Quantitative Imaging Biomarkers in Age-Related Macular Degeneration and Diabetic Eye Disease: A Step Closer to Precision Medicine
Abstract
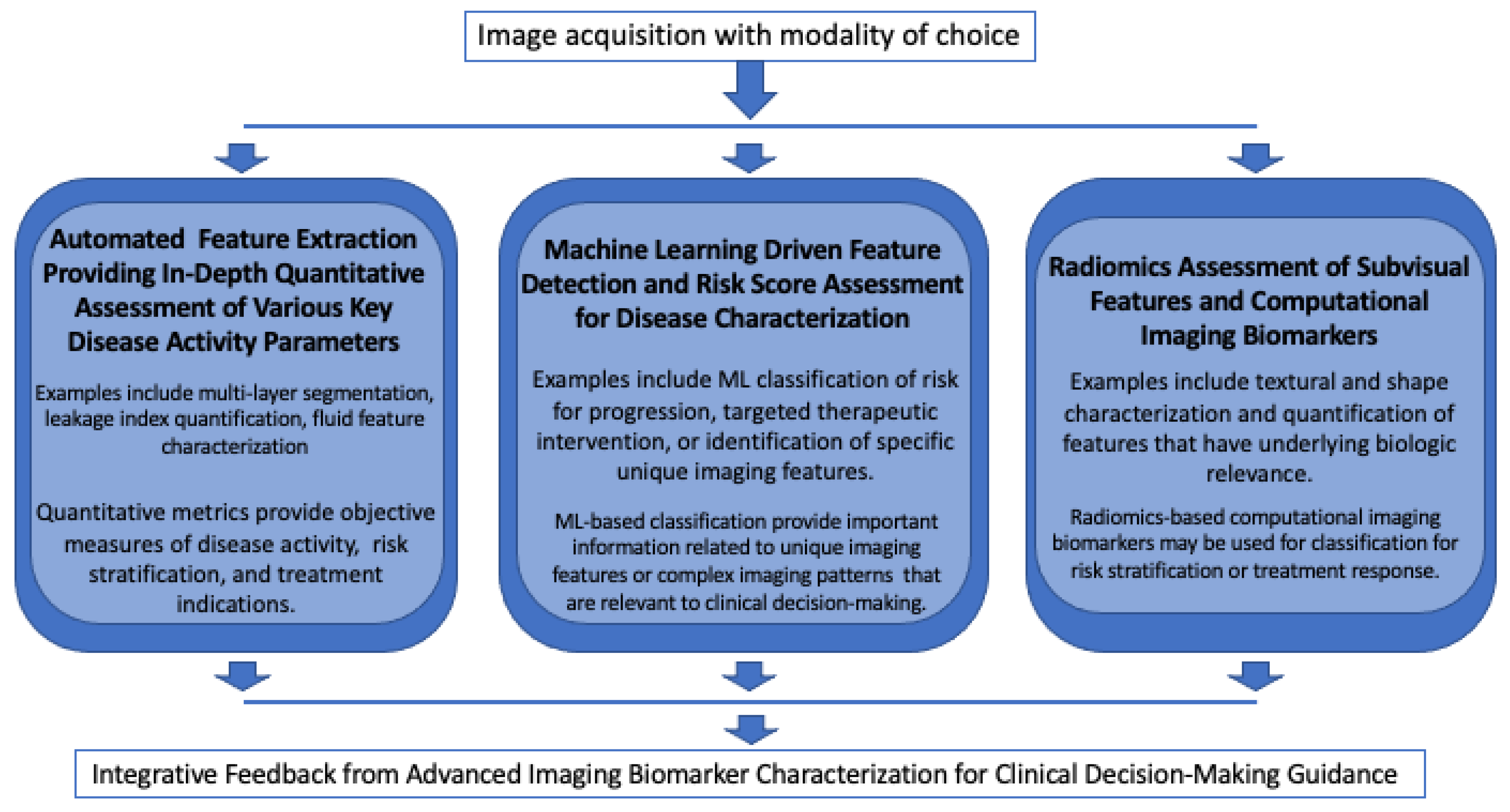
:1. Introduction
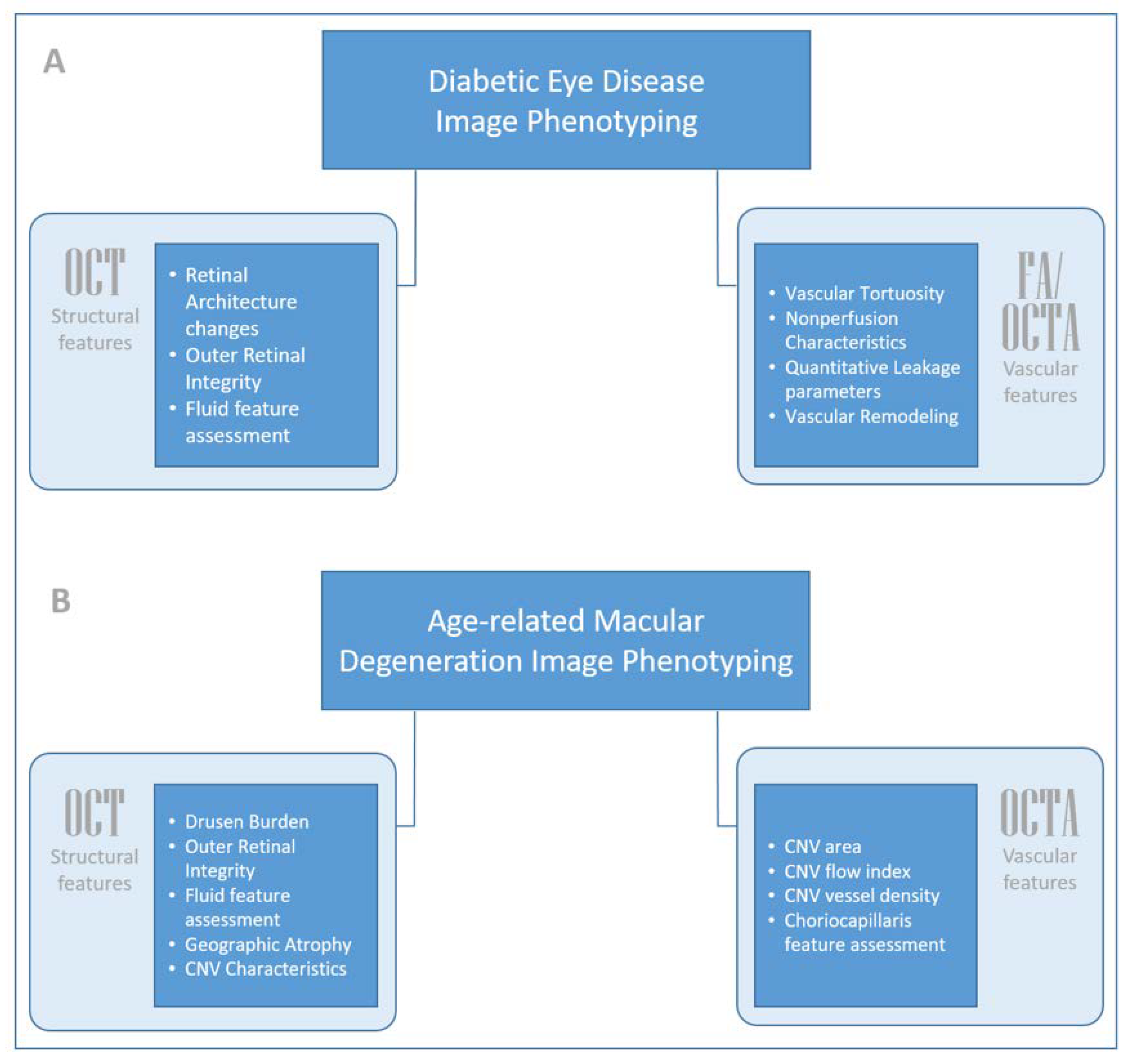
2. Diabetic Eye Disease: Diabetic Retinopathy and Diabetic Macular Edema
2.1. Structural Biomarkers: Optical Coherence Tomography (OCT)
2.1.1. Characterizing Disease Burden and Functional Significance
2.1.2. Imaging Biomarkers and Disease Pathway Expression
2.1.3. Predicting Future Treatment Need and Treatment Response Characteristics
2.2. Vascular Biomarkers: Ultra-Widefield Fluorescein Angiography (UWFA)
2.2.1. Biomarkers for Disease Severity and Disease Burden
2.2.2. Evaluating and Predicting Treatment Response Characteristics
2.2.3. Imaging Biomarkers and Disease Pathway Expression
2.2.4. Radiomics Angiographic Biomarkers for DR Severity
2.2.5. Angiographic Biomarkers for DME Presence
2.2.6. Evaluating and Predicting Therapeutic Response: From Quantitative UWFA to Radiomics
2.3. Vascular Biomarkers: OCTA
Biomarkers for Disease Severity and Burden: From Quantitative Features to Radiomics
3. Age-Related Macular Degeneration (AMD): Neovascular and Non-Neovascular AMD
3.1. Structural Biomarkers: Optical Coherence Tomography (OCT)
3.1.1. Features for Predicting Progression in AMD
3.1.2. Deep Learning and Radiomics Biomarkers in AMD
3.2. Vascular Biomarkers: OCTA
3.2.1. Quantitative Biomarkers of CNV Features
3.2.2. Choriocapillaris Biomarkers in Non-Neovascular AMD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ehlers, J.P.; Uchida, A.; Hu, M.; Figueiredo, N.; Kaiser, P.K.; Heier, J.S.; Brown, D.M.; Boyer, D.S.; Do, D.V.; Gibson, A.; et al. Higher-Order Assessment of OCT in Diabetic Macular Edema from the VISTA Study: Ellipsoid Zone Dynamics and the Retinal Fluid Index. Ophthalmol. Retin. 2019, 3, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Querques, L.; Parravano, M.; Sacconi, R.; Rabiolo, A.; Bandello, F.; Querques, G. Ischemic index changes in diabetic retinopathy after intravitreal dexamethasone implant using ultra-widefield fluorescein angiography: A pilot study. Acta Diabetol. 2017, 54, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.R.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H.; et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e888–e897. [Google Scholar] [CrossRef] [Green Version]
- Kurmann, T.; Yu, S.; Márquez-Neila, P.; Ebneter, A.; Zinkernagel, M.S.; Munk, M.R.; Wolf, S.; Sznitman, R. Expert-level Automated Biomarker Identification in Optical Coherence Tomography Scans. Sci. Rep. 2019, 9, 13605. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Klimscha, S.; Waldstein, S.M.; Bogunović, H. A view of the current and future role of optical coherence tomography in the management of age-related macular degeneration. Eye 2016, 31, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Clark, J.; Uchida, A.; Figueiredo, N.; Babiuch, A.; Talcott, K.E.; Lunasco, L.; Le, T.K.; Meng, X.; Hu, M.; et al. Longitudinal Higher-Order OCT Assessment of Quantitative Fluid Dynamics and the Total Retinal Fluid Index in Neovascular AMD. Transl. Vis. Sci. Technol. 2021, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Moraes, G.; Fu, D.J.; Wilson, M.; Khalid, H.; Wagner, S.K.; Korot, E.; Ferraz, D.; Faes, L.; Kelly, C.J.; Spitz, T.; et al. Quantitative Analysis of OCT for Neovascular Age-Related Macular Degeneration Using Deep Learning. Ophthalmology 2021, 128, 693–705. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Khan, M.; Petkovsek, D.; Stiegel, L.; Kaiser, P.; Singh, R.P.; Reese, J.L.; Srivastava, S.K. Outcomes of Intraoperative OCT–Assisted Epiretinal Membrane Surgery from the PIONEER Study. Ophthalmol. Retin. 2018, 2, 263–267. [Google Scholar] [CrossRef]
- Reznicek, L.; Kolb, J.P.; Klein, T.; Mohler, K.J.; Wieser, W.; Huber, R.; Kernt, M.; Märtz, J.; Neubauer, A.S. Wide-Field Megahertz OCT Imaging of Patients with Diabetic Retinopathy. J. Diabetes Res. 2015, 2015, 1–5. [Google Scholar] [CrossRef] [Green Version]
