Online Left-Hemispheric In-Phase Frontoparietal Theta tACS for the Treatment of Negative Symptoms of Schizophrenia
Abstract
:1. Introduction
2. Materials and Methods
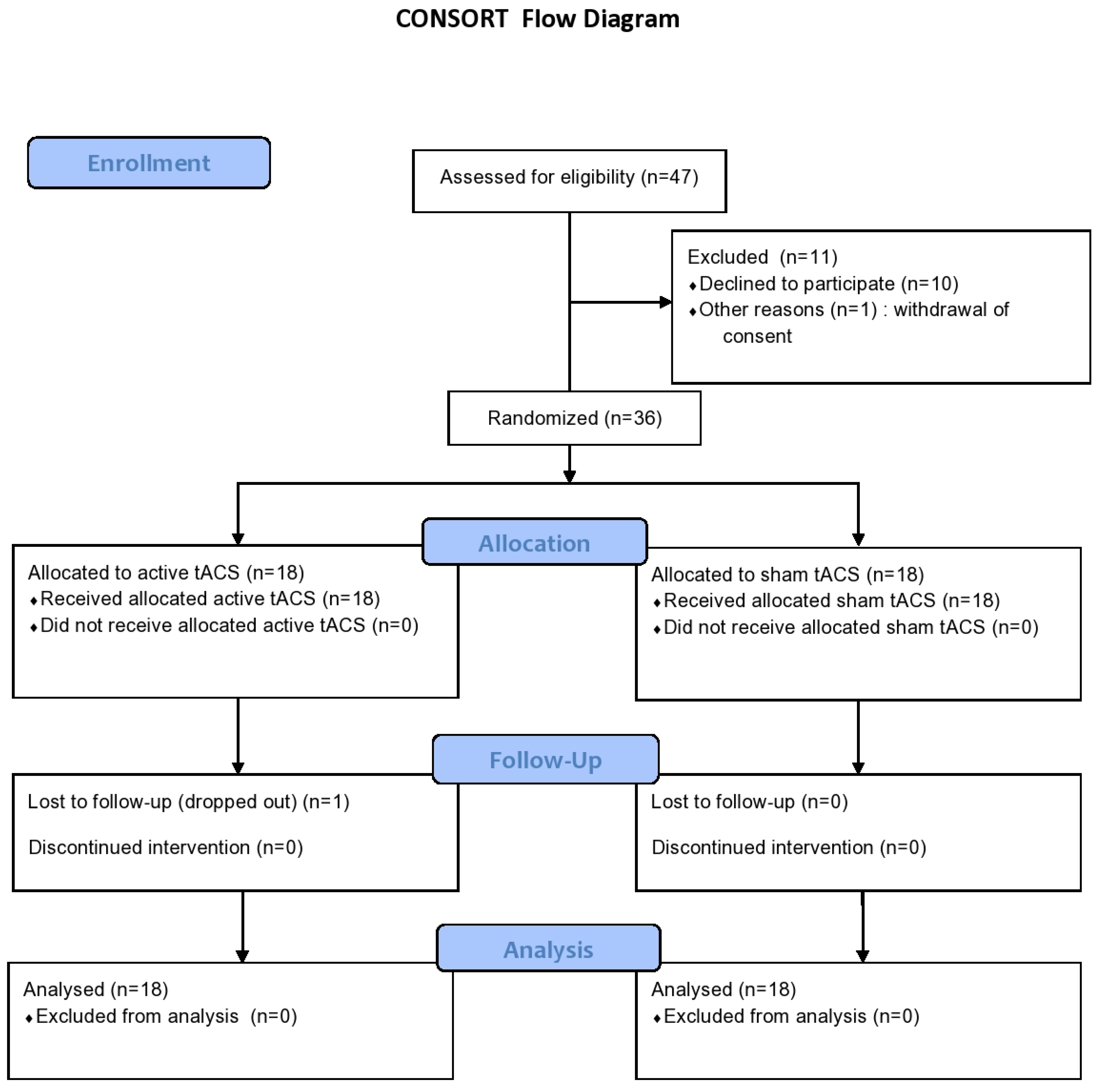
2.1. Participants
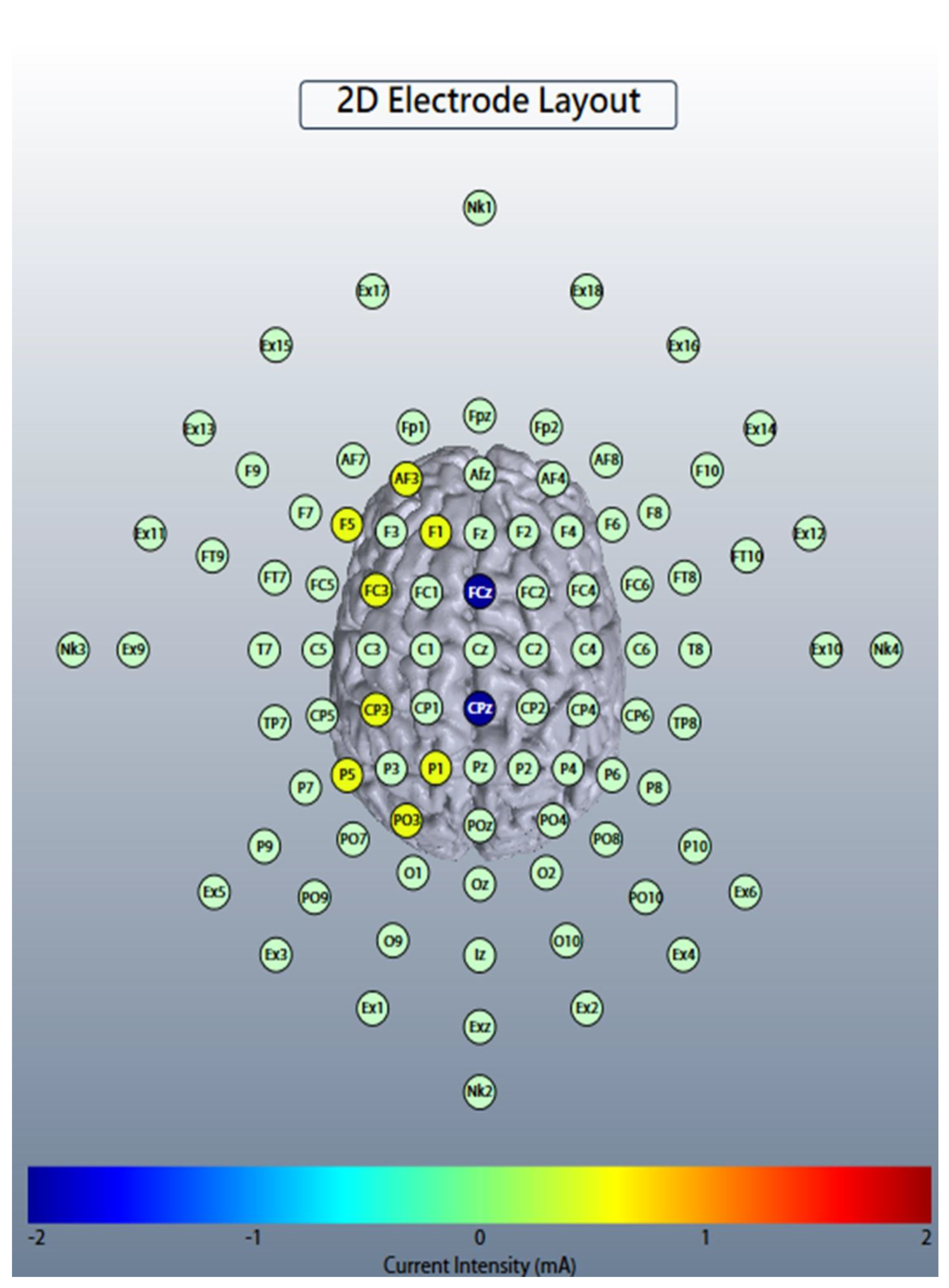
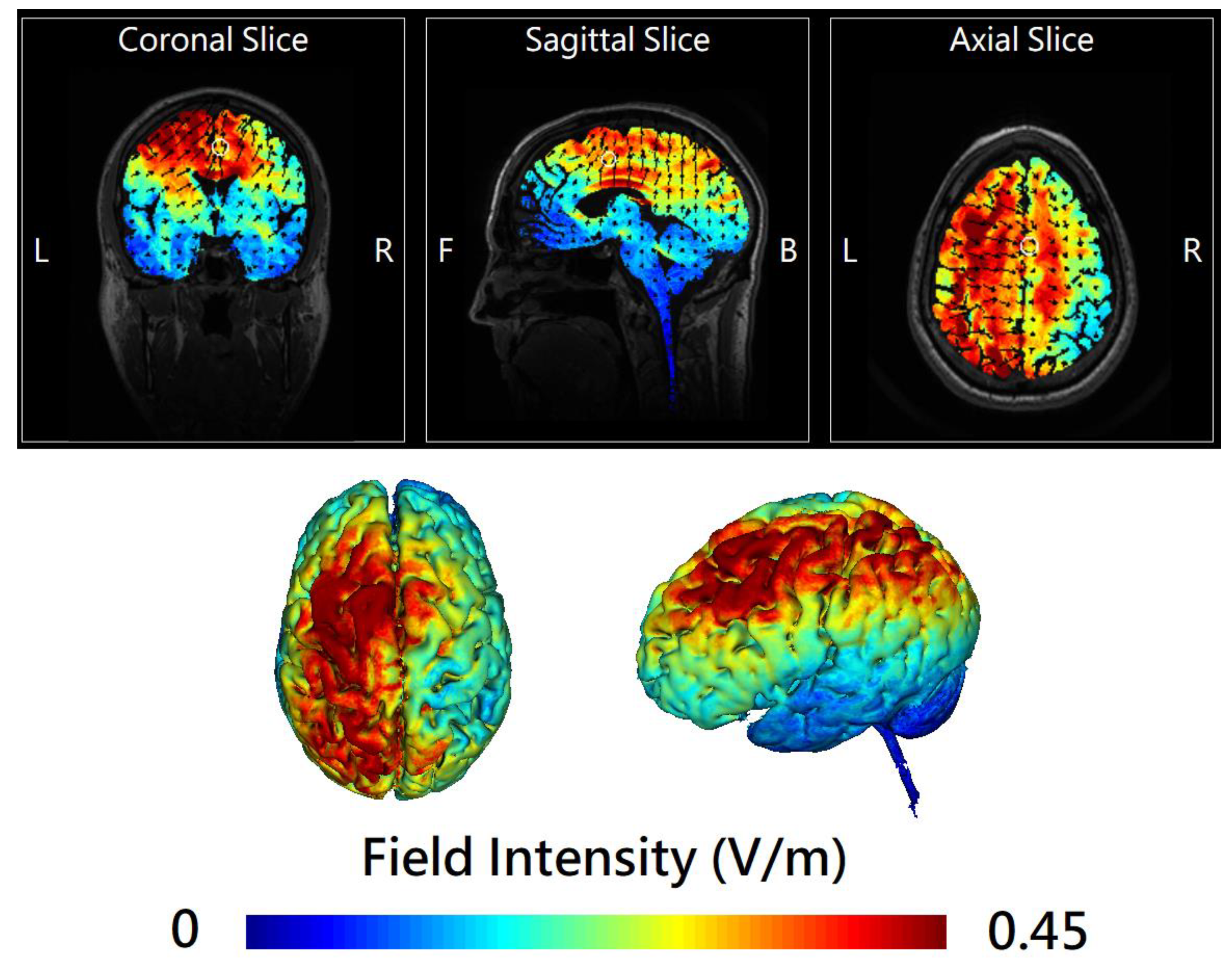
2.2. Online Left-Hemispheric In-Phase Frontoparietal Theta (6 Hz) tACS
2.3. Outcome Assessments
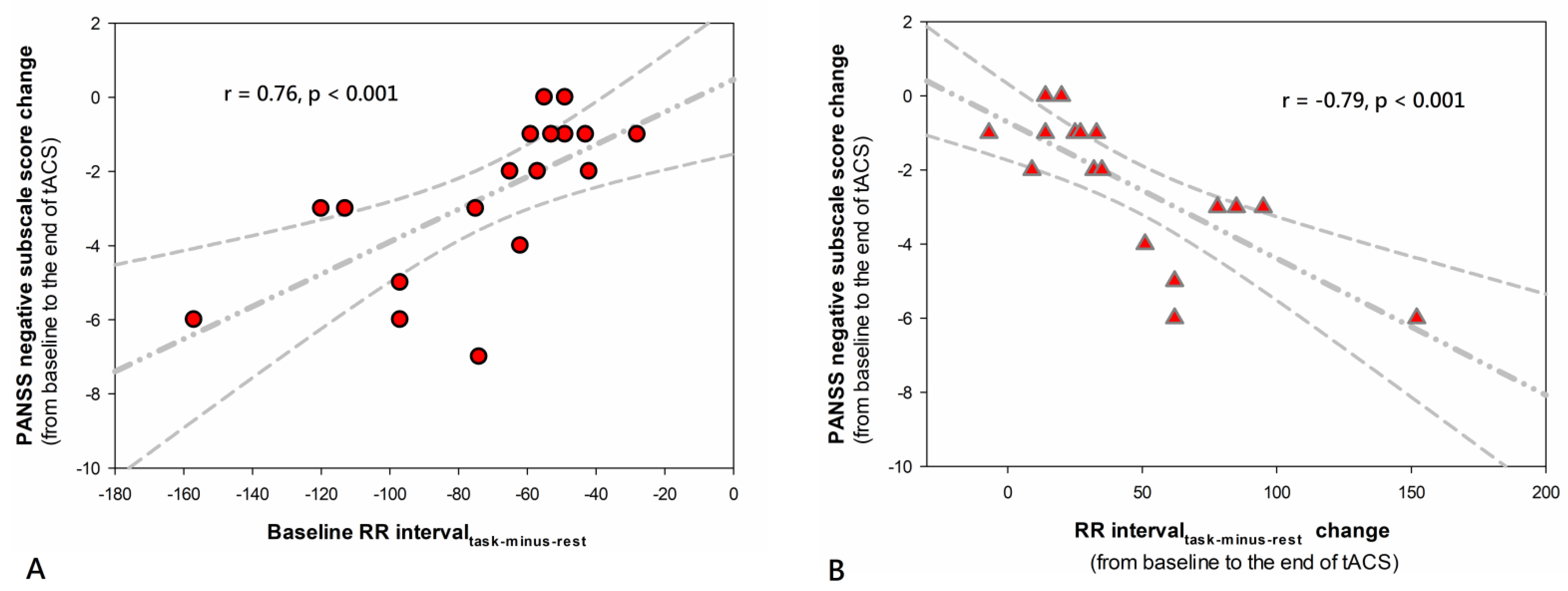
2.4. Biological Markers
2.5. Statistical Analyses
3. Results
3.1. Participant Characteristics
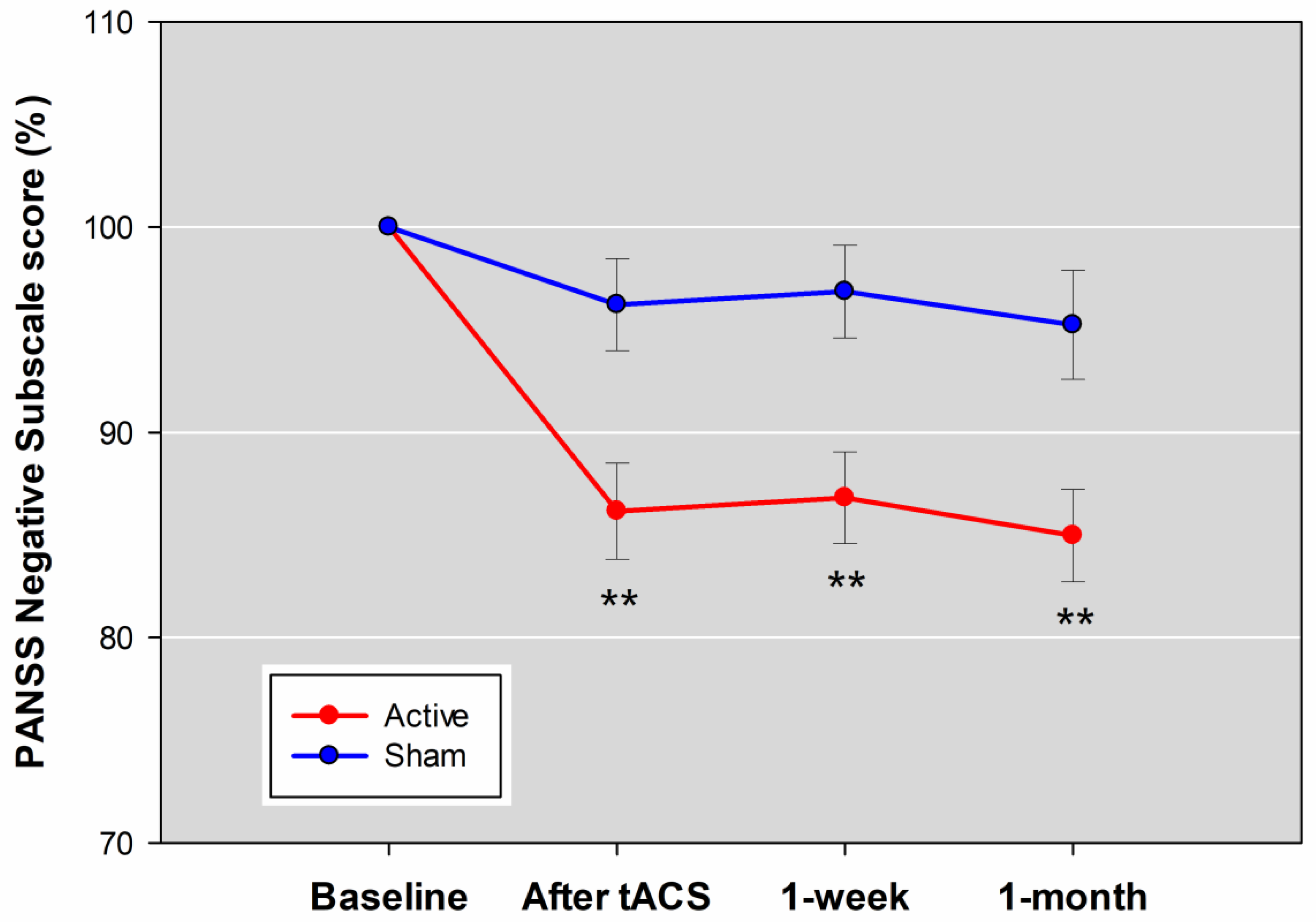
3.2. Primary Outcome
3.3. Adverse Events and Safety
3.4. Secondary Outcomes
3.5. Biologic Marker
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fusar-Poli, P.; Papanastasiou, E.; Stahl, D.; Rocchetti, M.; Carpenter, W.; Shergill, S.; McGuire, P. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr. Bull. 2015, 41, 892–899. [Google Scholar] [CrossRef]
- Farreny, A.; Aguado, J.; Ochoa, S.; Haro, J.M.; Usall, J. The role of negative symptoms in the context of cognitive remediation for schizophrenia. Schizophr. Res. 2013, 150, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Preti, A.; Edwards, C.; Dow, T.; Wykes, T. Cognitive remediation for negative symptoms of schizophrenia: A network meta-analysis. Clin. Psychol. Rev. 2017, 52, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Gold, J.M.; Barch, D.M.; Carter, C.S.; Dakin, S.; Luck, S.J.; MacDonald, A.W., 3rd; Ragland, J.D.; Ranganath, C.; Kovacs, I.; Silverstein, S.M.; et al. Clinical, functional, and intertask correlations of measures developed by the Cognitive Neuroscience Test Reliability and Clinical Applications for Schizophrenia Consortium. Schizophr. Bull. 2012, 38, 144–152. [Google Scholar] [CrossRef]
- Gold, J.M.; Waltz, J.A.; Prentice, K.J.; Morris, S.E.; Heerey, E.A. Reward processing in schizophrenia: A deficit in the representation of value. Schizophr. Bull. 2008, 34, 835–847. [Google Scholar] [CrossRef]
