.M.; Scott, R.A.; Thompson, S.G.; Walker, N.M.; Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: A randomised controlled trial. Lancet 2002, 360, 1531–1539. [Google Scholar] [CrossRef]
- Jones, G.T.; Tromp, G.; Kuivaniemi, H.; Gretarsdottir, S.; Baas, A.F.; Giusti, B.; Strauss, E.; Van’t Hof, F.N.; Webb, T.R.; Erdman, R.; et al. Meta-Analysis of Genome-Wide Association Studies for Abdominal Aortic Aneurysm Identifies Four New Disease-Specific Risk Loci. Circ. Res. 2017, 120, 341–353. [Google Scholar] [CrossRef]
- Tang, W.; Yao, L.; Roetker, N.S.; Alonso, A.; Lutsey, P.L.; Steenson, C.C.; Lederle, F.A.; Hunter, D.W.; Bengtson, L.G.; Guan, W.; et al. Lifetime Risk and Risk Factors for Abdominal Aortic Aneurysm in a 24-Year Prospective Study: The ARIC Study (Atherosclerosis Risk in Communities). Arter. Thromb. Vasc. Biol. 2016, 36, 2468–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsdahl, S.H.; Singh, K.; Solberg, S.; Jacobsen, B.K. Risk factors for abdominal aortic aneurysms: A 7-year prospective study: The Tromso Study, 1994–2001. Circulation 2009, 119, 2202–2208. [Google Scholar] [CrossRef] [Green Version]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindenauer, P.K.; Pekow, P.; Wang, K.; Gutierrez, B.; Benjamin, E.M. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004, 291, 2092–2099. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Wu, Z.; Qin, X.; Huang, Q.; Wang, X.; Qin, J.; Lu, X. Statins reduce mortality after abdominal aortic aneurysm repair: A systematic review and meta-analysis. J. Vasc. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Howatt, D.A.; Balakrishnan, A.; Graham, M.J.; Mullick, A.E.; Daugherty, A. Hypercholesterolemia Induced by a PCSK9 Gain-of-Function Mutation Augments Angiotensin II-Induced Abdominal Aortic Aneurysms in C57BL/6 Mice-Brief Report. Arter. Thromb. Vasc. Biol. 2016, 36, 1753–1757. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Liu, S.; Wang, X.; Deng, X.; Fan, Y.; Sun, C.; Wang, Y.; Mehta, J.L. Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxid. Redox Signal. 2015, 22, 760–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Yan, B.; Gui, Y.; Tang, Z.; Tai, S.; Zhou, S.; Zheng, X.L. Physiology and role of PCSK9 in vascular disease: Potential impact of localized PCSK9 in vascular wall. J. Cell. Physiol. 2021, 236, 2333–2351. [Google Scholar] [CrossRef]
- Dewey, F.E.; Gusarova, V.; Dunbar, R.L.; O’Dushlaine, C.; Schurmann, C.; Gottesman, O.; McCarthy, S.; Van Hout, C.V.; Bruse, S.; Dansky, H.M.; et al. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Harada-Shiba, M.; Ali, S.; Gipe, D.A.; Gasparino, E.; Son, V.; Zhang, Y.; Pordy, R.; Catapano, A.L. A randomized study investigating the safety, tolerability, and pharmacokinetics of evinacumab, an ANGPTL3 inhibitor, in healthy Japanese and Caucasian subjects. Atherosclerosis 2020, 314, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Tian, G.P.; Cheng, H.P.; Zhang, X.; Ou, X.; Yu, X.H.; Tan, R.Q.; Yang, F.Y.; Gong, D.; Huang, C.; et al. MicroRNA-134 Promotes the Development of Atherosclerosis Via the ANGPTL4/LPL Pathway in Apolipoprotein E Knockout Mice. J. Atheroscler. Thromb. 2018, 25, 244–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dix, F.P.; Titi, M.; Al-Khaffaf, H. The isolated internal iliac artery aneurysm—A review. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 119–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Huang, M.; Xuan, Y.; Li, K.; Xu, X.; Wang, L.; Sun, Y.; Xiao, L.; Xu, P.; Kong, W.; et al. Association between Lipid Levels and Risk for Different Types of Aneurysms: A Mendelian Randomization Study. J. Pers. Med. 2021, 11, 1171. https://doi.org/10.3390/jpm11111171
Chen Y, Huang M, Xuan Y, Li K, Xu X, Wang L, Sun Y, Xiao L, Xu P, Kong W, et al. Association between Lipid Levels and Risk for Different Types of Aneurysms: A Mendelian Randomization Study. Journal of Personalized Medicine. 2021; 11(11):1171. https://doi.org/10.3390/jpm11111171
Chicago/Turabian StyleChen, Yanghui, Man Huang, Yunling Xuan, Ke Li, Xin Xu, Linlin Wang, Yang Sun, Lei Xiao, Ping Xu, Wei Kong, and et al. 2021. "Association between Lipid Levels and Risk for Different Types of Aneurysms: A Mendelian Randomization Study" Journal of Personalized Medicine 11, no. 11: 1171. https://doi.org/10.3390/jpm11111171
APA StyleChen, Y., Huang, M., Xuan, Y., Li, K., Xu, X., Wang, L., Sun, Y., Xiao, L., Xu, P., Kong, W., & Wang, D. W. (2021). Association between Lipid Levels and Risk for Different Types of Aneurysms: A Mendelian Randomization Study. Journal of Personalized Medicine, 11(11), 1171. https://doi.org/10.3390/jpm11111171




