Effects of Therapeutic and Aerobic Exercise Programs on Pain, Neuromuscular Activation, and Bite Force in Patients with Temporomandibular Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
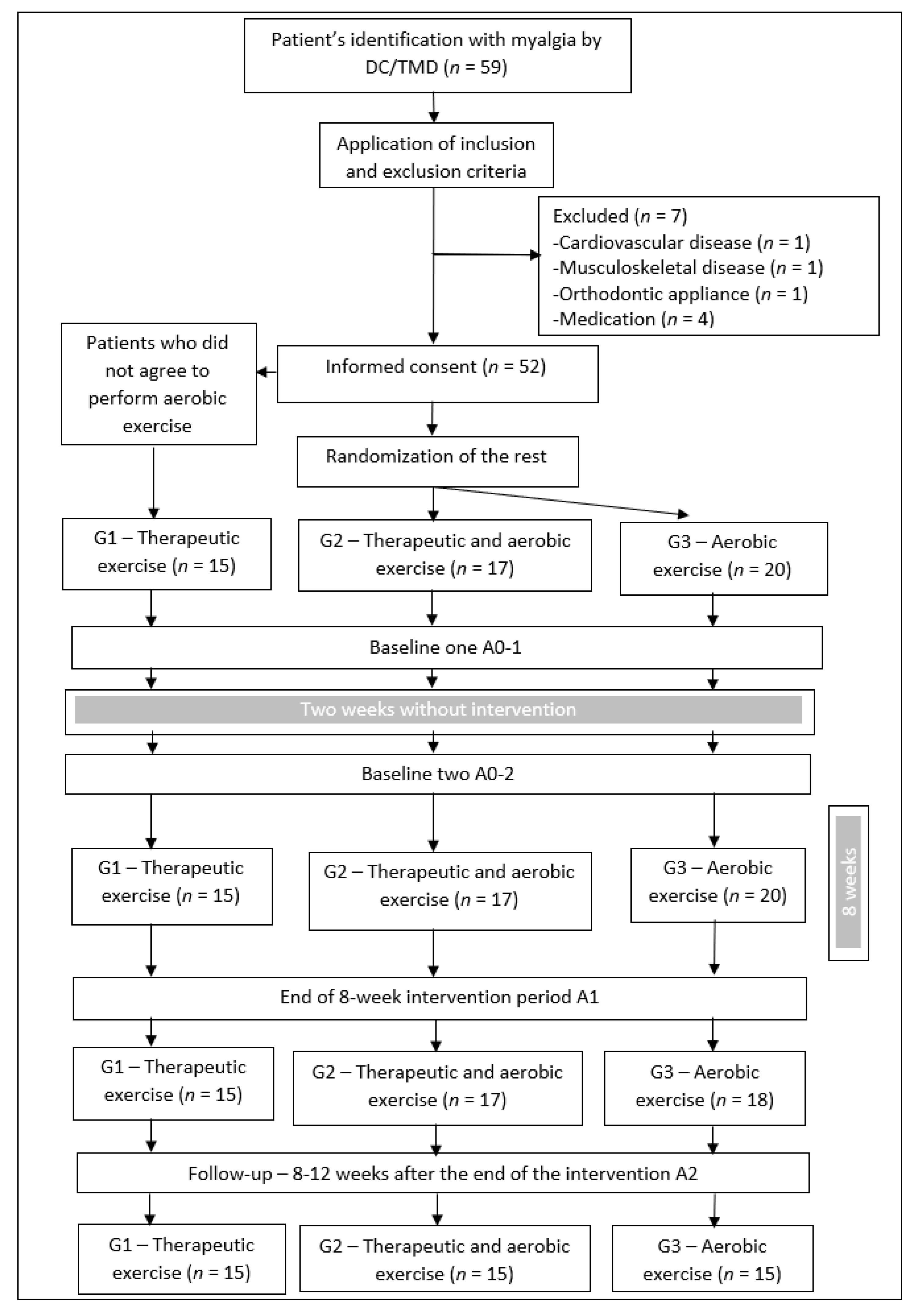
2.2. Sampling and Recruitment
2.3. Ethics and Procedures
2.4. Data Collection
2.5. Data Analysis
3. Results
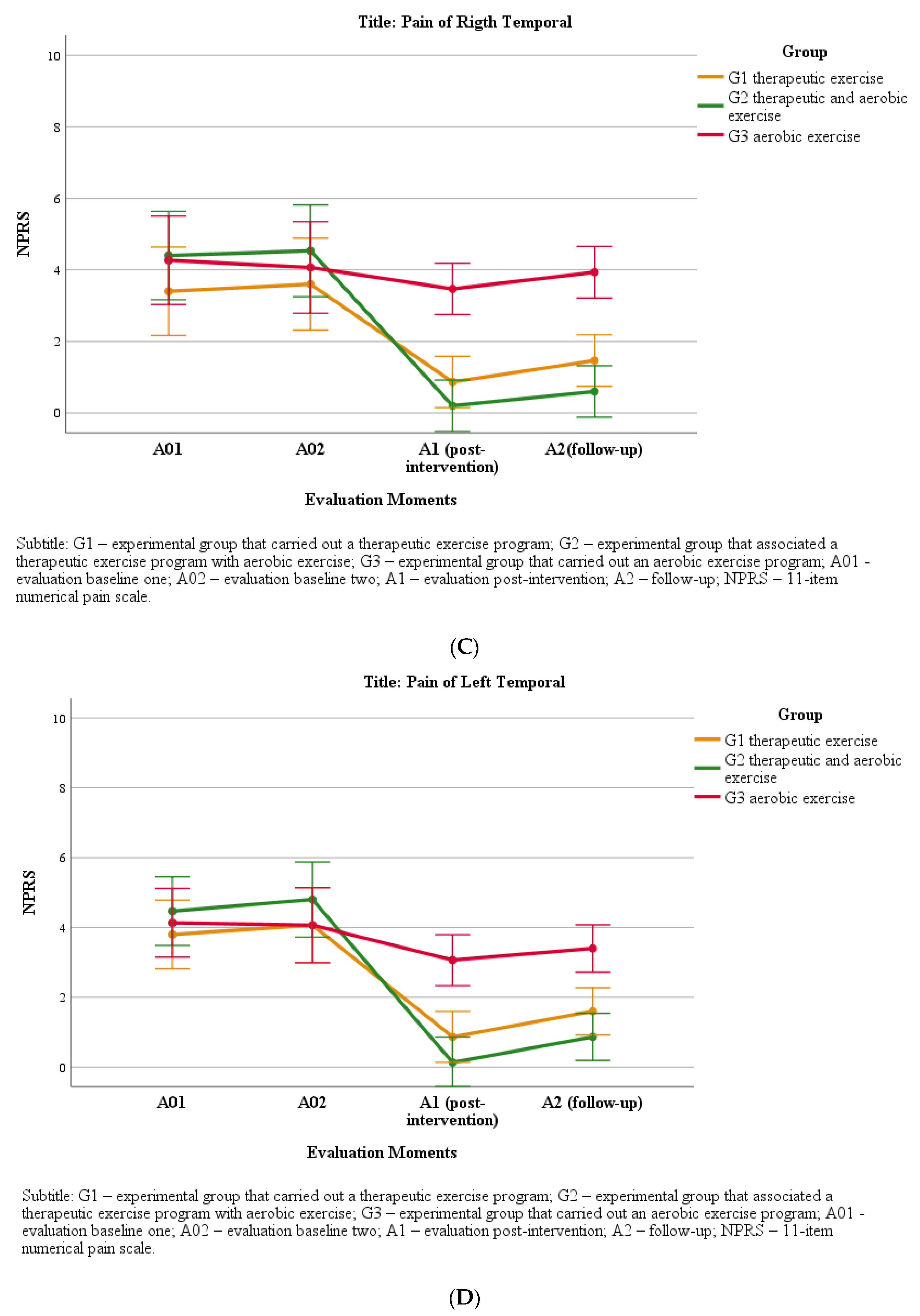
4. Pain Intensity
5. Neuromuscular Activity—sEMG
6. Bite Force
7. Discussion
8. Pain Intensity
9. Bite Force and Neuromuscular Activation of Masticatory Muscles
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Dworkin, S.F.; Massoth, D.L. Temporomandibular disorders and chronic pain: Disease or illness? J. Prosthet. Dent. 1994, 72, 29–38. [Google Scholar] [CrossRef]
- Lipton, J.A.; Ship, J.A.; Larach-Robinson, D. Estimated prevalence and distribution of reported orofacial pain in the United States. J. Am. Dent. Assoc. 1993, 124, 115–121. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Dental and Craniofacial Research. Facial Pain, July 2018. Available online: https://www.nidcr.nih.gov/research/data-statistics/facial-pain (accessed on 12 December 2020).
