Biomarkers in Ovarian Pathology: From Screening to Diagnosis. Review of the Literature
Abstract
:1. Introduction
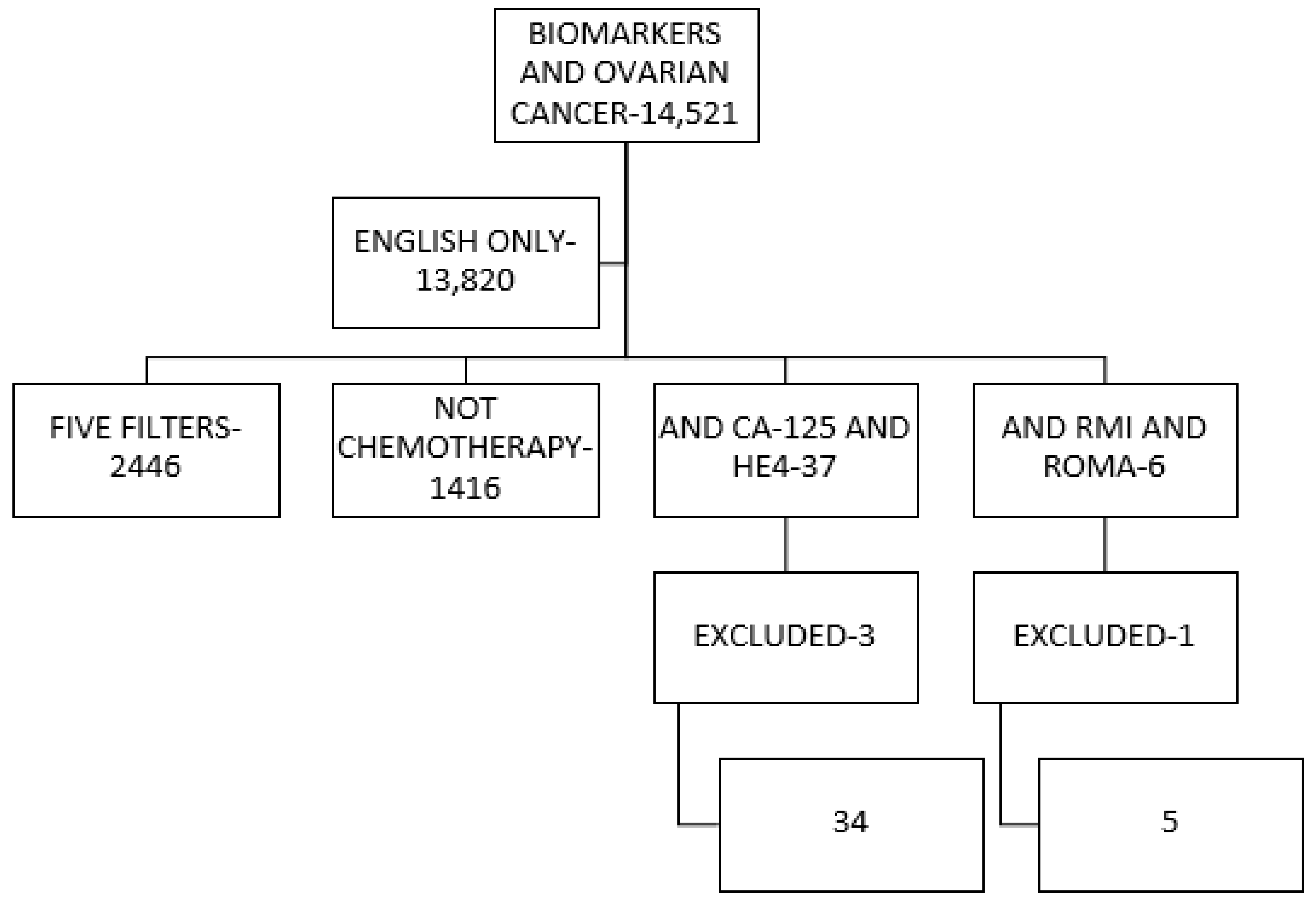
2. Methodology
3. Results
3.1. Tumour Marker CA125
3.2. Tumour Marker HE4
3.3. HE4 + CA125 Association
3.4. Multimodality Screening
3.5. Trial Description
3.6. Early Detection in the High-Risk Population
3.7. Tests Based on Prognostic and Predictive Biomarkers in Patients with Symptoms
3.8. New Screening Tools
3.9. Risk of Malignancy Index (RMI)
3.10. ROMA Algorithm
3.11. OVA 1 Test
3.12. Improving the Performance of Additional Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howlader, N.; Noone, A.M.; Krapcho, M.; Garshell, J.; Neyman, N.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2010; National Cancer Institute: Bethesda, MD, USA, 2012. Available online: https://seer.cancer.gov/archive/csr/1975_2010/ (accessed on 25 October 2013).