- De Pretto, L.R.; Moult, E.M.; Alibhai, A.Y.; Carrasco-Zevallos, O.M.; Chen, S.; Lee, B.K.; Witkin, A.J.; Baumal, C.R.; Reichel, E.; de Freitas, A.Z.; et al. Controlling for artifacts in widefield optical coherence tomography angiography measurements of non-perfusion area. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- de Carlo, T.E.; Bonini Filho, M.A.; Baumal, C.R.; Reichel, E.; Rogers, A.; Witkin, A.J.; Duker, J.S.; Waheed, N.K. Evaluation of preretinal neovascularization in proliferative diabetic retinopathy using optical coherence tomography angiography. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Sawada, O.; Ichiyama, Y.; Obata, S.; Ito, Y.; Kakinoki, M.; Sawada, T.; Saishin, Y.; Ohji, M. Comparison between wide-angle OCT angiography and ultra-wide field fluorescein angiography for detecting non-perfusion areas and retinal neovascularization in eyes with diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Kaines, A.; Tsui, I.; Sarraf, D.; Schwartz, S. The Use of Ultra Wide Field Fluorescein Angiography in Evaluation and Management of Uveitis. Semin. Ophthalmol. 2009, 24, 19–24. [Google Scholar] [CrossRef]
- Couturier, A.; Rey, P.-A.; Erginay, A.; Lavia, C.; Bonnin, S.; Dupas, B.; Gaudric, A.; Tadayoni, R. Widefield OCT-Angiography and Fluorescein Angiography Assessments of Nonperfusion in Diabetic Retinopathy and Edema Treated with Anti–Vascular Endothelial Growth Factor. Ophthalmology 2019, 126, 1685–1694. [Google Scholar] [CrossRef]
- Abraham, J.R.; Wykoff, C.C.; Arepalli, S.; Lunasco, L.; Hannah, J.Y.; Hu, M.; Reese, J.; Krovastava, S.K.; Brown, D.M.; Ehlers, J.P. Aqueous cytokine expression and higher order OCT biomarkers: Assessment of the Anatomic-Biologic bridge in the IMAGINE DME study. Am. J. Ophthalmol. 2021, 222, 328–339. [Google Scholar] [CrossRef]
- Abraham, J.R.; Wykoff, C.C.; Arepalli, S.; Lunasco, L.; Hannah, J.Y.; Martin, A.; Mugnaini, C.; Hu, M.; Reese, J.; Strivastava, S.K.; et al. Exploring the angiographic-biologic phenotype in the IMAGINE study: Quantitative UWFA and cytokine expression. Br. J. Ophthalmol. 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/34099465/ (accessed on 31 July 2021).
- Prasanna, P.; Bobba, V.; Figueiredo, N.; Sevgi, D.D.; Lu, C.; Braman, N.; Alilou, M.; Sharma, S.; Srivastava, S.K.; Madabhushi, A.; et al. Radiomics-based assessment of ultra-widefield leakage patterns and vessel network architecture in the PERMEATE study: Insights into treatment durability. Br. J. Ophthalmol. 2020, 105, 1155. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.S.; Sevgi, D.D.; Dong, V.; Srivastava, S.K.; Madabhushi, A.; Ehlers, J.P. Multi-Compartment Spatially-Derived Radiomics From Optical Coherence Tomography Predict Anti-VEGF Treatment Durability in Macular Edema Secondary to Retinal Vascular Disease: Preliminary Findings. IEEE J. Transl. Eng. Health Med. 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Sil, K.S.; Sevji, D.D.; Dong, V.; Srivastava, S.K.; Madabhushi, A.; Ehlers, J.P. Multi-Compartment OCT-derived Radiomics Features to predict Anti-VEGF Treatment Durability for Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2021, 62, 3. [Google Scholar]
- Akkus, Z.; Galimzianova, A.; Hoogi, A.; Rubin, D.L.; Erickson, B.J. Deep Learning for Brain MRI Segmentation: State of the Art and Future Directions. J. Digit. Imaging 2017, 30, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Sumathipala, Y.; Lay, N.S.; Turkbey, B. Prostate cancer detection from multi-institution multiparametric MRIs using deep convolutional neural networks. J. Med. Imaging 2018, 5, 044507. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; De Sisternes, L.; Hallak, J.A.; Leng, T.; Osborne, A.; Rosenfeld, P.J.; Gregori, G.; Durbin, M.; Rubin, D. Prediction of age-related macular degeneration disease using a sequential deep learning approach on longitudinal SD-OCT imaging bi-omarkers. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lundervold, A.; Lundervold, A. An overview of deep learning in medical imaging focusing on MRI. Z. Med. Phys. 2019, 29, 102–127. [Google Scholar] [CrossRef] [PubMed]
- Dong, V.; Sevgi, D.D.; Kar, S.S.; Srivastava, S.K.; Ehlers, J.P.; Madabhushi, A. Evaluating the utility of deep learning using ultra-widefield fluorescein angiography for predicting need for anti-VEGF therapy in diabetic eye disease. Investig. Ophthalmol. Visual Sci. 2021, 62, 2114. [Google Scholar]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 36. [Google Scholar] [CrossRef]