- Strauss, G.P. Translating basic emotion research into novel psychosocial interventions for anhedonia. Schizophr. Bull. 2013, 39, 737–739. [Google Scholar] [CrossRef] [Green Version]
- Vinograd, M.; Craske, M.G. Using Neuroscience to Augment Behavioral Interventions for Depression. Harv. Rev. Psychiatry 2020, 28, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Kringelbach, M.L.; Berridge, K.C. Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn. Sci. 2009, 13, 479–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rottschy, C.; Langner, R.; Dogan, I.; Reetz, K.; Laird, A.R.; Schulz, J.B.; Fox, P.T.; Eickhoff, S.B. Modelling neural correlates of working memory: A coordinate-based meta-analysis. Neuroimage 2012, 60, 830–846. [Google Scholar] [CrossRef] [Green Version]
- Nejad, A.B.; Madsen, K.H.; Ebdrup, B.H.; Siebner, H.R.; Rasmussen, H.; Aggernaes, B.; Glenthoj, B.Y.; Baare, W.F. Neural markers of negative symptom outcomes in distributed working memory brain activity of antipsychotic-naive schizophrenia patients. Int. J. Neuropsychopharmacol. 2013, 16, 1195–1204. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xiao, Y.H.; Zou, L.Q.; Li, H.H.; Yang, Z.Y.; Shi, H.S.; Lui, S.S.; Cheung, E.F.; Chan, R.C. The effects of working memory training on enhancing hedonic processing to affective rewards in individuals with high social anhedonia. Psychiatry Res. 2016, 245, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Li, K.; Zeng, Y.W.; Shi, H.S.; Xie, W.L.; Yang, Z.Y.; Lui, S.S.; Cheung, E.F.; Leung, A.W.; et al. The neural transfer effect of working memory training to enhance hedonic processing in individuals with social anhedonia. Sci. Rep. 2016, 6, 35481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Chu, M.Y.; Lv, Q.Y.; Hu, H.X.; Li, Z.; Yi, Z.H.; Wang, J.H.; Zhang, J.Y.; Lui, S.S.Y.; Cheung, E.F.C.; et al. The remediation effects of working memory training in schizophrenia patients with prominent negative symptoms. Cogn. Neuropsychiatry 2019, 24, 434–453. [Google Scholar] [CrossRef]
