Pharmaco-Metabolomics of Inhaled Corticosteroid Response in Individuals with Asthma
Abstract
:1. Introduction
2. Methods
2.1. Study Population: Mass General Brigham Biobank
2.2. Metabolomic Profiling for Mass General Brigham Biobank
2.3. Statistical Analysis
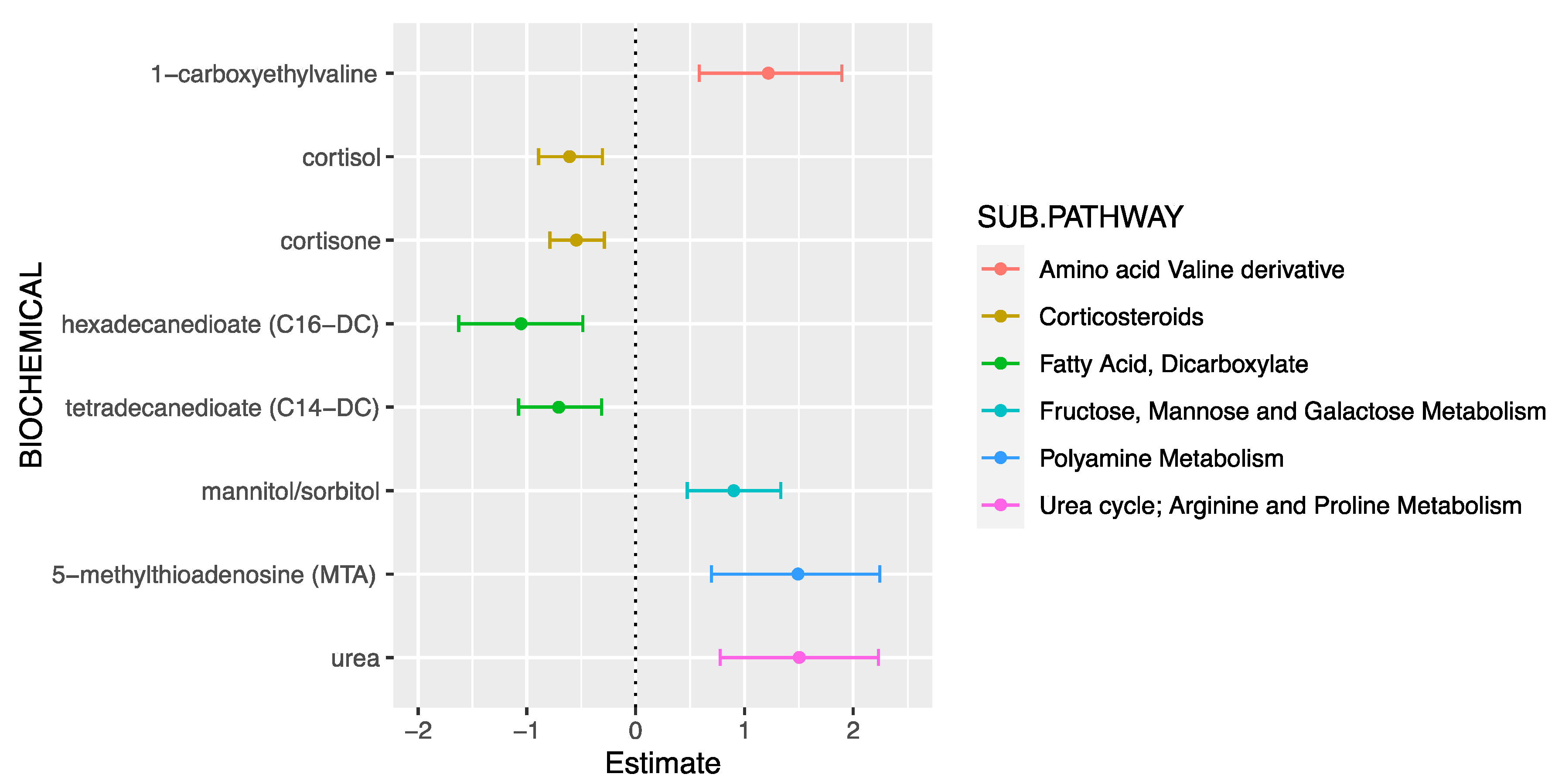
Metabolite Associations with Exacerbation and Their Effect Modification by Age
3. Results
Metabolite Associations with Exacerbation and Their Effect Modification by Age and Sex
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2021. Available online: www.ginasthma.org (accessed on 12 October 2021).