- Wu, G.; Chen, Y.; Wang, Y.; Yu, J.; Lv, X.; Ju, X.; Shi, Z.; Chen, L.; Chen, Z. Sparse Representation-Based Radiomics for the Diagnosis of Brain Tumors. IEEE Trans. Med. Imaging 2018, 37, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Parekh, V.S.; Jacobs, M.A. Integrated radiomic framework for breast cancer and tumor biology using advanced machine learning and multiparametric MRI. NPJ Breast Cancer 2017, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Penzias, G.; Singanamalli, A.; Elliott, R.; Gollamudi, J.; Shih, N.; Feldman, M.; Stricker, P.; Delprado, W.; Tiwari, S.; Böhm, M.; et al. Identifying the morphologic basis for radiomic features in distinguishing different Gleason grades of prostate cancer on MRI: Preliminary findings. PLoS ONE 2018, 13, e0200730. [Google Scholar] [CrossRef] [Green Version]
- Vujosevic, S.; Midena, E. Retinal Layers Changes in Human Preclinical and Early Clinical Diabetic Retinopathy Support Early Retinal Neuronal and Müller Cells Alterations. J. Diabetes Res. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Shi, R.; Guo, Z.; Wang, F.; Lin, R.; Zhao, L. Alterations in retinal nerve fiber layer thickness in early stages of diabetic reti-nopathy and potential risk factors. Curr. Eye Res. 2018, 43, 244–253. [Google Scholar] [CrossRef]
- Deák, G.G.; Schmidt-Erfurth, U.; Jampol, L.M. Correlation of Central Retinal Thickness and Visual Acuity in Diabetic Macular Edema. JAMA Ophthalmol. 2018, 136, 1215–1216. [Google Scholar] [CrossRef]
- Joltikov, K.; Sesi, C.A.; De Castro, V.M.; Davila, J.R.; Anand, R.; Khan, S.M.; Farbman, N.; Jackson, G.R.; Johnson, C.A.; Gardner, T.W. Disorganization of Retinal Inner Layers (DRIL) and Neuroretinal Dysfunction in Early Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2018, 59, 5481–5486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.K.; Lin, M.M.; Lammer, J.; Prager, S.; Sarangi, R.; Silva, P.S.; Aiello, L.P. Disorganization of the Retinal Inner Layers as a Predictor of Visual Acuity in Eyes With Center-Involved Diabetic Macular Edema. JAMA Ophthalmol. 2014, 132, 1309–1316. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, L.; Ramu, J.; Triantafyllopoulou, I.; Patrao, N.V.; Comyn, O.; Hykin, P.; Sivaprasad, S. Diagnostic accuracy of disorganization of the retinal inner layers in detecting macular capillary non-perfusion in diabetic retinopathy. Clin. Exp. Ophthalmol. 2015, 43, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Eliwa, T.F.; Hussein, M.A.; Zaki, M.A.; Raslan, O.A. Outer retinal layer thickness as good visual predictor in patients with diabetic macular edema. Retina 2018, 38, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Bolz, M.; Schmidt-Erfurth, U.; Deak, G.; Mylonas, G.; Kriechbaum, K.; Scholda, C. Optical Coherence Tomographic Hyperreflective Foci: A Morphologic Sign of Lipid Extravasation in Diabetic Macular Edema. Ophthalmology 2009, 116, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Bini, S.; Midena, G.; Berton, M.; Pilotto, E.; Midena, E. Hyperreflective Intraretinal Spots in Diabetics without and with Nonproliferative Diabetic Retinopathy: AnIn VivoStudy Using Spectral Domain OCT. J. Diabetes Res. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Jang, H.; A Choi, Y.; Kim, H.C.; Chung, H. Association Between Soluble CD14 in the Aqueous Humor and Hyperreflective Foci on Optical Coherence Tomography in Patients With Diabetic Macular Edema. Investig. Opthalmol. Vis. Sci. 2018, 59, 715–721. [Google Scholar] [CrossRef] [Green Version]