- Lett, T.A.; Voineskos, A.N.; Kennedy, J.L.; Levine, B.; Daskalakis, Z.J. Treating working memory deficits in schizophrenia: A review of the neurobiology. Biol. Psychiatry 2014, 75, 361–370. [Google Scholar] [CrossRef]
- Sathappan, A.V.; Luber, B.M.; Lisanby, S.H. The Dynamic Duo: Combining noninvasive brain stimulation with cognitive interventions. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Elyamany, O.; Leicht, G.; Herrmann, C.S.; Mulert, C. Transcranial alternating current stimulation (tACS): From basic mechanisms towards first applications in psychiatry. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Polania, R.; Nitsche, M.A.; Korman, C.; Batsikadze, G.; Paulus, W. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr. Biol. 2012, 22, 1314–1318. [Google Scholar] [CrossRef] [Green Version]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Sreeraj, V.S.; Shivakumar, V.; Sowmya, S.; Bose, A.; Nawani, H.; Narayanaswamy, J.C.; Venkatasubramanian, G. Online Theta Frequency Transcranial Alternating Current Stimulation for Cognitive Remediation in Schizophrenia: A Case Report and Review of Literature. J. ECT 2019, 35, 139–143. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Amadera, J.; Berbel, B.; Volz, M.S.; Rizzerio, B.G.; Fregni, F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int. J. Neuropsychopharmacol. 2011, 14, 1133–1145. [Google Scholar] [CrossRef]
- Quintana, D.S.; Westlye, L.T.; Kaufmann, T.; Rustan, O.G.; Brandt, C.L.; Haatveit, B.; Steen, N.E.; Andreassen, O.A. Reduced heart rate variability in schizophrenia and bipolar disorder compared to healthy controls. Acta Psychiatr. Scand. 2016, 133, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Roy, H.; Wasylyshyn, N.; Spangler, D.P.; Gamble, K.R.; Patton, D.; Brooks, J.R.; Garcia, J.O.; Vettel, J.M. Linking Emotional Reactivity Between Laboratory Tasks and Immersive Environments Using Behavior and Physiology. Front. Hum. Neurosci. 2019, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Y.; Bruya, B. Mechanisms of Mind-Body Interaction and Optimal Performance. Front. Psychol. 2017, 8, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, Y.C.; Tzeng, N.S.; Chao, C.Y.; Chang, C.C.; Chang, H.A. Modulation of self-appraisal of illness, medication adherence, life quality and autonomic functioning by transcranial direct current stimulation in schizophrenia patients. Clin. Neurophysiol. 2020, 131, 1997–2007. [Google Scholar] [CrossRef]
- Chang, C.C.; Kao, Y.C.; Chao, C.Y.; Tzeng, N.S.; Chang, H.A. The Effects of Bi-Anodal tDCS Over the Prefrontal Cortex Regions With Extracephalic Reference Placement on Insight Levels and Cardio-Respiratory and Autonomic Functions in Schizophrenia Patients and Exploratory Biomarker Analyses for Treatment Response. Int. J. Neuropsychopharmacol. 2021, 24, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. The polyvagal perspective. Biol. Psychol. 2007, 74, 116–143. [Google Scholar] [CrossRef]
- Sreeraj, V.S.; Shanbhag, V.; Nawani, H.; Shivakumar, V.; Damodharan, D.; Bose, A.; Narayanaswamy, J.C.; Venkatasubramanian, G. Feasibility of Online Neuromodulation Using Transcranial Alternating Current Stimulation in Schizophrenia. Indian J. Psychol. Med. 2017, 39, 92–95. [Google Scholar] [CrossRef] [Green Version]
- Brunelin, J.; Fecteau, S.; Suaud-Chagny, M.F. Abnormal striatal dopamine transmission in schizophrenia. Curr. Med. Chem. 2013, 20, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Goto, Y.; Otani, S.; Grace, A.A. The Yin and Yang of dopamine release: A new perspective. Neuropharmacology 2007, 53, 583–587. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Liu, C.L.; Yang, S.; Jin, G.Z.; Bunney, B.S.; Shi, W.X. Functional coupling between the prefrontal cortex and dopamine neurons in the ventral tegmental area. J. Neurosci. 2007, 27, 5414–5421. [Google Scholar] [CrossRef]