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy 2004, 59, 469–478. [Google Scholar] [CrossRef]
- Becker, A.B.; Abrams, E.M. Asthma guidelines: The Global Initiative for Asthma in relation to national guidelines. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 99–103. [Google Scholar] [CrossRef] [PubMed]
- CDC. Data, Statistics, and Surveillance—Asthma Surveillance Data; CDC: Atlanta, GA, USA, 2018.
- Greally, M.; Jagoe, W.S.; Greally, J. The genetics of asthma. Ir. Med. J. 1982, 75, 403–405. [Google Scholar]
- Dold, S.; Wjst, M.; von Mutius, E.; Reitmeir, P.; Stiepel, E. Genetic risk for asthma, allergic rhinitis, and atopic dermatitis. Arch. Dis. Child. 1992, 67, 1018–1022. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, M.A.; Hopper, J.L.; Giles, G.G. Regressive logistic modeling of familial aggregation for asthma in 7,394 population-based nuclear families. Genet. Epidemiol. 1997, 14, 317–332. [Google Scholar] [CrossRef]
- Sharma, S.; Chhabra, D.; Kho, A.T.; Hayden, L.; Tantisira, K.G.; Weiss, S.T. The genomic origins of asthma. Thorax 2014, 69, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Louisias, M.; Ramadan, A.; Naja, A.S.; Phipatanakul, W. The Effects of the Environment on Asthma Disease Activity. Immunol. Allergy Clin. N. Am. 2019, 39, 163–175. [Google Scholar] [CrossRef]
- Crompton, G. A brief history of inhaled asthma therapy over the last fifty years. Prim. Care Respir. J. 2006, 15, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.J.; Szefler, S.J.; King, T.S.; Kraft, M.; Boushey, H.A.; Chinchilli, V.M.; Craig, T.J.; DiMango, E.A.; Deykin, A.; Fahy, J.V.; et al. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. J. Allergy Clin. Immunol. 2007, 119, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-F.; Su, M.-W.; Chiang, B.-L.; Yang, Y.-H.; Tsai, C.-H.; Lee, Y.L. A simple prediction tool for inhaled corticosteroid response in asthmatic children. BMC Pulm. Med. 2017, 17, 176. [Google Scholar] [CrossRef] [Green Version]
- Duplantier, J.E.; Nelson, R.P.J.; Morelli, A.R.; Good, R.A.; Kornfeld, S.J. Hypothalamic-pituitary-adrenal axis suppression associated with the use of inhaled fluticasone propionate. J. Allergy Clin. Immunol. 1998, 102, 699–700. [Google Scholar] [CrossRef]
- Guilbert, T.W.; Morgan, W.J.; Zeiger, R.; Mauger, D.T.; Boehmer, S.J.; Szefler, S.J.; Bacharier, L.B.; Lemanske, R.F.; Strunk, R.C.; Allen, D.B.; et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N. Engl. J. Med. 2006, 354, 1985–1997. [Google Scholar] [CrossRef] [Green Version]
- Allen, D.B. Effects of inhaled steroids on growth, bone metabolism, and adrenal function. Adv. Pediatr. 2006, 53, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Lapi, F.; Kezouh, A.; Suissa, S.; Ernst, P. The use of inhaled corticosteroids and the risk of adrenal insufficiency. Eur. Respir. J. 2013, 42, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keeley, D. Inhaled corticosteroids for asthma: Guidance is inconsistent. BMJ 2019, 367, l6934. [Google Scholar] [CrossRef] [PubMed]
- Gurnell, M.; Heaney, L.G.; Price, D.; Menzies-Gow, A. Long-term corticosteroid use, adrenal insufficiency and the need for steroid-sparing treatment in adult severe asthma. J. Intern. Med. 2021, 290, 240–256. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, S.; Zhang, S.; Ouyang, Z.; Wang, G.; Wang, F. Research Progress of Metabolomics in Asthma. Metabolites 2021, 11, 567. [Google Scholar] [CrossRef]