- Hinojo, C.; Jimeno, R.; De Juan, A. Prevención de los tumores ginecológicos. In Manual SEOM de Prevención y Diagnóstico Precoz del Cáncer. Cap26, 210-8. Grupo de Trabajo SEOM de Prevención y Diagnóstico Precoz del Cáncer; Sociedad Española de Oncología Médica (SEOM): Madrid, Spain, 2017; ISBN 978-84-697-4630-1. [Google Scholar]
- Kurman, R.J.; Shih, I.-M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—Shifting the paradigm. Hum. Pathol. 2011, 42, 918–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Committee on the State of the Science in Ovarian Cancer Research; Board on Health Care Services; Institute of Medicine; National Academies of Sciences, Engineering, and Medicine. Ovarian Cancers: Evolving Paradigms in Research and Care; National Academies Press (US): Washington, DC, USA, 2016. [Google Scholar]
- Scully, R.E.; Young, R.H.; Clement, P.B. Tumors of the Ovary, Maldeveloped Gonads, Fallopian Tube, and Broad Ligament. Atlas of Tumor Pathology, Third Series. Fascicle 23; Armed Forces Institute of Pathology: Washington, DC, USA, 1998; pp. 51–79. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCI (National Cancer Institute). NCI Dictionary of Cancer Terms. 2015. [16 September 2015]. Available online: http://www.cancer.gov/publications/dictionaries/cancer-terms (accessed on 11 January 2021).
- Consensus Study Report. IOM (Institute of Medicine). Cancer Biomarkers the Promises and Challenges of Improving Detection and Treatment; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Bast, R.; Feeney, M.; Lazarus, H.; Nadler, L.M.; Colvin, R.B.; Knapp, R.C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J. Clin. Investig. 1981, 68, 1331–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niloff, J.M.; Knapp, R.C.; Schaetzl, E.; Reynolds, C.; Bast, R. CA125 antigen levels in obstetric and gynecologic patients. Obstet. Gynecol. 1984, 64, 6208522. [Google Scholar]
- Bast, R.; Siegal, F.; Runowicz, C.D.; Klug, T.; Zurawski, V.; Schonholz, D.; Cohen, C.; Knapp, R. Elevation of serum CA 125 prior to diagnosis of an epithelial ovarian carcinoma. Gynecol. Oncol. 1985, 22, 115–120. [Google Scholar] [CrossRef]
- Boivin, M.; Lane, D.; Piché, A.; Rancourt, C. CA125 (MUC16) tumor antigen selectively modulates the sensitivity of ovarian cancer cells to genotoxic drug-induced apoptosis. Gynecol. Oncol. 2009, 115, 407–413. [Google Scholar] [CrossRef]
- Felder, M.; Kapur, A.; Gonzalez-Bosquet, J.; Horibata, S.; Heintz, J.; Albrecht, R.; Fass, L.; Kaur, J.; Hu, K.; Shojaei, H.; et al. MUC16 (CA125): Tumor biomarker to cancer therapy, a work in progress. Mol. Cancer 2014, 13, 129. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.G. In 2014, can we do better than CA125 in the early detection of ovarian cancer? World J. Biol. Chem. 2014, 5, 286–300. [Google Scholar] [CrossRef]
- Schummer, M.; Ng, W.V.; Bumgarner, R.; Nelson, P.S.; Schummer, B.; Bednarski, D.W.; Hassell, L.; Baldwin, R.L.; Karlan, B.Y.; Hood, L. Comparative hybridization of an array of 21 500 ovarian cDNAs for the discovery of genes overexpressed in ovarian carcinomas. Gene 1999, 238, 375–385. [Google Scholar] [CrossRef]
- Bast, R.; Badgwell, D.; Lu, Z.; Marquez, R.; Rosen, D.; Liu, J.; Baggerly, K.; Atkinson, E.; Skates, S.; Zhang, Z.; et al. New tumor markers: CA125 and beyond. Int. J. Gynecol. Cancer 2005, 15, 274–281. [Google Scholar] [CrossRef]
- Karlsen, N.S.; Karlsen, M.A.; Høgdall, C.; Høgdall, E.V. HE4 Tissue Expression and Serum HE4 Levels in Healthy Individuals and Patients with Benign or Malignant Tumors: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2285–2295. [Google Scholar] [CrossRef] [Green Version]
- Hertlein, L.; Stieber, P.; Kirschenhofer, A.; Fürst, S.; Mayr, D.; Hofmann, K.; Krocker, K.; Nagel, D.; Lenhard, M.; Burges, A. Human epididymis protein 4 (HE4) in benign and malignant diseases. Clin. Chem. Lab. Med. 2012, 50, 2181–2188. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, S.; Braga, F.; Lanzoni, M.; Boracchi, P.; Biganzoli, E.; Panteghini, M. Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: A systematic review. J. Clin. Pathol. 2013, 66, 273–281. [Google Scholar] [CrossRef]