- Blanco-Hungria, A.; Blanco-Aguilera, A.; Blanco-Aguilera, E.; Serrano-del-Rosal, R.; Biedma-Velázquez, L.; Rodríguez-Torronteras, A.; Segura-Saint-Gerons, R. Prevalence of the different Axis I clinical subtypes in a sample of patients with orofacial pain and temporomandibular disorders in the Andalusian Healthcare Service. Med. Oral Patol. Oral Cir. Bucal 2016, 21, 169–177. [Google Scholar] [CrossRef]
- Oral, K.; Küçük, B.B.; Ebeoğlu, B.; Dinçer, S. Etiology of temporomandibular disorder pain. J. Turk. Soc. Algol. 2009, 21, 89–94. [Google Scholar]
- Fricton, J.R.; Ouyang, W.; Nixdorf, D.R.; Schiffman, E.L.; Velly, A.M.; Look, J.O. Critical appraisal of methods used in randomized controlled trials of treatments for temporomandibular disorders. J. Orofac. Pain 2010, 24, 139–151. [Google Scholar]
- Cho, G.; Lee, Y. Analysis of Masticatory Muscle Activity Based on Presence of Temporomandibular Joint Disorders. Med. Sci. Monit. 2020, 26, e921337. [Google Scholar] [CrossRef]
- Todic, J.; Martinovic, B.; Pavlovic, J.; Tabakovic, S.; Staletovic, M. Assessment of the impact of temporomandibular disorders on maximum bite force. Biomed. Pap. 2019, 163, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Pficer, K.J.; Dodic, S.; Lazic, V.; Trajkovic, G.; Milic, N.; Milicic, B. Occlusal stabilization splint for patients with temporomandibular disorders: Meta-analysis of short and long term effects. PLoS ONE 2017, 12, e0171296. [Google Scholar] [CrossRef]
- Xu, G.Z.; Jia, J.; Jin, L.; Li, J.H.; Wang, Z.Y.; Cao, D.Y. Low-Level Laser Therapy for Temporomandibular Disorders: A Systematic Review with Meta-Analysis. Pain Res. Manag. 2018, 2018, 4230583. [Google Scholar] [CrossRef] [Green Version]
- Al-Moraissi, E.A.; Alradom, J.; Aladashi, O.; Goddard, G.; Christidis, N. Needling therapies in the management of myofascial pain of the masticatory muscles: A network meta-analysis of randomised clinical trials. J. Oral Rehabil. 2020, 47, 910–922. [Google Scholar] [CrossRef]
- Al-Moraissi, E.A.; Conti, P.C.R.; Alyahya, A.; Alkebsi, K.; Elsharkawy, A.; Christidis, N. The hierarchy of different treatments for myogenous temporomandibular disorders: A systematic review and network meta-analysis of randomized clinical trials. Oral Maxillofac. Surg. 2021, 1–15. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Pitance, L.; Singh, V.; Neto, F.; Thie, N.; Michelotti, A. Effectiveness of manual therapy and therapeutic exercise for temporomandibular disorders: Systematic review and meta-analysis. Phys. Ther. 2016, 96, 9–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickerson, S.M.; Weaver, J.M.; Boyson, A.N.; Thacker, J.A.; Junak, A.A.; Ritzline, P.D.; Donaldson, M.B. The effectiveness of exercise therapy for temporomandibular dysfunction: A systematic review and meta-analysis. Clin. Rehabil. 2017, 31, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Paço, M.; Peleteiro, B.; Duarte, J.; Pinho, T. The effectiveness of physiotherapy in the management of temporomandibular disorders: A systematic review and meta-analysis. J. Oral Facial Pain Headache 2016, 30, 210–220. [Google Scholar] [CrossRef]