- Santos, A.; Pité, H.; Chaves-Loureiro, C.; Rocha, S.M.; Taborda-Barata, L. Metabolic Phenotypes in Asthmatic Adults: Relationship with Inflammatory and Clinical Phenotypes and Prognostic Implications. Metabolites 2021, 11, 534. [Google Scholar] [CrossRef]
- Kaddurah-Daouk, R.; Kristal, B.S.; Weinshilboum, R.M. Metabolomics: A global biochemical approach to drug response and disease. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 653–683. [Google Scholar] [CrossRef] [Green Version]
- di Palmo, E.; Cantarelli, E.; Catelli, A.; Ricci, G.; Gallucci, M.; Miniaci, A.; Pession, A. The Predictive Role of Biomarkers and Genetics in Childhood Asthma Exacerbations. Int. J. Mol. Sci. 2021, 22, 4651. [Google Scholar] [CrossRef]
- Kaddurah-Daouk, R.; Weinshilboum, R.M. Pharmacometabolomics: Implications for clinical pharmacology and systems pharmacology. Clin. Pharmacol. Ther. 2014, 95, 154–167. [Google Scholar] [CrossRef]
- Sordillo, J.E.; McGeachie, M.; Lutz, S.M.; Lasky-Su, J.; Tantisira, K.; Tsai, C.H.; Dahlin, A.; Kelly, R.; Wu, A.C. Longitudinal analysis of bronchodilator response in asthmatics and effect modification of age-related trends by genotype. Pediatr. Pulmonol. 2019, 54, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liao, K.P.; Shaw, S.Y.; Gainer, V.S.; E Churchill, S.; Szolovits, P.; Murphy, S.N.; Kohane, I.S.; Cai, T. Toward high-throughput phenotyping: Unbiased automated feature extraction and selection from knowledge sources. J. Am. Med. Inform. Assoc. 2015, 22, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.S.; Virkud, Y.; Giorgio, R.; Celedón, J.C.; Weiss, S.T.; Lasky-Su, J. Metabolomic profiling of lung function in Costa-Rican children with asthma. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1590–1595. [Google Scholar] [CrossRef]
- Li, M.-X.; Yeung, J.M.Y.; Cherny, S.S.; Sham, P.C. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum. Genet. 2012, 131, 747–756. [Google Scholar] [CrossRef] [Green Version]
- Nyholt, D.R. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am. J. Hum. Genet. 2004, 74, 765–769. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.S.; Boulin, A.; Laranjo, N.; Lee-Sarwar, K.; Chu, S.; Yadama, A.P.; Carey, V.; Litonjua, A.A.; Lasky-Su, J.; Weiss, S.T. Metabolomics and Communication Skills Development in Children; Evidence from the Ages and Stages Questionnaire. Metabolites 2019, 9, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.; Kelly, R.S.; Kachroo, P.; Chu, S.H.; Lee-Sarwar, K.; Chawes, B.L.; Bisgaard, H.; Litonjua, A.A.; Weiss, S.T.; Lasky-Su, J. Plasma 25-Hydroxyvitamin D Concentrations are Associated with Polyunsaturated Fatty Acid Metabolites in Young Children: Results from the Vitamin D Antenatal Asthma Reduction Trial. Metabolites 2020, 10, 151. [Google Scholar] [CrossRef]
- Kelly, R.S.; Bayne, H.; Spiro, A.; Vokonas, P.; Sparrow, D.; Weiss, S.T.; Schwartz, J.; Nassan, F.L.; Lee-Sarwar, K.; Huang, M.; et al. Metabolomic signatures of lead exposure in the VA Normative Aging Study. Environ. Res. 2020, 190, 110022. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Kelly, R.S.; Chu, S.H.; Kachroo, P.; Gürdeniz, G.; Chawes, B.L.; Bisgaard, H.; Weiss, S.T.; Lasky-Su, J. Maternal Metabolome in Pregnancy and Childhood Asthma or Recurrent Wheeze in the Vitamin D Antenatal Asthma Reduction Trial. Metabolites 2021, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- RC Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Beger, R.D.; A Schmidt, M.; Kaddurah-Daouk, R. Current Concepts in Pharmacometabolomics, Biomarker Discovery, and Precision Medicine. Metabolites 2020, 10, 129. [Google Scholar] [CrossRef] [Green Version]