- Wang, J.; Gao, J.; Yao, H.; Wu, Z.; Wang, M.; Qi, J. Diagnostic accuracy of serum HE4, CA125 and ROMA in patients with ovarian cancer: A meta-analysis. Tumor Biol. 2014, 35, 6127–6138. [Google Scholar] [CrossRef]
- Zhen, S.; Bian, L.-H.; Chang, L.-L.; Gao, X. Comparison of serum human epididymis protein 4 and carbohydrate antigen 125 as markers in ovarian cancer: A meta-analysis. Mol. Clin. Oncol. 2014, 2, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhou, H.; Chen, R.; He, J.; Wang, Y.; Huang, L.; Sun, L.; Duan, C.; Luo, X.; Yan, H. Development of a multimarker assay for differential diagnosis of benign and malignant pelvic masses. Clin. Chim. Acta 2015, 440, 57–63. [Google Scholar] [CrossRef]
- Moore, R.G.; Brown, A.K.; Miller, M.C.; Skates, S.; Allard, W.J.; Verch, T.; Steinhoff, M.; Messerlian, G.; DiSilvestro, P.; Granai, C.; et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol. Oncol. 2008, 108, 402–408. [Google Scholar] [CrossRef]
- Dochez, V.; Caillon, H.; Vaucel, E.; Dimet, J.; Winer, N.; Ducarme, G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J. Ovarian Res. 2019, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Goff, B.A.; Agnew, K.; Neradilek, M.B.; Gray, H.J.; Liao, J.B.; Urban, R.R. Combining a symptom index, CA125 and HE4 (triple screen) to detect ovarian cancer in women with a pelvic mass. Gynecol. Oncol. 2017, 147, 291–295. [Google Scholar] [CrossRef]
- Anton, C.; Carvalho, F.; Oliveira, E.I.; Maciel, G.A.R.; Baracat, E.C.; Carvalho, J. A comparison of CA125, HE4, risk ovarian malignancy algorithm (ROMA), and risk malignancy index (RMI) for the classification of ovarian masses. Clinics 2012, 67, 437–441. [Google Scholar] [CrossRef]
- Meys, E.; Kaijser, J.; Kruitwagen, R.; Slangen, B.; Van Calster, B.; Aertgeerts, B.; Verbakel, J.; Timmerman, D.; Van Gorp, T. Subjective assessment versus ultrasound models to diagnose ovarian cancer: A systematic review and meta-analysis. Eur. J. Cancer 2016, 58, 17–29. [Google Scholar] [CrossRef]
- Li, F.; Tie, R.; Chang, K.; Wang, F.; Deng, S.; Lu, W.; Yu, L.; Chen, M. Does risk for ovarian malignancy algorithm excel human epididymis protein 4 and ca125 in predicting epithelial ovarian cancer: A meta-analysis. BMC Cancer 2012, 12, 258. [Google Scholar] [CrossRef] [Green Version]
- Al Musalhi, K.; Al Kindi, M.; Al Aisary, F.; Ramadhan, F.; Al Rawahi, T.; Al Hatali, K.; Mula-Abed, W.-A. Evaluation of HE4, CA-125, Risk of Ovarian Malignancy Algorithm (ROMA) and Risk of Malignancy Index (RMI) in the Preoperative Assessment of Patients with Adnexal Mass. Oman Med. J. 2016, 31, 336–344. [Google Scholar] [CrossRef]
- Hua, S.; Williams, C.C.; Dimapasoc, L.M.; Ro, G.S.; Ozcan, S.; Miyamoto, S.; Lebrilla, C.B.; An, H.J.; Leiserowitz, G.S. Isomer-specific chromatographic profiling yields highly sensitive and specific potential N-glycan biomarkers for epithelial ovarian cancer. J. Chromatogr. A 2013, 1279, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Buys, S.S.; Partridge, E.; Black, A.; Johnson, C.C.; Lamerato, L.; Isaacs, C.; Reding, D.J.; Greenlee, R.T.; Yokochi, L.A.; Kessel, B.; et al. Effect of Screening on Ovarian Cancer Mortality. JAMA 2011, 305, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.J.; Menon, U.; Ryan, A.; Gentry-Maharaj, A.; Burnell, M.; Kalsi, J.K.; Amso, N.; Apostolidou, S.; Benjamin, E.; Cruickshank, D.; et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2016, 387, 945–956. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food and Drug Administration. The FDA Recommends against Using Screening Tests for Ovarian Cancer Screening: FDA Safety Communication. Available online: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm519413.htm (accessed on 11 January 2021).