- Naugle, K.M.; Fillingim, R.B.; Riley, J.L., III. A meta-analytic review of the hypoalgesic effects of exercise. J. Pain 2012, 13, 1139–1150. [Google Scholar] [CrossRef] [Green Version]
- Koltyn, K.F.; Garvin, A.W.; Gardiner, R.L.; Nelson, T.F. Perception of pain following aerobic exercise. Med. Sci. Sports Exerc. 1996, 28, 1418–1421. [Google Scholar] [CrossRef]
- Meeus, M.; Roussel, N.A.; Truijen, S.; Nijs, J. Reduced pressure pain thresholds in response to exercise in Chronic Fatigue Syndrome but not in Chronic Low Back Pain: An experimental study. J. Rehabil. Med. 2010, 42, 884–890. [Google Scholar] [CrossRef] [Green Version]
- Vierck Jr, C.J.; Staud, R.; Price, D.D.; Cannon, R.L.; Mauderli, A.P.; Martin, A.D. The effect of maximal exercise on temporal summation of second pain (windup) in patients with Fibromyalgia Syndrome. J. Pain 2001, 2, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Koltyn, K.F. Analgesia following exercise: A review. Sports Med. 2000, 29, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Koltyn, K.F. Exercise-induced hypoalgesia and intensity of exercise. Sports Med. 2002, 32, 477–487. [Google Scholar] [CrossRef]
- Koltyn, K.F.; Brellenthin, A.G.; Cook, D.B.; Sehgal, N.; Hillard, C. Mechanisms of Exercise-Induced Hypoalgesia. J. Pain 2014, 15, 1294–1304. [Google Scholar] [CrossRef] [Green Version]
- Naugle, K.M.; Naugle, K.E.; Fillingim, R.B.; Samuels, B.; Riley, J.L., 3rd. Intensity Thresholds for Aerobic Exercise-Induced Hypoalgesia. Med. Sci. Sports Exerc. 2014, 46, 817–825. [Google Scholar] [CrossRef] [Green Version]
- Bidone, J.; Busch, A.J.; Schachter, C.L.; Overend, T.J.; Kim, S.Y.; Góes, S.M.; Boden, C.B.; Foulds, H.J. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst. Rev. 2017, 21, CD012700. [Google Scholar] [CrossRef]
- Polaski, A.M.; Phelps, A.L.; Kostek, M.C.; Szucs, K.A.; Kolber, B.J. Exercise-induced hypoalgesia: A meta-analysis of exercise dosing for the treatment of chronic pain. PLoS ONE 2019, 14, e0210418. [Google Scholar] [CrossRef] [Green Version]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Gay, C.W.; Alappattu, M.J.; Coronado, R.A.; Horn, M.E.; Bishop, M.D. Effect of a single session of muscle-biased therapy on pain sensitivity: A systematic review and meta-analysis of randomized controlled trials. J. Pain Res. 2013, 6, 7–22. [Google Scholar] [CrossRef] [Green Version]
- Okeson, J. Management of Temporomandibular Disorders and Occlusion, 8th ed.; Elsevier: Maryland Heights, MO, USA, 2019. [Google Scholar]
- Simons, D.G. Understanding effective treatments of myofascial trigger points. J. Bodyw. Mov. Ther. 2002, 6, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Shimada, A.; Ishigaki, S.; Matsuka, Y.; Komiyama, O.; Torisu, T.; Oono, Y.; Sato, H.; Naganawa, T.; Mine, A.; Yamazaki, Y.; et al. Effects of exercise therapy on painful temporomandibular disorders. J. Oral Rehabil. 2019, 46, 475–481. [Google Scholar] [CrossRef]