- De Benedetto, U.; Sacconi, R.; Pierro, L.; Lattanzio, R.; Bandello, F. Optical coherence tomographic hyperreflective foci in early stages of diabetic retinopathy. Retina 2015, 35, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Okuwobi, I.P.; Ji, Z.; Fan, W.; Yuan, S.; Bekalo, L.; Chen, Q. Automated Quantification of Hyperreflective Foci in SD-OCT With Diabetic Retinopathy. IEEE J. Biomed. Health Inform. 2019, 24, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Rübsam, A.; Wernecke, L.; Rau, S.; Pohlmann, D.; Müller, B.; Zeitz, O.; Joussen, A.M. Behavior of SD-OCT Detectable Hyperreflective Foci in Diabetic Macular Edema Patients after Therapy with Anti-VEGF Agents and Dexamethasone Implants. J. Diabetes Res. 2021, 2021, 8820216. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.K.; Vogl, W.D.; Gerendas, B.S.; Glassman, A.R.; Bogunovic, H.; Jampol, L.M.; Schmidt-Erfurth, U.M. Quantification of fluid resolution and visual acuity gain in patients with diabetic macular edema using deep learning: A post hoc analysis of a randomized clinical trial. JAMA Ophthalmol. 2020, 138, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Uchida, A.; Sevgi, D.D.; Hu, M.; Reed, K.; Berliner, A.; Vitti, R.; Chu, K.; Srivastava, S.K. Retinal Fluid Volatility Associated with Interval Tolerance and Visual Outcomes in Diabetic Macular Edema in the VISTA Phase III Trial. Am. J. Ophthalmol. 2021, 224, 217–227. [Google Scholar] [CrossRef]
- Rasti, R.; Allingham, M.J.; Mettu, P.S.; Kavusi, S.; Govind, K.; Cousins, S.W.; Farsiu, S. Deep learning-based single-shot pre-diction of differential effects of anti-VEGF treatment in patients with diabetic macular edema. Biomed. Opt. Express 2020, 11, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Prahs, P.; Radeck, V.; Mayer, C.; Cvetkov, Y.; Cvetkova, N.; Helbig, H.; Märker, D. OCT-based deep learning algorithm for the evaluation of treatment indication with anti-vascular endothelial growth factor medications. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 91–98. [Google Scholar] [CrossRef]
- Manivannan, A.; Plskova, J.; Farrow, A.; Mckay, S.; Sharp, P.F.; Forrester, J.V. Ultra-Wide-Field Fluorescein Angiography of the Ocular Fundus. Am. J. Ophthalmol. 2005, 140, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Falavarjani, K.G.; Wang, K.; Khadamy, J.; Sadda, S.R. Ultra-wide-field imaging in diabetic retinopathy; an overview. J. Curr. Ophthalmol. 2016, 28, 57–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabbani, H.; Allingham, M.J.; Mettu, P.S.; Cousins, S.W.; Farsiu, S. Fully Automatic Segmentation of Fluorescein Leakage in Subjects With Diabetic Macular Edema. Investig. Opthalmol. Vis. Sci. 2015, 56, 1482–1492. [Google Scholar] [CrossRef] [Green Version]
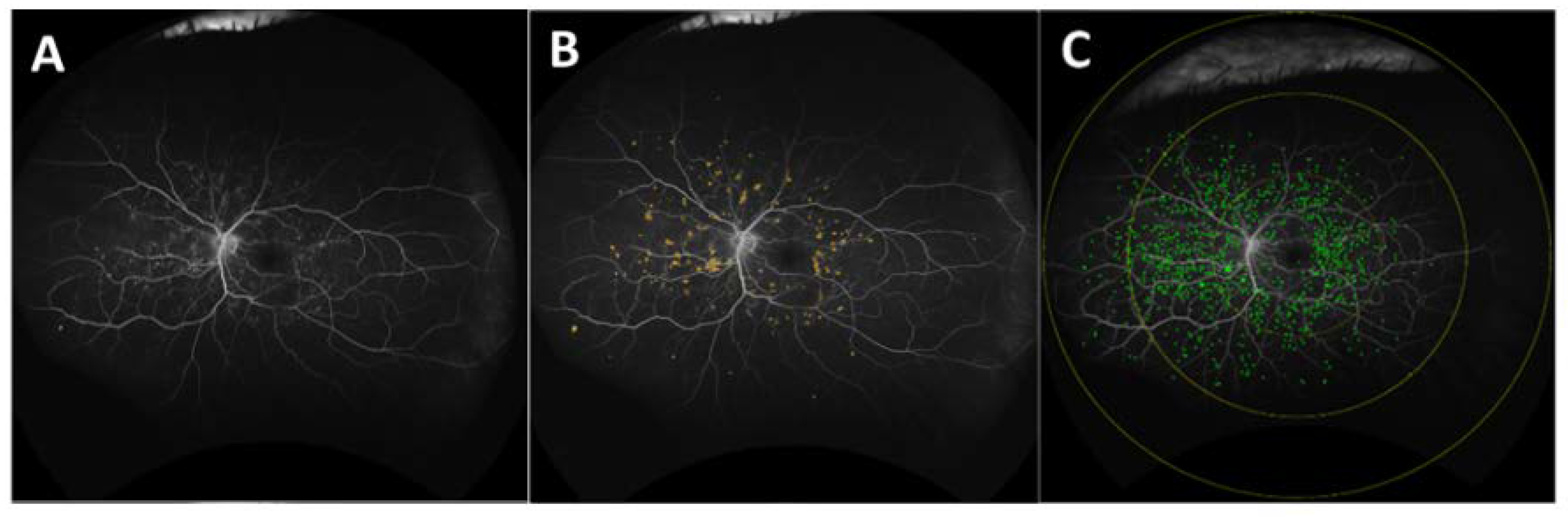
- Ehlers, J.P.; Wang, K.; Vasanji, A.; Hu, M.; Srivastava, S.K. Automated quantitative characterisation of retinal vascular leakage and microaneurysms in ultra-widefield fluorescein angiography. Br. J. Ophthalmol. 2017, 101, 696–699. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.; Sevgi, D.D.; Srivastava, S.K.; Whitney, J.; Hach, J.M.; Atwood, R.; Springer, Q.; Williams, J.; Vasanji, A.; Reese, J.; et al. Longitudinal precision of vasculature parameter assessment on ultra-widefield fluorescein angiography using a deep-learning model for vascular segmentation in eyes without vascular pathology. Investig. Ophthalmol. Vis. Sci. 2020, 61, 2010. [Google Scholar]
- Sevgi, D.D.; Hach, J.; Srivastava, S.K.; Wykoff, C.; O’connell, M.; Whitney, J.; Reese, J.; Ehlers, J.P. Automated quality optimized phase selection in ultra-widefield angiography using retinal vessel segmentation with deep neural networks. Investig. Ophthalmol. Vis. Sci. 2020, 61, PB00125. [Google Scholar]
- Sevgi, D.D.; Scott, A.W.; Martin, A.; Mugnaini, C.; Patel, S.; Linz, M.O.; Nti, A.; Reese, J.; Ehlers, J.P. Longitudinal assessment of quantitative ultra-widefield ischaemic and vascular parameters in sickle cell retinopathy. Br. J. Ophthalmol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.C.; Srivastava, S.K.; Hu, M.; Figueiredo, N.; Babiuch, A.; Boss, J.D.; Reese, J.L.; Ehlers, J.P. Quantitative Ultra-Widefield Angiographic Features and Associations with Diabetic Macular Edema. Ophthalmol. Retin. 2020, 4, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Jiang, A.C.; Boss, J.D.; Hu, M.; Figueiredo, N.; Babiuch, A.; Talcott, K.; Sharma, S.; Hach, J.; Le, T.K.; et al. Quantitative Ultra-Widefield Angiography and Diabetic Retinopathy Severity. Ophthalmology 2019, 126, 1527–1532. [Google Scholar] [CrossRef]