- Miura, Y. The biological significance of ω-oxidation of fatty acids. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2013, 89, 370–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Khami, A.A.; Ghonim, M.A.; Del Valle, L.; Ibba, S.V.; Zheng, L.; Pyakurel, K.; Okpechi, S.C.; Garay, J.; Wyczechowska, D.; Sanchez-Pino, M.D.; et al. Fuelling the mechanisms of asthma: Increased fatty acid oxidation in inflammatory immune cells may represent a novel therapeutic target. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2017, 47, 1170–1184. [Google Scholar] [CrossRef]
- Esteves, P.; Blanc, L.; Celle, A.; Dupin, I.; Maurat, E.; Amoedo, N.; Cardouat, G.; Ousova, O.; Gales, L.; Bellvert, F.; et al. Crucial role of fatty acid oxidation in asthmatic bronchial smooth muscle remodelling. Eur. Respir. J. 2021, 58, 2004252. [Google Scholar] [CrossRef]
- Tian, M.; Chen, M.; Bao, Y.-L.; Xu, C.-D.; Qin, Q.-Z.; Zhang, W.-X.; He, Y.-T.; Shao, Q. Sputum metabolomic profiling of bronchial asthma based on quadruple time-of-flight mass spectrometry. Int. J. Clin. Exp. Pathol. 2017, 10, 10363–10373. [Google Scholar]
- Quinn, K.D.; Schedel, M.; Nkrumah-Elie, Y.; Joetham, A.; Armstrong, M.; Cruickshank-Quinn, C.; Reisdorph, N.; Gelfand, E.W. Dysregulation of metabolic pathways in a mouse model of allergic asthma. Allergy 2017, 72, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Lara, A.; Khatri, S.B.; Wang, Z.; Comhair, S.A.A.; Xu, W.; Dweik, R.A.; Bodine, M.; Levison, B.S.; Hammel, J.; Bleecker, E.; et al. Alterations of the arginine metabolome in asthma. Am. J. Respir. Crit. Care Med. 2008, 178, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Comhair, S.A.A.; Janocha, A.J.; Lara, A.; Mavrakis, L.A.; Bennett, C.D.; Kalhan, S.C.; Erzurum, S.C. Arginine metabolic endotypes related to asthma severity. PLoS ONE 2017, 12, e0183066. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, A.M.; Park, Y.; Brown, L.A.S.; Jones, D.P. Children with severe asthma have unique oxidative stress-associated metabolomic profiles. J. Allergy Clin. Immunol. 2014, 133, 258. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.H.; Fitzpatrick, A.M.; Medriano, C.A.; Jones, D.P. High-resolution metabolomics to identify urine biomarkers in corticosteroid-resistant asthmatic children. J. Allergy Clin. Immunol. 2017, 139, 1518–1524.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, G.J. Serum lactate increase during acute asthma treatment: A new piece of the puzzle. Chest 2014, 145, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, C.C.; Duarte, I.; Gomes, J.; Carrola, J.; Barros, A.S.; Gil, A.; Bousquet, J.; Todo-Bom, A.; Rocha, S. Urinary metabolomic changes as a predictive biomarker of asthma exacerbation. J. Allergy Clin. Immunol. 2014, 133, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Quan-Jun, Y.; Jian-Ping, Z.; Jian-Hua, Z.; Yong-Long, H.; Bo, X.; Jing-Xian, Z.; Bona, D.; Yuan, Z.; Cheng, G. Distinct Metabolic Profile of Inhaled Budesonide and Salbutamol in Asthmatic Children during Acute Exacerbation. Basic Clin. Pharmacol. Toxicol. 