- Karvonen, M.; Kentala, E.; Mustala, O. The Effects of Training on Heart Rate: A Longitudinal Study. Ann. Med. Exp. Biol. Fenn. 1957, 35, 307–315. [Google Scholar]
- Farrar, J.T.; Young Jr, J.P.; LaMoreaux, L.W.; Werth, J.L.; Poole, M.R. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Tartaglia, G.M.; Lodetti, G.; Paiva, G.; De Felicio, C.M.; Sforza, C. Surface electromyographic assessment of patients with long lasting temporomandibular joint disorder pain. J. Electromyogr. Kinesiol. 2011, 21, 659–664. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for sEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Kalamir, A.; Pollard, H.; Vitiello, A.; Bonello, R. Intra-oral myofascial therapy for chronic myogenous temporomandibular disorders: A randomized, controlled pilot study. J. Man. Manip. Ther. 2010, 18, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Chichorro, J.G.; Porreca, F.; Sessle, B. Mechanisms of craniofacial pain. Cephalalgia 2017, 37, 613–626. [Google Scholar] [CrossRef]
- Svensson, P.; Kumar, A. Assessment of risk factors for oro-facial pain and recent developments in classification: Implications for management. J. Oral Rehabil. 2016, 43, 977–989. [Google Scholar] [CrossRef]
- Pfau, D.; Rolke, R.; Nickel, R.; Treede, R.; Daublaender, M. Somatosensory profiles in subgroups of patients with myogenic temporomandibular disorders and Fibromyalgia Syndrome. Pain 2009, 147, 72–83. [Google Scholar] [CrossRef]
- Thorén, P.; Floras, J.; Hoffmann, P.; Seals, D.R. Endorphins and exercise: Physiological mechanisms and clinical implications. Med. Sci. Sports Exerc. 1990, 22, 417–428. [Google Scholar]
- Dietrich, A.; McDaniel, W.F. Endocannabinoids and exercise. Br. J. Sports Med. 2004, 38, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Koltyn, K.; Trine, M.; Stegner, A.; Tobar, D.A. Effect of Isometric Exercise on Pain Perception and Blood Pressure in Men and Women. Med. Sci. Sports Exerc. 2001, 33, 282–290. [Google Scholar] [CrossRef]
- Villemure, C.; Bushnell, M. Cognitive modulation of pain: How do attention and emotion influence pain processing? Pain 2002, 95, 195–199. [Google Scholar] [CrossRef]
- Herpich, C.; Leal-Junior, E.; Gomes, C.; Gloria, I.; Amaral, A.; Amaral, M.; Politti, F.; Biasotto-Gonzalez, D. Immediate and short-term effects of phototherapy on pain, muscle activity, and joint mobility in women with temporomandibular disorder: A randomized, double-blind, placebo-controlled, clinical trial. Disabil. Rehabil. 2017, 40, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Siriani, A.; Bérzin, F. Effect of conventional TENS on pain and electromyographic activity of masticatory muscles in TMD patients. Braz. Oral Res. 2004, 18, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Hara, S.; Svensson, P. Effect of experimental jaw muscle pain on EMG activity and bite force distribution at different level of clenching. J. Oral Rehabil. 2013, 40, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; Zuim, P.R.J.; Moreno, A.; Dos Santos, D.M.; da Silva, E.V.F.; de Caxias, F.P.; Turcio, K.H.L. Does pain in the masseter and anterior temporal muscles influence maximal bite force? Arch. Oral Biol. 2017, 83, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Testa, M.; Geri, T.; Pitance, L.; Lentz, P.; Gizzi, L.; Erlenwein, J.; Petkze, F.; Falla, D. Alterations in jaw clenching force control in people with myogenic temporomandibular disorders. J. Electromyogr. Kinesiol. 2018, 43, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Peck, C.; Murray, G.; Gerzina, T. How does pain affect jaw muscle activity? The Integrated Pain Adaptation Model. Aust. Dent. J. 2008, 53, 201–207. [Google Scholar] [CrossRef]
- Maulina, T.; Amhamed, M.; Whittle, T.; Gal, J.; Akhter, R.; Murray, G.M. The Effects of Experimental Temporalis Muscle Pain on Jaw Muscle Electromyographic Activity During Jaw Movements and Relationships with Some Psychological Variables. J. Oral Facial Pain Headache 2018, 32, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, P.; Sandoval, I.; Whittle, T.; Mojaver, Y.N.; Murray, G.M. Reorganization of masseter and temporalis muscles single motor unit activity during experimental masseter muscle pain. J. Oral Facial Pain Headache 2020, 34, 40–52. [Google Scholar] [CrossRef]
- Mellor, R.; Hodges, P. Motor unit synchronization between medial and lateral vasti muscles. Clin. Neurophysiol. 2005, 116, 1585–1595. [Google Scholar] [CrossRef]
- Cormie, P.; McGuigan, M.R.; Newton, R.U. Developing Maximal Neuromuscular Power. Sports Med. 2011, 41, 17–38. [Google Scholar] [CrossRef]
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, S.P.; Bojsen-Møller, F.; Dyhre-Poulsen, P. Antagonist muscle coactivation during isokinetic knee extension. Scand. J. Med. Sci. Sports 2000, 10, 58–67. [Google Scholar] [CrossRef]
- Milner, T.E.; Cloutier, C.; Leger, A.B.; Franklin, D.W. Inability to activate muscles maximally during cocontraction and the effect of joint stiffness. Exp. Brain Res. 1995, 107, 293–305. [Google Scholar] [CrossRef]
- Lauriti, L.; Motta, L.J.; Godoy, C.H.L.; Biasotto-Gonzalez, D.A.; Politti, F.; Mesquita-Ferrari, R.A.; Fernandes, K.P.S.; Bussadori, S.K. Influence of temporomandibular disorder on temporal and masseter muscles and occlusal contacts in adolescents: An electromyographic study. BMC Musculoskelet. Disord. 2014, 15, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, B.; Biasotto-Gonzalez, D.; Bussadori, S.K.; Gomes, C.; Politti, F. Is there a difference in the electromyographic activity of the masticatory muscles between individuals with temporomandibular disorder and healthy controls? A systematic review with meta-analysis. J. Oral Rehabil. 2020, 47, 672–682. [Google Scholar] [CrossRef] [PubMed]


| G1 (Mean) | Lower Bound | Upper Bound | G2 (Mean) | Lower Bound | Upper Bound | G3 (Mean) | Lower Bound | Upper Bound | ||
|---|---|---|---|---|---|---|---|---|---|---|
| sEMG of MVC and RP | 95% Confidence Interval | 95% Confidence Interval | 95% Confidence Interval | |||||||