- Fujisawa, S.; Buzsaki, G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron 2011, 72, 153–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bor, J.; Brunelin, J.; Rivet, A.; d’Amato, T.; Poulet, E.; Saoud, M.; Padberg, F. Effects of theta burst stimulation on glutamate levels in a patient with negative symptoms of schizophrenia. Schizophr. Res. 2009, 111, 196–197. [Google Scholar] [CrossRef]
- Brunelin, J.; Szekely, D.; Costes, N.; Mondino, M.; Bougerol, T.; Saoud, M.; Suaud-Chagny, M.F.; Poulet, E.; Polosan, M. Theta burst stimulation in the negative symptoms of schizophrenia and striatal dopamine release. An iTBS-[11C]raclopride PET case study. Schizophr. Res. 2011, 131, 264–265. [Google Scholar] [CrossRef]
- Kallel, L.; Mondino, M.; Brunelin, J. Effects of theta-rhythm transcranial alternating current stimulation (4.5 Hz-tACS) in patients with clozapine-resistant negative symptoms of schizophrenia: A case series. J. Neural. Transm. 2016, 123, 1213–1217. [Google Scholar] [CrossRef]
- Tremblay, S.; Lepage, J.F.; Latulipe-Loiselle, A.; Fregni, F.; Pascual-Leone, A.; Theoret, H. The uncertain outcome of prefrontal tDCS. Brain Stimul. 2014, 7, 773–783. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yi, Z.H.; Lv, Q.Y.; Chu, M.Y.; Hu, H.X.; Wang, J.H.; Zhang, J.Y.; Cheung, E.E.F.; Chan, R.C.K. Clinical utility of the dual n-back task in schizophrenia: A functional imaging approach. Psychiatry Res. Neuroimaging 2019, 284, 37–44. [Google Scholar] [CrossRef]
- Jaeggi, S.M.; Buschkuehl, M.; Jonides, J.; Perrig, W.J. Improving fluid intelligence with training on working memory. Proc. Natl. Acad. Sci. USA 2008, 105, 6829–6833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweizer, S.; Grahn, J.; Hampshire, A.; Mobbs, D.; Dalgleish, T. Training the emotional brain: Improving affective control through emotional working memory training. J. Neurosci. 2013, 33, 5301–5311. [Google Scholar] [CrossRef] [Green Version]
- Liemburg, E.; Castelein, S.; Stewart, R.; van der Gaag, M.; Aleman, A.; Knegtering, H.; Genetic, R.; Outcome of Psychosis, I. Two subdomains of negative symptoms in psychotic disorders: Established and confirmed in two large cohorts. J. Psychiatr. Res. 2013, 47, 718–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galderisi, S.; Mucci, A.; Buchanan, R.W.; Arango, C. Negative symptoms of schizophrenia: New developments and unanswered research questions. Lancet Psychiatry 2018, 5, 664–677. [Google Scholar] [CrossRef]
- Roth, M.M.; Dahmen, J.C.; Muir, D.R.; Imhof, F.; Martini, F.J.; Hofer, S.B. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nat. Neurosci. 2016, 19, 299–307. [Google Scholar] [CrossRef]
- Chou, X.L.; Fang, Q.; Yan, L.; Zhong, W.; Peng, B.; Li, H.; Wei, J.; Tao, H.W.; Zhang, L.I. Contextual and cross-modality modulation of auditory cortical processing through pulvinar mediated suppression. eLife 2020, 9, e54157. [Google Scholar] [CrossRef]
- Fang, Q.; Chou, X.L.; Peng, B.; Zhong, W.; Zhang, L.I.; Tao, H.W. A Differential Circuit via Retino-Colliculo-Pulvinar Pathway Enhances Feature Selectivity in Visual Cortex through Surround Suppression. Neuron 2020, 105, 355–369.e6. [Google Scholar] [CrossRef]
- Ibrahim, L.A.; Mesik, L.; Ji, X.Y.; Fang, Q.; Li, H.F.; Li, Y.T.; Zingg, B.; Zhang, L.I.; Tao, H.W. Cross-Modality Sharpening of Visual Cortical Processing through Layer-1-Mediated Inhibition and Disinhibition. Neuron 2016, 89, 1031–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, N.; Masterson, S.P.; Damron, J.K.; Guido, W.; Bickford, M.E. The Mouse Pulvinar Nucleus Links the Lateral Extrastriate Cortex, Striatum, and Amygdala. J. Neurosci. 2018, 38, 347–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.I.K.; Kwak, Y.B.; Hwang, W.J.; Lee, J.; Kim, M.; Lee, T.Y.; Kwon, J.S. Microstructural Changes in Higher-Order Nuclei of the Thalamus in Patients With First-Episode Psychosis. Biol. Psychiatry 2019, 85, 70–78. [Google Scholar] [CrossRef]
- Shen, L.; Liu, D.; Huang, Y. Hypothesis of subcortical visual pathway impairment in schizophrenia. Med. Hypotheses 2021, 156, 110686. [Google Scholar] [CrossRef] [PubMed]