- Abcodia. Abcodia Statement on the ROCA Test for Ovarian Cancer Screening. Available online: https://www.rocatest.com/about-us/news-events/news-item/abcodia-statement-roca-test-ovarian-cancer-screening/ (accessed on 11 January 2021).
- Kobayashi, H.; Yamada, Y.; Sado, T.; Sakata, M.; Yoshida, S.; Kawaguchi, R.; Kanayama, S.; Shigetomi, H.; Haruta, S.; Tsuji, Y.; et al. A randomized study of screening for ovarian cancer: A multicenter study in Japan. Int. J. Gynecol. Cancer 2008, 18, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.N. Ovarian Cancer Screening in the High-Risk Population—The UK Familial Ovarian Cancer Screening Study (UKFOCSS). Int. J. Gynecol. Cancer 2012, 22, S27–S28. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.M.; Duffy, M.J.; Stenman, U.-H.; Lilja, H.; Brünner, N.; Chan, D.W.; Babaian, R.; Bast, R.; Dowell, B.; Esteva, F.; et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for Use of Tumor Markers in Testicular, Prostate, Colorectal, Breast, and Ovarian Cancers. Clin. Chem. 2008, 54, e11–e79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallardo-Rincón, D.; Cantú-de-León, D.; Alanís-López, P.; Alvarez-Avitia, M.A.; Bañuelos-Flores, J.; Herbert-Núñez, G.S.; Oñate-Ocaña, L.F.; Pérez-Montiel, M.D.; Rodríguez-Trejo, A.; Ruvalcaba-Limón, E.; et al. Tercer Consenso Nacional de Cáncer de Ovario 2011.Grupo de Investigación en Cáncer de Ovario y Tumores Ginecológicos de México “GICOM”. Rev. Investig. Clin. 2011, 63, 665–702. [Google Scholar]
- Jacobs, I.; Oram, D.; Fairbanks, J.; Turner, J.; Frost, C.; Grudzinskas, J.G. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. BJOG: Int. J. Obstet. Gynaecol. 1990, 97, 922–929. [Google Scholar] [CrossRef]
- Tingulstad, S.; Hagen, B.; Skjeldestad, F.E.; Halvorsen, T.; Nustad, K.; Onsrud, M. The risk-of-malignancy index to evaluate potential ovarian cancers in local hospitals. Obstet. Gynecol. 1999, 93, 448–452. [Google Scholar] [CrossRef]
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; DiSilvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast, R.C.; Skates, S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2009, 112, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Hunter, M.I.; Ziogas, A.; Flores, F.; Brewster, W.R. Epithelial Ovarian Cancer and Low Malignant Potential (LMP) Tumors Associated with a Lower Incidence of Second Primary Breast Cancer. Am. J. Clin. Oncol. 2007, 30, 1–7. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/510k-program-evaluating-substantial-equivalence-premarket-notifications-510k (accessed on 12 January 2021).
- Bristow, R.E.; Smith, A.; Zhang, Z.; Chan, D.W.; Crutcher, G.; Fung, E.T.; Munroe, D.G. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol. Oncol. 2013, 128, 252–259. [Google Scholar] [CrossRef]
- Halvorsen, A.R.; Kristensen, G.; Embleton, A.; Adusei, C.; Barretina-Ginesta, M.P.; Beale, P.; Helland, A. Evaluation of Prognostic and Predictive Significance of Circulating MicroRNAs in Ovarian Cancer Patients. Dis. Mark. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Available online: https://edrn.nci.nih.gov/ (accessed on 12 January 2021).