- Babiuch, A.S.; Wykoff, C.C.; Srivastava, S.K.; Talcott, K.; Zhou, B.; Hach, J.; Hu, M.; Reese, J.L.; Ehlers, J.P. Retinal Leakage Index Dynamics On Ultra-Widefield Fluorescein Angiography In Eyes Treated With Intravitreal Aflibercept For Proliferative Diabetic Retinopathy In The Recovery Study. Retina 2020, 40, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Indian Retina Research Associates (IRRA); Alagorie, A.R.; Ramasamy, K.; van Hemert, J.; Yadav, N.; Pappuru, R.R.; Tufail, A.; Nittala, M.G.; Sadda, S.R.; et al. Distribution of peripheral lesions identified by mydriatic ultra-wide field fundus imaging in diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 725–733. [Google Scholar] [CrossRef]
- Silva, P.S.; Cruz, A.J.D.; Ledesma, M.G.; van Hemert, J.; Radwan, A.; Cavallerano, J.; Aiello, L.M.; Sun, J.K. Diabetic Retinopathy Severity and Peripheral Lesions Are Associated with Nonperfusion on Ultrawide Field Angiography. Ophthalmology 2015, 122, 2465–2472. [Google Scholar] [CrossRef]
- Figueiredo, N.; Srivastava, S.K.; Singh, R.P.; Babiuch, A.; Sharma, S.; Rachitskaya, A.; Talcott, K.; Reese, J.; Hu, M.; Ehlers, J.P. Longitudinal Panretinal Leakage and Ischemic Indices in Retinal Vascular Disease after Aflibercept Therapy. Ophthalmol. Retin. 2020, 4, 154–163. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Nittala, M.G.; Zhou, B.; Fan, W.; Velaga, S.B.; Lampen, S.I.; Rusakevich, A.; Ehlers, J.P.; Babiuch, A.; Brown, D.M.; et al. Intravitreal Aflibercept for Retinal Nonperfusion in Proliferative Diabetic Retinopathy. Ophthalmol. Retin. 2019, 3, 1076–1086. [Google Scholar] [CrossRef]
- Yu, H.J.; Ehlers, J.P.; Sevgi, D.D.; Hach, J.; O’Connell, M.; Reese, J.L.; Srivastava, S.K.; Wykoff, C.C. Real-Time Photographic- and Fluorescein Angiographic-Guided Management of Diabetic Retinopathy: Randomized PRIME Trial Outcomes. Am. J. Ophthalmol. 2021, 226, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Nittala, M.G.; Velaga, S.B.; Hirano, T.; Wykoff, C.C.; Ip, M.; Lampen, S.I.; van Hemert, J.; Fleming, A.; Verhoek, M.; et al. Distribution of Nonperfusion and Neovascularization on Ultrawide-Field Fluorescein Angiography in Proliferative Diabetic Retinopathy (RECOVERY Study): Report 1. Am. J. Ophthalmol. 2019, 206, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Mainster, M.A. The fractal properties of retinal vessels: Embryological and clinical implications. Eye 1990, 4, 235–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, W.; Uji, A.; Wang, K.; Falavarjani, K.G.; Wykoff, C.C.; Brown, D.M.; Van Hemert, J.; Sagong, M.; Sadda, S.R.; Ip, M. Severity Of Diabetic Macular Edema Correlates With Retinal Vascular Bed Area On Ultra-Wide Field Fluorescein Angiography: DAVE Study. Retina 2020, 40, 1029–1037. [Google Scholar] [CrossRef]
- Fan, W.; Nittala, M.G.; Fleming, A.; Robertson, G.; Uji, A.; Wykoff, C.C.; Brown, D.M.; van Hemert, J.; Ip, M.; Wang, K.; et al. Relationship Between Retinal Fractal Dimension and Nonperfusion in Diabetic Retinopathy on Ultrawide-Field Fluorescein Angiography. Am. J. Ophthalmol. 2020, 209, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Sevgi, D.D.; Srivastava, S.K.; Whitney, J.; O’Connell, M.; Kar, S.S.; Hu, M.; Reese, J.; Madabhushi, A.; Ehlers, J.P. Characterization of Ultra-Widefield Angiographic Vascular Features in Diabetic Retinopathy with Automated Severity Classification. Ophthalmol. Sci. 2021, 1, 100049. [Google Scholar] [CrossRef]
- Fang, M.; Fan, W.; Shi, Y.; Ip, M.S.; Wykoff, C.C.; Wang, K.; Falavarjani, K.G.; Brown, D.M.; van Hemert, J.; Sadda, S.R. Classification of Regions of Nonperfusion on Ultra-widefield Fluorescein Angiography in Patients with Diabetic Macular Edema. Am. J. Ophthalmol. 2019, 206, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, A.; Figueiredo, N.; Prasanna, P.; Srivastava, S.K.; Sharma, S.; Madabhushi, A.; Ehlers, J.P. Imaging Features of Vessels and Leakage Patterns Predict Extended Interval Aflibercept Dosing Using Ultra-Widefield Angiography in Retinal Vascular Disease: Findings From the PERMEATE Study. IEEE Trans. Biomed. Eng. 2021, 68, 1777–1786. [Google Scholar] [CrossRef]