- Zaehle, T.; Rach, S.; Herrmann, C.S. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS ONE 2010, 5, e13766. [Google Scholar] [CrossRef] [Green Version]
- Vossen, A.; Gross, J.; Thut, G. Alpha Power Increase After Transcranial Alternating Current Stimulation at Alpha Frequency (alpha-tACS) Reflects Plastic Changes Rather Than Entrainment. Brain Stimul. 2015, 8, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Battleday, R.M.; Muller, T.; Clayton, M.S.; Cohen Kadosh, R. Mapping the mechanisms of transcranial alternating current stimulation: A pathway from network effects to cognition. Front. Psychiatry 2014, 5, 162. [Google Scholar] [CrossRef] [Green Version]
- Mulcahy, J.S.; Larsson, D.E.O.; Garfinkel, S.N.; Critchley, H.D. Heart rate variability as a biomarker in health and affective disorders: A perspective on neuroimaging studies. Neuroimage 2019, 202, 116072. [Google Scholar] [CrossRef] [PubMed]
- Mandrick, K.; Peysakhovich, V.; Remy, F.; Lepron, E.; Causse, M. Neural and psychophysiological correlates of human performance under stress and high mental workload. Biol. Psychol. 2016, 121, 62–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleman, A.; Enriquez-Geppert, S.; Knegtering, H.; Dlabac-de Lange, J.J. Moderate effects of noninvasive brain stimulation of the frontal cortex for improving negative symptoms in schizophrenia: Meta-analysis of controlled trials. Neurosci. Biobehav. Rev. 2018, 89, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Jeon, D.W.; Jung, D.U.; Kim, S.J.; Shim, J.C.; Moon, J.J.; Seo, Y.S.; Jung, S.S.; Seo, B.J.; Kim, J.E.; Oh, M.; et al. Adjunct transcranial direct current stimulation improves cognitive function in patients with schizophrenia: A double-blind 12-week study. Schizophr. Res. 2018, 197, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Wykes, T.; Huddy, V.; Cellard, C.; McGurk, S.R.; Czobor, P. A meta-analysis of cognitive remediation for schizophrenia: Methodology and effect sizes. Am. J. Psychiatry 2011, 168, 472–485. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Active tACS | Sham | t/U or χ2/Fisher’s | p-Value |