- Moore, R.G.; Blackman, A.; Miller, M.C.; Robison, K.; DiSilvestro, P.A.; Eklund, E.E.; Strongin, R.; Messerlian, G. Multiple biomarker algorithms to predict epithelial ovarian cancer in women with a pelvic mass: Can additional makers improve performance? Gynecol. Oncol. 2019, 154, 150–155. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Raja, F.A.; Fotopoulou, C.; Martín, A.G.; Colombo, N.; Sessa, C. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi24–vi32. [Google Scholar] [CrossRef]
- Salvador, S.; Gilks, B.; Köbel, M.; Huntsman, D.; Rosen, B.; Miller, D. The Fallopian Tube: Primary Site of Most Pelvic High-grade Serous Carcinomas. Int. J. Gynecol. Cancer 2009, 19, 58–64. [Google Scholar] [CrossRef]
- Freedman, D.M.; Curtis, R.E.; Travis, L.B.; Fraumeni, J.F., Jr. New Malignancies Following Cancer of the Uterine Corpus and Ovary. In New Malignancies Among Cancer Survivors: SEER Cancer Registries; Curtis, R.E., Freedman, D.M., Ron, E., Ries, L.A.G., Hacker, D.G., Edwards, B.K., Tucker, M.A., Fraumeni, J.F., Jr., Eds.; 1973–2000; NIH Publ. No. 05-5302; National Cancer Institute: Bethesda, MD, USA, 2006. Available online: http://seer.cancer.gov/archive/publications/mpmono/MPMonograph_complete.pdf (accessed on 12 January 2021).
- Jacobs, I.; Bast, R.C., Jr. The CA 125 tumour-associated antigen: A review of the literature. Hum. Reprod. 1989, 4, 1–12. [Google Scholar] [CrossRef]
- Bast, R.C.; Lu, Z.; Han, C.Y.; Lu, K.H.; Anderson, K.S.; Drescher, C.W.; Skates, S.J. Biomarkers and Strategies for Early Detection of Ovarian Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2504–2512. [Google Scholar] [CrossRef]
- Etzioni, R.; Gulati, R.; Mallinger, L.; Mandelblatt, J. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann. Intern. Medicine. 2013, 158, 831–838. [Google Scholar] [CrossRef] [Green Version]
- Bonifácio, V.D.B. Ovarian Cancer Biomarkers: Moving Forward in Early Detection. Adv. Exp. Med. Biol. 2020, 1219, 355–363. [Google Scholar]
| Reference. Author and Year | The Systemic Revision or Metanalysis | CA125 | HE4 | CA125 + HE4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Se (%) (95% IC) | Sp (%) (95% IC) | PPV (%) | NPV (%) | AUC (95% IC) | Se (%) (95% IC) | Sp (%) (95% IC) | PPV (%) | NPV (%) | AUC (95% IC) | Se (%) (95% IC) | Sp (%) (95% IC) | PPV (%) | NPV (%) | AUC (95% IC) | ||
| [19] Ferraro S. et al., 2013 | X | 79 (77–82) | 78 (76–80) | 79 (76–81) | 93 (92–94) | 82 (78–86) | 76 (72–80) | |||||||||
| [20] Wang J et al. 2014 | X | 79 (74–84) | 82 (77–87) | 0.87 (0.84–0.90) | 76 (72–80) | 94 (90–96) | 0.89 (0.86–0.92) | |||||||||
| [21] Zhen et al. 2014 | X | 74 (72–76) | 83 (81–84) | 0.85 | 74 (72–76) | 90 (89–91) | 0.89 | |||||||||
| [27] Meys et al. 2016 | X | |||||||||||||||
| [28] Li et al. 2012 | X | 77 (58–89) | 84 (76–90) | 0.88 (0.85–0.91) | 79 (74–84) | 93 (87–96) | 0.82 (0.78–0.85) | |||||||||
| RMI | ROMA | |||||||||||||||
| [20] Wang J et al. 2014 | X | 85 (81–89) | 82 (77–87) | 0.91 (0.88–0.93) | ||||||||||||
| [27] Meys et al. 2016 | X | 75 (72–79) | 92 (88–94) | |||||||||||||
| Al Musalhi et al. [29] 2016 | X | 77 | 82 | 56 | 93 | 0.85 | 75 | 88 | 65 | 92 | 0.84 | |||||
| [28] Li et al. 2012 | X | 89 (84–93) | 83 (77–88) | 0.93 (0.90–0.95) | ||||||||||||
| Name of the Study | Study Design | Screening Cohort Size | Screening Strategy | Interpretation CA125 | Sensitivity (%) | Mortality/Subrogated Results | Author, Year. (Reference) |
|---|---|---|---|---|---|---|---|
| PLCO | RCT, on general population | 30,630 | CA125 + TVU | Fixed cut, 35 U/mL | IOC/FT: 69.5 IOC/FT: 68.2 | No mortality benefit | Buys et al. 2011 [31] |
| UKCTO | RCT, on | 101,247 | 1. | ROCA | OC/FT: | Relative | Jacobs |
| CS | general population, | CA125 following | longitudinal sampling | MMS (89.4)/ | mortality reduction of | et al. 2015 [32] | |
| two arms | for MMS | TVU (84.9) | MMS (14%) | ||||
| IOC/FT: | and groups of | ||||||
| MMS | USS (11%) | ||||||
| 2. Only | (84.9)/ | about no | |||||
| TVU | TVU (75.0) | action, but | |||||
| IOC/FT: | the reductions | ||||||
| MMS | were not | ||||||
| (88.6)/ | significant in | ||||||
| TVU (65.8) | the primary | ||||||
| analysis | |||||||
| Japanes e cohort Shizuoka | RCT, low risk post-menop ausal | 41,688 | Physical examinat ion, CA125 and TVU | Fixed cut, 35 U/mL | OC/FT: 77.1 | Change of stage; Stage I OC in screening (63%) versus | Kobayashi et al. 2008 [35] |
| Control (38%) |
| Evaluated Marker or Combination of Markers | ROC-AUC (95% CI) | p Value vs. ROMA |
|---|---|---|
| CA125 | 86.6% (80.6–92.6%) | 0.039 |
| HE4 | 90.8% (85.7–95.8%) | 0.671 |
| ROMA (CA-125 + HE4 + menopausal) | 91.2% (86.0–96.4%) | ––– |
| CA125 + Transthyretin + ApoA1 + Beta2 Microglobulin + Transferrin + menopausal | 89.7% (84.4–95.0%) | 0.502 |
| CA125 + HE4 + ApoA1 + Transferrin + menopausal | 93.2% (88.7–97.6%) | 0.153 |
| CA125 + HE4 + YKL-40 + Transthyretin + ApoA1 + Beta2 Microglobulin + Transferrin + LPA + menopausal | 94.6% (90.1–99.2%) | 0.078 |
| Analysed Value | ROMA (95% CI) | 8 Markers Trial (95% CI) |
|---|---|---|
| Sensitivity | 90.0% (79.5–96.2%) | 94.0% (83.5–98.7%) |
| Specificity | 76.7% (67.3–84.5%) | 76.3% (66.4–84.5%) |
| PPV | 69.2% (57.8–79.2%) | 68.1% (55.8–78.8%) |
| NPV | 92.9% (85.3–97.4%) | 95.9% (88.6–99.2%) |
| Accuracy | 81.6% (74.8–87.2%) | 82.5% (75.3–88.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elorriaga, M.Á.; Neyro, J.L.; Mieza, J.; Cristóbal, I.; Llueca, A. Biomarkers in Ovarian Pathology: From Screening to Diagnosis. Review of the Literature. J. Pers. Med. 2021, 11, 1115. https://doi.org/10.3390/jpm11111115
Elorriaga MÁ, Neyro JL, Mieza J, Cristóbal I, Llueca A. Biomarkers in Ovarian Pathology: From Screening to Diagnosis. Review of the Literature. Journal of Personalized Medicine. 2021; 11(11):1115. https://doi.org/10.3390/jpm11111115
Chicago/Turabian StyleElorriaga, Miguel Ángel, José Luis Neyro, Jon Mieza, Ignacio Cristóbal, and Antoni Llueca. 2021. "Biomarkers in Ovarian Pathology: From Screening to Diagnosis. Review of the Literature" Journal of Personalized Medicine 11, no. 11: 1115. https://doi.org/10.3390/jpm11111115
APA StyleElorriaga, M. Á., Neyro, J. L., Mieza, J., Cristóbal, I., & Llueca, A. (2021). Biomarkers in Ovarian Pathology: From Screening to Diagnosis. Review of the Literature. Journal of Personalized Medicine, 11(11), 1115. https://doi.org/10.3390/jpm11111115