- Hormel, T.T.; Jia, Y.; Jian, Y.; Hwang, T.S.; Bailey, S.T.; Pennesi, M.E.; Wilson, D.J.; Morrison, J.C.; Huang, D. Plexus-specific retinal vascular anatomy and pathologies as seen by projection-resolved optical coherence tomographic angiography. Prog. Retin. Eye Res. 2021, 80, 100878. [Google Scholar] [CrossRef]
- Shahlaee, A.; Pefkianaki, M.; Hsu, J.; Ho, A.C. Measurement of Foveal Avascular Zone Dimensions and its Reliability in Healthy Eyes Using Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2016, 161, 50–55.e1. [Google Scholar] [CrossRef] [PubMed]
- Barraso, M.; Alé-Chilet, A.; Hernández, T.; Oliva, C.; Vinagre, I.; Ortega, E.; Figueras-Roca, M.; Sala-Puigdollers, A.; Esquinas, C.; Esmatjes, E.; et al. Optical Coherence Tomography Angiography in Type 1 Diabetes Mellitus. Report 1: Diabetic Retinopathy. Transl. Vis. Sci. Technol. 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Salz, D.A.; De Carlo, T.E.; Adhi, M.; Moult, E.M.; Choi, W.; Baumal, C.R.; Witkin, A.J.; Duker, J.S.; Fujimoto, J.G.; Waheed, N.K. Select Features of Diabetic Retinopathy on Swept-Source Optical Coherence Tomographic Angiography Compared with Fluorescein Angiography and Normal Eyes. JAMA Ophthalmol. 2016, 134, 644–650. [Google Scholar] [CrossRef]
- Freiberg, F.J.; Pfau, M.; Wons, J.; Wirth, M.A.; Becker, M.D.; Michels, S. Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1051–1058. [Google Scholar] [CrossRef] [Green Version]
- Balaratnasingam, C.; Inoue, M.; Ahn, S.; McCann, J.; Dhrami-Gavazi, E.; Yannuzzi, L.A.; Freund, K.B. Visual Acuity Is Correlated with the Area of the Foveal Avascular Zone in Diabetic Retinopathy and Retinal Vein Occlusion. Ophthalmology 2016, 123, 2352–2367. [Google Scholar] [CrossRef] [PubMed]
- Samara, W.A.; Shahlaee, A.; Adam, M.; Khan, M.A.; Chiang, A.; Maguire, J.I.; Hsu, J.; Ho, A.C. Quantification of Diabetic Macular Ischemia Using Optical Coherence Tomography Angiography and Its Relationship with Visual Acuity. Ophthalmology 2017, 124, 235–244. [Google Scholar] [CrossRef]
- Lee, H.; Lee, M.; Chung, H.; Kim, H.C. Quantification Of Retinal Vessel Tortuosity In Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Retina 2018, 38, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Zarranz-Ventura, J.; Barraso, M.; Alé-Chilet, A.; Hernandez, T.; Oliva, C.; Gascón, J.; Sala-Puigdollers, A.; Figueras-Roca, M.; Vinagre, I.; Ortega, E.; et al. Evaluation of microvascular changes in the perifoveal vascular network using optical coherence tomography angiography (OCTA) in type I diabetes mellitus: A large scale prospective trial. BMC Med. Imaging 2019, 19, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Z.; Lin, J.; Gao, C.; Xin, C.; Zhang, Q.; Chen, C.-L.; Roisman, L.; Gregori, G.; Rosenfeld, P.J.; Wang, R. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J. Biomed. Opt. 2016, 21, 066008. [Google Scholar] [CrossRef] [Green Version]
- Dupas, B.; Minvielle, W.; Bonnin, S.; Couturier, A.; Erginay, A.; Massin, P.; Gaudric, A.; Tadayoni, R. Association Between Vessel Density and Visual Acuity in Patients with Diabetic Retinopathy and Poorly Controlled Type 1 Diabetes. JAMA Ophthalmol. 2018, 136, 721–728. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wang, J.J.; Sharrett, A.R.; Islam, F.A.; Klein, R.; Klein, B.E.; Cotch, M.F.; Wong, T.Y. Relationship of Retinal Vascular Caliber with Diabetes and Retinopathy: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2007, 31, 544–549. [Google Scholar] [CrossRef] [Green Version]
- Tsai, A.S.; Wong, T.Y.; Lavanya, R.; Zhang, R.; Hamzah, H.; Tai, E.S.; Cheung, C. Differential association of retinal arteriolar and venular caliber with diabetes and retinopathy. Diabetes Res. Clin. Pr. 2011, 94, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Ng, D.S.; Lam, A.; Luk, F.; Wong, R.; Chan, C.; Mohamed, S.; Fong, A.; Lok, J.; Tso, T.; et al. Determinants of Quantitative Optical Coherence Tomography Angiography Metrics in Patients with Diabetes. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maloca, P.M.; IOB Study Group; Spaide, R.F.; De Carvalho, E.R.; Studer, H.P.; Hasler, P.W.; Scholl, H.P.N.; Heeren, T.; Schottenhamml, J.; Balaskas, K.; et al. Novel biomarker of sphericity and cylindricity indices in volume-rendering optical coherence tomography angiography in normal and diabetic eyes: A preliminary study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 711–723. [Google Scholar] [CrossRef]
- Le, D.; Alam, M.; Miao, B.A.; Lim, J.I.; Yao, X. Fully automated geometric feature analysis in optical coherence tomography angiography for objective classification of diabetic retinopathy. Biomed. Opt. Express 2019, 10, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Nassisi, M.; Lei, J.; Abdelfattah, N.; Karamat, A.; Balasubramanian, S.; Fan, W.; Uji, A.; Marion, K.M.; Baker, K.; Huang, X.; et al. OCT Risk Factors for Development of Late Age-Related Macular Degeneration in the Fellow Eyes of Patients Enrolled in the HARBOR Study. Ophthalmology 2019, 126, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Toth, C.A.; Tai, V.; Chiu, S.J.; Winter, K.; Sevilla, M.B.; Daniel, E.; Grunwald, J.E.; Jaffe, G.J.; Martin, D.F.; Ying, G.-S.; et al. Linking OCT, Angiographic, and Photographic Lesion Components in Neovascular Age-Related Macular Degeneration. Ophthalmol. Retin. 2017, 2, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Lunasco, L.; Abraham, J.R.; Sarici, K.; Sevgi, D.D.; Hanumanthu, A.; Cetin, H.; Hu, M.; Srivastava, S.; Reese, J.; Ehlers, J.P. Comparative Assessment of Long-Term Longitudinal Multi-Layer Retinal Dynamics in Non-neovascular Age-Related Macular Degeneration in Eyes Progressing to Subfoveal Geographic Atrophy and Eyes without Progression. Investig. Ophthalmol. Vis. Sci. 2021, 62, 2548. [Google Scholar]
- Hanumanthu, A.; Sarici, K.; Abraham, J.R.; Whitney, J.; Lunasco, L.; Sevgi, D.D.; Cetin, H.; Srivastava, S.K.; Reese, J.; Ehlers, J.P. Utilizing Higher-Order Quantitative SD-OCT Biomarkers in a Machine Learning Prediction Model for the Development of Subfoveal Geographic Atrophy in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2021, 62, 98. [Google Scholar]
- Lunasco, L.; Abraham, J.R.; Sarici, K.; Sevgi, D.D.; Hanumanthu, A.; Cetin, H.; Hu, M.; Srivastava, S.K.; Reese, J.; Ehlers, J.P. Risk Classification for Progression to Subfoveal Geographic Atrophy in Dry Age-Related Macular Degeneration Using Machine Learning-Enabled Outer Retinal Feature Extraction. OSLI Retin. 2021, in press. [Google Scholar]
- Abdelfattah, N.; Zhang, H.; Boyer, D.S.; Rosenfeld, P.J.; Feuer, W.J.; Gregori, G.; Sadda, S.R. Drusen Volume as a Predictor of Disease Progression in Patients with Late Age-Related Macular Degeneration in the Fellow Eye. Investig. Opthalmol. Vis. Sci. 2016, 57, 1839–1846. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Zahid, R.; Kaiser, P.K.; Heier, J.S.; Brown, D.M.; Meng, X.; Reese, J.; Le, T.K.; Lunasco, L.; Hu, M.; et al. Longitudinal Assessment of Ellipsoid Zone Integrity, Subretinal Hyperreflective Material, and Subretinal Pigment Epithelium Disease in Neovascular Age-Related Macular Degeneration. Ophthalmol. Retin. 2021. [Google Scholar] [CrossRef]
- Waldstein, S.; Philip, A.-M.; Leitner, R.; Simader, C.; Langs, G.; Gerendas, B.S.; Schmidt-Erfurth, U. Correlation of 3-Dimensionally Quantified Intraretinal and Subretinal Fluid with Visual Acuity in Neovascular Age-Related Macular Degeneration. JAMA Ophthalmol. 2016, 134, 182–190. [Google Scholar] [CrossRef] [PubMed]
- De Fauw, J.; Ledsam, J.R.; Romera-Paredes, B.; Nikolov, S.; Tomasev, N.; Blackwell, S.; Askham, H.; Glorot, X.; O’Donoghue, B.; Visentin, D.; et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat. Med. 2018, 24, 1342–1350. [Google Scholar] [CrossRef]
- Lee, C.S.; Baughman, D.M.; Lee, A.Y. Deep Learning Is Effective for Classifying Normal versus Age-Related Macular Degeneration OCT Images. Ophthalmol. Retin. 2017, 1, 322–327. [Google Scholar] [CrossRef] [PubMed]
- De Sisternes, L.; Simon, N.; Tibshirani, R.; Leng, T.; Rubin, D.L. Quantitative SD-OCT Imaging Biomarkers as Indicators of Age-Related Macular Degeneration Progression. Investig. Opthalmology Vis. Sci. 2014, 55, 7093–7103. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Waldstein, S.M.; Klimscha, S.; Sadeghipour, A.; Hu, X.; Gerendas, B.S.; Osborne, A.; Bogunović, H. Prediction of Individual Disease Conversion in Early AMD Using Artificial Intelligence. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3199–3208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Freund, K.B.; Yannuzzi, L.A.; Sorenson, J.A. Age-related Macular Degeneration and Choroidal Neovascularization. Am. J. Ophthalmol. 1993, 115, 786–791. [Google Scholar] [CrossRef]