2017, 120, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Lee-Sarwar, K.A.; Lasky-Su, J.; Kelly, R.S.; Litonjua, A.A.; Weiss, S.T. Gut Microbial-Derived Metabolomics of Asthma. Metabolites 2020, 10, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shore, S.A.; Cho, Y. Obesity and Asthma: Microbiome-Metabolome Interactions. Am. J. Respir. Cell Mol. Biol. 2016, 54, 609–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Clinical Characteristics | All Subjects (n = 170) | Exacerbation | p-Value * | |
|---|---|---|---|---|
| Presence (n = 80) | Absence (n = 90) | |||
| Sex, n (%) | 0.03 | |||
| Female | 136 (80.0) | 70 (87.5) | 66 (73.3) | |
| Male | 34 (20.0) | 10 (12.5) | 24 (26.7) | |
| Race, n (%) | 0.03 | |||
| African American | 23 (13.5) | 15 (18.8) | 8 (8.9) | |
| White | 132 (77.6) | 55 (68.8) | 77 (85.6) | |
| Others | 15 (8.8) | 10 (12.5) | 5 (5.6) | |
| Smoking, n (%) | 0.25 | |||
| No | 127 (74.7) | 56 (70.0) | 71 (78.9) | |
| Yes | 43 (25.3) | 24 (30.0) | 19 (21.1) | |
| Exacerbation counts, mean (SD) | 2.2 (4.6) | 4.6 (5.8) | 0.0 (0.0) | NA |
| Age, mean (SD) | 33.5 (7.1) | 34.6 (5.9) | 32.6 (7.8) | 0.06 |
| BMI kg/m2, mean (SD) | 29.8 (8.6) | 30.6 (8.6) | 29.0 (8.5) | 0.23 |
| Metabolite | Super-Pathway | Sub-Pathway | β (95%CI) | p-Value |
|---|---|---|---|---|
| Cortisone | Lipid | Corticosteroids | −0.55 (−0.79, −0.29) | 2.90 × 10−5 |
| Cortisol | Lipid | Corticosteroids | −0.61 (−0.89, −0.30) | 7.11 × 10−5 |
| Tetradecanedioate (C14-DC) | Lipid | Fatty acid, dicarboxylate | −0.71 (−1.08, −0.31) | 3.6 × 10−4 |
| Hexadecanedioate (C16-DC) | Lipid | Fatty acid, dicarboxylate | −1.05 (−1.62, −0.49) | 3.7 × 10−4 |
| Mannitol/Sorbitol | Carbohydrate | Fructose, mannose, and galactose metabolism | 0.90 (0.47, 1.33) | 5.93 × 10−5 |
| Urea | Amino Acid | Urea cycle; arginine and proline metabolism | 1.50 (0.78, 2.23) | 7.78 × 10−5 |
| 5-methylthioadenosine (MTA) | Amino Acid | Polyamine metabolism | 1.49 (0.70, 2.24) | 2.2 × 10−4 |
| 1-carboxyethylvaline | Amino Acid | Valine derivative | 1.22 (0.58, 1.89) | 3.8 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kachroo, P.; Sordillo, J.E.; Lutz, S.M.; Weiss, S.T.; Kelly, R.S.; McGeachie, M.J.; Wu, A.C.; Lasky-Su, J.A. Pharmaco-Metabolomics of Inhaled Corticosteroid Response in Individuals with Asthma. J. Pers. Med. 2021, 11, 1148. https://doi.org/10.3390/jpm11111148
Kachroo P, Sordillo JE, Lutz SM, Weiss ST, Kelly RS, McGeachie MJ, Wu AC, Lasky-Su JA. Pharmaco-Metabolomics of Inhaled Corticosteroid Response in Individuals with Asthma. Journal of Personalized Medicine. 2021; 11(11):1148. https://doi.org/10.3390/jpm11111148
Chicago/Turabian StyleKachroo, Priyadarshini, Joanne E. Sordillo, Sharon M. Lutz, Scott T. Weiss, Rachel S. Kelly, Michael J. McGeachie, Ann Chen Wu, and Jessica A. Lasky-Su. 2021. "Pharmaco-Metabolomics of Inhaled Corticosteroid Response in Individuals with Asthma" Journal of Personalized Medicine 11, no. 11: 1148. https://doi.org/10.3390/jpm11111148
APA StyleKachroo, P., Sordillo, J. E., Lutz, S. M., Weiss, S. T., Kelly, R. S., McGeachie, M. J., Wu, A. C., & Lasky-Su, J. A. (2021). Pharmaco-Metabolomics of Inhaled Corticosteroid Response in Individuals with Asthma. Journal of Personalized Medicine, 11(11), 1148. https://doi.org/10.3390/jpm11111148