|---|---|---|---|---|
| (n = 18) | (n = 18) | |||
| Schizophrenia/schizoaffective disorder | 13/5 | 15/3 | 0.64 | 0.69 |
| Females, n (%) | 8 (44.40) | 10 (55.60) | 0.44 | 0.51 |
| Age, years | 41.78 ± 8.84 | 43.17 ± 11.20 | 0.41 | 0.68 |
| Education level, years | 14.44 ± 3.13 | 12.67 ± 2.79 | 112.5 | 0.1 |
| BMI, kg/m2 | 26.50 ± 6.14 | 27.30 ± 5.37 | 141 | 0.51 |
| Weekly regular exercise, hours | 2.63 ± 3.79 | 2.45 ± 2.67 | 154 | 0.8 |
| Handedness (right-left) | 17/1 | 15/3 | 1.13 | 0.6 |
| Smoker, n (%) | 8 (44.40) | 3 (16.70) | 3.27 | 0.15 |
| Hypertension, n (%) | 3 (16.70%) | 3 (16.70%) | 0 | 1 |
| Diabetes mellitus, n (%) | 5 (27.80%) | 1 (5.60%) | 3.2 | 0.18 |
| Onset age, years | 27.44 ± 6.99 | 25.94 ± 7.44 | −0.62 | 0.54 |
| Length of illness, years | 15.28 ± 10.31 | 17.28 ± 10.58 | 136.5 | 0.42 |
| Antipsychotic dosage, mg/day a | 21.50 ± 14.04 | 19.03 ± 13.46 | 177 | 0.65 |
| Anticholinergic dosage, mg/day b | 0.72 ± 1.60 | 1.06 ± 1.47 | 136.5 | 0.42 |
| Sedative-hypnotic dosage, mg/day c | 37.22 ± 42.50 | 13.89 ± 17.37 | 200 | 0.24 |
| PANSS total score | 72.44 ± 9.72 | 74.11 ± 7.30 | 0.58 | 0.57 |
| PANSS negative subscale score | 19.22 ± 3.86 | 19.83 ± 3.63 | 0.49 | 0.63 |
| PANSS five-factor score | ||||
| PANSS-FSNS | 21.67 ± 4.96 | 21.94 ± 4.18 | 0.18 | 0.86 |
| PANSS-FSPS | 13.56 ± 5.22 | 13.94 ± 3.93 | 148.5 | 0.67 |
| Excited | 5.78 ± 2.34 | 5.83 ± 1.98 | 147 | 0.62 |
| Cognitive | 9.94 ± 1.70 | 10.50 ± 1.69 | −0.98 | 0.33 |
| Emotional/depressed | 6.78 ± 2.46 | 6.39 ± 1.58 | 161.5 | 0.99 |
| PANSS two-subdomain score of negative symptoms | ||||
| Exp Neg | 16.50 ± 3.59 | 17.17 ± 3.20 | 145.5 | 0.6 |
| Soc Amot | 8.94 ± 2.39 | 9.11 ± 1.60 | 0.25 | 0.81 |
| SANS | 51.94 ± 11.87 | 52.61 ± 10.05 | −0.18 | 0.86 |
| Active tACS | Sham | Cohen’s d | 95% CI | p a | |
|---|---|---|---|---|---|
| Variables | Mean ± SD | Mean ± SD | |||
| