Relationship between the Corticospinal and Corticocerebellar Tracts and Their Role in Upper Extremity Motor Recovery in Stroke Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. MRI Data Acquisition
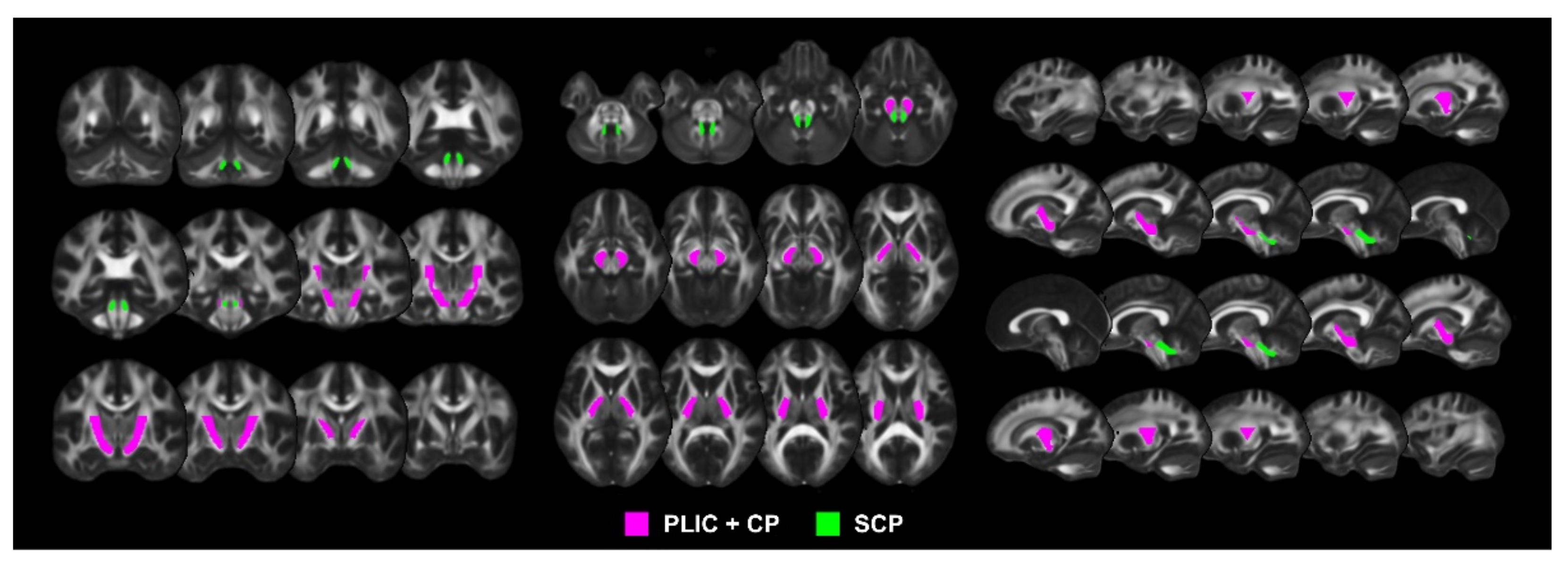
2.3. DTI Data Processing for Extraction of CST and CCT FA Values
2.4. Statistical Analysis
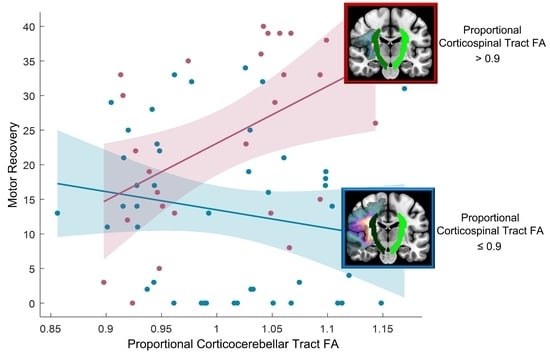
3. Results
4. Discussion
4.1. The Corticospinal Tract Injury Associated with Motor Recovery
4.2. The Corticocerebellar Tract Injury Associated with Motor Recovery
4.3. The Relationship between the Corticospinal and Corticocerebellar Tracts for Motor Recovery
4.4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van der Vliet, R.; Selles, R.W.; Andrinopoulou, E.R.; Nijland, R.; Ribbers, G.M.; Frens, M.A.; Meskers, C.; Kwakkel, G. Predicting upper limb motor impairment recovery after stroke: A mixture model. Ann. Neurol. 2020, 87, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of motor function after stroke: A multiple systematic review focused on techniques to stimulate upper extremity recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef] [Green Version]
- Stinear, C.M. Prediction of motor recovery after stroke: Advances in biomarkers. Lancet Neurol. 2017, 16, 826–836. [Google Scholar] [CrossRef]
- Welniarz, Q.; Dusart, I.; Roze, E. The corticospinal tract: Evolution, development, and human disorders. Dev. Neurobiol. 2017, 77, 810–829. [Google Scholar] [CrossRef]
- Bostan, A.C.; Dum, R.P.; Strick, P.L. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn. Sci. 2013, 17, 241–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manto, M.; Bower, J.M.; Conforto, A.B.; Delgado-García, J.M.; Da Guarda, S.N.F.; Gerwig, M.; Habas, C.; Hagura, N.; Ivry, R.B.; Mariën, P. Consensus paper: Roles of the cerebellum in motor control—The diversity of ideas on cerebellar involvement in movement. Cerebellum 2012, 11, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Penhune, V.B.; Steele, C.J. Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav. Brain Res. 2012, 226, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Brooks, V. Cerebellar functions in motor control. Hum. Neurobiol. 1984, 2, 251–260. [Google Scholar]
- Boyd, L.A.; Hayward, K.S.; Ward, N.S.; Stinear, C.M.; Rosso, C.; Fisher, R.J.; Carter, A.R.; Leff, A.P.; Copland, D.A.; Carey, L.M. Biomarkers of stroke recovery: Consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Neurorehabilit. Neural Repair 2017, 31, 864–876. [Google Scholar] [CrossRef]
- Buch, E.R.; Rizk, S.; Nicolo, P.; Cohen, L.G.; Schnider, A.; Guggisberg, A.G. Predicting motor improvement after stroke with clinical assessment and diffusion tensor imaging. Neurology 2016, 86, 1924–1925. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Park, E.; Lee, A.; Chang, W.H.; Kim, D.-S.; Kim, Y.-H. Prediction of motor recovery using indirect connectivity in a lesion network after ischemic stroke. Ther. Adv. Neurol. Disord. 2020, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, J.; Chhatbar, P.Y.; Doughty, C.; Landsittel, D.; Lioutas, V.A.; Kautz, S.A.; Schlaug, G. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann. Neurol. 2015, 78, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Lindenberg, R.; Alexander, M.P.; Schlaug, G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 2010, 41, 910–915. [Google Scholar] [CrossRef] [Green Version]
- Marchina, S.; Zhu, L.L.; Norton, A.; Zipse, L.; Wan, C.Y.; Schlaug, G. Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke 2011, 42, 2251–2256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Lee, A.; Kim, H.; Chang, W.H.; Kim, Y.H. Differences in motor network dynamics during recovery between supra- and infra-tentorial ischemic strokes. Hum. Brain Mapp. 2018, 39, 4976–4986. [Google Scholar] [CrossRef] [Green Version]
- Schulz, R.; Frey, B.M.; Koch, P.; Zimerman, M.; Bönstrup, M.; Feldheim, J.; Timmermann, J.E.; Schön, G.; Cheng, B.; Thomalla, G. Cortico-cerebellar structural connectivity is related to residual motor output in chronic stroke. Cereb. Cortex 2017, 27, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Small, S.L.; Hlustik, P.; Noll, D.; Genovese, C.; Solodkin, A. Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain 2002, 125, 1544–1557. [Google Scholar] [CrossRef]
- Scholz, J.; Tomassini, V.; Johansen-Berg, H. Chapter 14—Individual differences in white matter microstructure in the healthy brain. In Diffusion MRI, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2014; pp. 301–306. [Google Scholar] [CrossRef]
- Jang, S.H. Prediction of motor outcome for hemiparetic stroke patients using diffusion tensor imaging: A review. NeuroRehabilitation 2010, 27, 367–372. [Google Scholar] [CrossRef]
- Lindenberg, R.; Zhu, L.L.; Rüber, T.; Schlaug, G. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum. Brain Mapp. 2012, 33, 1040–1051. [Google Scholar] [CrossRef] [Green Version]
- Werring, D.J.; Toosy, A.T.; Clark, C.A.; Parker, G.J.; Barker, G.J.; Miller, D.H.; Thompson, A.J. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J. Neurol. Neurosurg. Psychiatry 2000, 69, 269–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woytowicz, E.J.; Rietschel, J.C.; Goodman, R.N.; Conroy, S.S.; Sorkin, J.D.; Whitall, J.; Waller, S.M. Determining levels of upper extremity movement impairment by applying a cluster analysis to the Fugl-Meyer assessment of the upper extremity in chronic stroke. Arch. Phys. Med. Rehabil. 2017, 98, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Mori, S.; Oishi, K.; Jiang, H.; Jiang, L.; Li, X.; Akhter, K.; Hua, K.; Faria, A.V.; Mahmood, A.; Woods, R. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008, 40, 570–582. [Google Scholar] [CrossRef] [Green Version]
- Puig, J.; Pedraza, S.; Blasco, G.; Daunis-I-Estadella, J.; Prats, A.; Prados, F.; Boada, I.; Castellanos, M.; Sánchez-González, J.; Remollo, S. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. Am. J. Neuroradiol. 2010, 31, 1324–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.; Su, Z.; Hu, J.; Xia, H.; Ma, H.; Wang, X.; Yan, J.; Ma, D. The value of diffusion tensor tractography delineating corticospinal tract in glioma in rat: Validation via correlation histology. PeerJ 2019, 7, e6453. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Sui, R.; Zhang, L. Diffusion Tensor Tractography Characteristics of White Matter Tracts are Associated with Post-Stroke Depression. Neuropsychiatr. Dis. Treat. 2021, 17, 167. [Google Scholar] [CrossRef] [PubMed]
- Farahat, A.H.; Lgohary, M.E.; Hafez, H. The Role of the Diffusion Tensor Imaging and the MR Tractography in the Evaluation of the Ischemic Cerebral Strokes. Int. J. Radiol. Imaging Technol. 2018, 4, 1–7. [Google Scholar] [CrossRef]
- Puig, J.; Pedraza, S.; Blasco, G.; Daunis-I-Estadella, J.; Prados, F.; Remollo, S.; Prats-Galino, A.; Soria, G.; Boada, I.; Castellanos, M. Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. Am. J. Neuroradiol. 2011, 32, 857–863. [Google Scholar] [CrossRef] [Green Version]
- Stinear, C.M.; Barber, P.A.; Petoe, M.; Anwar, S.; Byblow, W.D. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain 2012, 135, 2527–2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stinear, C.M.; Barber, P.A.; Smale, P.R.; Coxon, J.P.; Fleming, M.K.; Byblow, W.D. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 2007, 130, 170–180. [Google Scholar] [CrossRef]
- Burke Quinlan, E.; Dodakian, L.; See, J.; McKenzie, A.; Le, V.; Wojnowicz, M.; Shahbaba, B.; Cramer, S.C. Neural function, injury, and stroke subtype predict treatment gains after stroke. Ann. Neurol. 2015, 77, 132–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehme, A.K.; Eickhoff, S.B.; Rottschy, C.; Fink, G.R.; Grefkes, C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. Neuroimage 2012, 59, 2771–2782. [Google Scholar] [CrossRef] [PubMed]
- Johansen-Berg, H.; Dawes, H.; Guy, C.; Smith, S.M.; Wade, D.T.; Matthews, P.M. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain 2002, 125, 2731–2742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Várkuti, B.; Guan, C.; Pan, Y.; Phua, K.S.; Ang, K.K.; Kuah, C.W.K.; Chua, K.; Ang, B.T.; Birbaumer, N.; Sitaram, R. Resting state changes in functional connectivity correlate with movement recovery for BCI and robot-assisted upper-extremity training after stroke. Neurorehabilit. Neural Repair 2013, 27, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Kucinski, T.; Tüscher, O.; Pawlas, F.; Bäumer, T.; Weiller, C. Motor cortex excitability after cerebellar infarction. Stroke 2004, 35, 2484–2488. [Google Scholar] [CrossRef] [Green Version]
- Park, C.-h.; Chang, W.H.; Ohn, S.H.; Kim, S.T.; Bang, O.Y.; Pascual-Leone, A.; Kim, Y.-H. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke 2011, 42, 1357–1362. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, P.; Liang, F.; Huang, W. The microstructural status of the corpus callosum is associated with the degree of motor function and neurological deficit in stroke patients. PLoS ONE 2015, 10, e0122615. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Park, E.; Lee, A.; Chang, W.H.; Kim, D.S.; Kim, Y.H. Recovery-related indicators of motor network plasticity according to impairment severity after stroke. Eur. J. Neurol. 2017, 24, 1290–1299. [Google Scholar] [CrossRef]
- Schulz, R.; Park, E.; Lee, J.; Chang, W.H.; Lee, A.; Kim, Y.-H.; Hummel, F.C. Interactions between the corticospinal tract and premotor–motor pathways for residual motor output after stroke. Stroke 2017, 48, 2805–2811. [Google Scholar] [CrossRef]
- Ramnani, N. The primate cortico-cerebellar system: Anatomy and function. Nat. Rev. Neurosci. 2006, 7, 511–522. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Sherman, J.C. The cerebellar cognitive affective syndrome. Brain A J. Neurol. 1998, 121, 561–579. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D.; Guell, X.; Stoodley, C.J.; Halko, M.A. The theory and neuroscience of cerebellar cognition. Annu. Rev. Neurosci. 2019, 42, 337–364. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | n = 70 |
|---|---|
| Age (years) | |
| Mean ± SD | 59.1 ± 12.9 |
| Sex (n) | |
| Male | 42 |
| Female | 28 |
| Lesion side (n) | |
| Right | 34 |
| Left | 36 |
| Lesion location (n) | |
| Supratentorial | 57 |
| Infratentorial | 13 |
| Lesion volume (cm3) | |
| Mean ± SD | 49.9 ± 78.1 |
| Initial impairment, mean ± SD | |
| FMA-UE | 13.8 ± 9.4 |
| NIHSS | 8.9 ± 4.4 |
| MMSE | 23.0 ± 9.1 |
| Model | Estimate | t | p | R2 | Adjusted R2 |
|---|---|---|---|---|---|
| CST*CCT | 0.358 | 0.329 | |||
| Intercept | 281.42 | 2.12 | 0.038 | ||
| CST | −340.6 | −2.19 | 0.032 | ||
| CCT | −313.5 | −2.44 | 0.018 | ||
| CST*CCT | 397.9 | 2.63 | 0.011 |
| Tracts | Proportional CST FA > 0.9 (n = 26) | Proportional CST FA ≤ 0.9 (n = 44) | ||||
|---|---|---|---|---|---|---|
| t | p | R2 | t | p | R2 | |
| CST | −0.89 | 0.382 | 0.032 | 4.75 | <0.001 | 0.349 * |
| CCT | 2.63 | 0.015 | 0.223 * | −1.16 | 0.252 | 0.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Chang, W.H.; Kim, Y.-H. Relationship between the Corticospinal and Corticocerebellar Tracts and Their Role in Upper Extremity Motor Recovery in Stroke Patients. J. Pers. Med. 2021, 11, 1162. https://doi.org/10.3390/jpm11111162
Lee J, Chang WH, Kim Y-H. Relationship between the Corticospinal and Corticocerebellar Tracts and Their Role in Upper Extremity Motor Recovery in Stroke Patients. Journal of Personalized Medicine. 2021; 11(11):1162. https://doi.org/10.3390/jpm11111162
Chicago/Turabian StyleLee, Jungsoo, Won Hyuk Chang, and Yun-Hee Kim. 2021. "Relationship between the Corticospinal and Corticocerebellar Tracts and Their Role in Upper Extremity Motor Recovery in Stroke Patients" Journal of Personalized Medicine 11, no. 11: 1162. https://doi.org/10.3390/jpm11111162
APA StyleLee, J., Chang, W. H., & Kim, Y.-H. (2021). Relationship between the Corticospinal and Corticocerebellar Tracts and Their Role in Upper Extremity Motor Recovery in Stroke Patients. Journal of Personalized Medicine, 11(11), 1162. https://doi.org/10.3390/jpm11111162