- Jia, Y.; Bailey, S.T.; Hwang, T.S.; McClintic, S.M.; Gao, S.S.; Pennesi, M.E.; Flaxel, C.J.; Lauer, A.K.; Wilson, D.J.; Hornegger, J.; et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc. Natl. Acad. Sci. USA 2015, 112, E2395–E2402. [Google Scholar] [CrossRef] [Green Version]
- Spaide, R.F.; Klancnik, J.M.; Cooney, M.J. Retinal Vascular Layers Imaged by Fluorescein Angiography and Optical Coherence Tomography Angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Rabiolo, A.; Sacconi, R.; Carnevali, A.; Querques, L.; Bandello, F.; Querques, G. Optical coherence tomography angiography in dry age-related macular degeneration. Surv. Ophthalmol. 2018, 63, 236–244. [Google Scholar] [CrossRef]
- Jia, Y.; Bailey, S.T.; Wilson, D.J.; Tan, O.; Klein, M.L.; Flaxel, C.J.; Potsaid, B.; Liu, J.J.; Lu, C.D.; Kraus, M.F.; et al. Quantitative Optical Coherence Tomography Angiography of Choroidal Neovascularization in Age-Related Macular Degeneration. Ophthalmology 2014, 121, 1435–1444. [Google Scholar] [CrossRef] [Green Version]
- Uchida, A.; Hu, M.; Babiuch, A.; Srivastava, S.K.; Singh, R.P.; Kaiser, P.K.; Talcott, K.; Rachitskaya, A.; Ehlers, J.P. Optical coherence tomography angiography characteristics of choroidal neovascularization requiring varied dosing frequencies in treat-and-extend management: An analysis of the AVATAR study. PLoS ONE 2019, 14, e0218889. [Google Scholar] [CrossRef]
- Chatziralli, I.; Theodossiadis, G.; Panagiotidis, D.; Pousoulidi, P.; Theodossiadis, P. Choriocapillaris Vascular Density Changes in Patients with Drusen: Cross-Sectional Study Based on Optical Coherence Tomography Angiography Findings. Ophthalmol. Ther. 2018, 7, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, M.; Moult, E.M.; Novais, E.A.; Louzada, R.N.; Cole, E.D.; Lee, B.; Husvogt, L.; Keane, P.A.; Denniston, A.K.; Witkin, A.J.; et al. Visualizing the Choriocapillaris Under Drusen: Comparing 1050-nm Swept-Source Versus 840-nm Spectral-Domain Optical Coherence Tomography Angiography. Investig. Opthalmol. Vis. Sci. 2016, 57, OCT585–OCT590. [Google Scholar] [CrossRef] [PubMed]
- Byon, I.; Ji, Y.; Alagorie, A.R.; Tiosano, L.; Sadda, S.R. Topographic Assessment Of Choriocapillaris Flow Deficits In The Intermediate Age-Related Macular Degeneration Eyes With Hyporeflective Cores Inside Drusen. Retina 2021, 41, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Moult, E.M.; Waheed, N.K.; Adhi, M.; Lee, B.; Lu, C.D.; de Carlo, T.E.; Jayaraman, V.; Rosenfeld, P.J.; Duker, J.S.; et al. Ultrahigh-Speed, Swept-Source Optical Coherence Tomography Angiography in Nonexudative Age-Related Macular Degeneration with Geographic Atrophy. Ophthalmology 2015, 122, 2532–2544. [Google Scholar] [CrossRef] [PubMed]
- Camino, A.; Guo, Y.; You, Q.S.; Wang, J.; Huang, D.; Bailey, S.T.; Jia, Y. Detecting and measuring areas of choriocapillaris low perfusion in intermediate, non-neovascular age-related macular degeneration. Neurophotonics 2019, 6, 041108. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalra, G.; Kar, S.S.; Sevgi, D.D.; Madabhushi, A.; Srivastava, S.K.; Ehlers, J.P. Quantitative Imaging Biomarkers in Age-Related Macular Degeneration and Diabetic Eye Disease: A Step Closer to Precision Medicine. J. Pers. Med. 2021, 11, 1161. https://doi.org/10.3390/jpm11111161
Kalra G, Kar SS, Sevgi DD, Madabhushi A, Srivastava SK, Ehlers JP. Quantitative Imaging Biomarkers in Age-Related Macular Degeneration and Diabetic Eye Disease: A Step Closer to Precision Medicine. Journal of Personalized Medicine. 2021; 11(11):1161. https://doi.org/10.3390/jpm11111161
Chicago/Turabian StyleKalra, Gagan, Sudeshna Sil Kar, Duriye Damla Sevgi, Anant Madabhushi, Sunil K. Srivastava, and Justis P. Ehlers. 2021. "Quantitative Imaging Biomarkers in Age-Related Macular Degeneration and Diabetic Eye Disease: A Step Closer to Precision Medicine" Journal of Personalized Medicine 11, no. 11: 1161. https://doi.org/10.3390/jpm11111161