| A01 | sEMG Right MVC | 93.3 | 91.672 | 95.005 | 92.5 | 90.858 | 94.192 | 92.4 | 90.736 | 94.069 |
| sEMG Left MVC | 90.8 | 89.246 | 92.265 | 91.4 | 89.924 | 92.943 | 91.2 | 89.663 | 92.682 | |
| Right RP | 6.1 | 4.031 | 8.044 | 5.0 | 2.950 | 6.963 | 5.4 | 3.355 | 7.367 | |
| Left RP | 7.2 | 4.920 | 9.560 | 5.5 | 3.154 | 7.794 | 6.2 | 3.881 | 8.521 | |
| A02 | sEMG Right MVC | 90.8 | 88.938 | 92.583 | 91.9 | 90.068 | 93.713 | 90.8 | 88.978 | 92.623 |
| sEMG Left MVC | 90.5 | 88.844 | 92.161 | 90.6 | 88.901 | 92.218 | 89.5 | 87.834 | 91.151 | |
| Right RP | 6.0 | 3.892 | 8.144 | 5.1 | 3.015 | 7.267 | 5.6 | 3.478 | 7.730 | |
| Left RP | 7.3 | 4.967 | 9.553 | 5.6 | 3.352 | 7.938 | 6.3 | 3.971 | 8.557 | |
| A1 | sEMG Right MVC | 92.0 | 90.353 | 93.732 | 92.3 | 90.570 | 93.949 | 92.3 | 90.653 | 94.032 |
| sEMG Left MVC | 90.8 | 88.986 | 92.668 | 91.8 | 89.977 | 93.658 | 90.5 | 88.666 | 92.347 | |
| Right RP | 4.4 | 2.740 | 6.115 | 3.2 | 1.469 | 4.844 | 4.6 | 2.900 | 6.276 | |
| Left RP | 4.6 | 2.821 | 6.385 | 3.6 | 1.788 | 5.352 | 5.3 | 3.481 | 7.045 | |
| A2 | sEMG Right MVC | 92.3 | 90.584 | 93.916 | 92.1 | 90.445 | 93.778 | 91.9 | 90.221 | 93.553 |
| sEMG Left MVC | 90.9 | 89.056 | 92.667 | 91.3 | 89.474 | 93.084 | 91.7 | 89.921 | 93.531 | |
| Right RP | 4.6 | 2.800 | 6.431 | 3.5 | 1.660 | 5.292 | 5.1 | 3.315 | 6.947 | |
| Left RP | 4.7 | 2.979 | 6.560 | 3.7 | 1.902 | 5.484 | 5.8 | 4.068 | 7.650 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moleirinho-Alves, P.M.M.; Cebola, P.M.T.C.; dos Santos, P.D.G.; Correia, J.P.; Godinho, C.; Oliveira, R.A.N.d.S.; Pezarat-Correia, P.L.C. Effects of Therapeutic and Aerobic Exercise Programs on Pain, Neuromuscular Activation, and Bite Force in Patients with Temporomandibular Disorders. J. Pers. Med. 2021, 11, 1170. https://doi.org/10.3390/jpm11111170
Moleirinho-Alves PMM, Cebola PMTC, dos Santos PDG, Correia JP, Godinho C, Oliveira RANdS, Pezarat-Correia PLC. Effects of Therapeutic and Aerobic Exercise Programs on Pain, Neuromuscular Activation, and Bite Force in Patients with Temporomandibular Disorders. Journal of Personalized Medicine. 2021; 11(11):1170. https://doi.org/10.3390/jpm11111170
Chicago/Turabian StyleMoleirinho-Alves, Paula Manuela Mendes, Pedro Miguel Teixeira Cravas Cebola, Paulo Duarte Guia dos Santos, José Pedro Correia, Catarina Godinho, Raul Alexandre Nunes da Silva Oliveira, and Pedro Luís Cemacelha Pezarat-Correia. 2021. "Effects of Therapeutic and Aerobic Exercise Programs on Pain, Neuromuscular Activation, and Bite Force in Patients with Temporomandibular Disorders" Journal of Personalized Medicine 11, no. 11: 1170. https://doi.org/10.3390/jpm11111170
APA StyleMoleirinho-Alves, P. M. M., Cebola, P. M. T. C., dos Santos, P. D. G., Correia, J. P., Godinho, C., Oliveira, R. A. N. d. S., & Pezarat-Correia, P. L. C. (2021). Effects of Therapeutic and Aerobic Exercise Programs on Pain, Neuromuscular Activation, and Bite Force in Patients with Temporomandibular Disorders. Journal of Personalized Medicine, 11(11), 1170. https://doi.org/10.3390/jpm11111170








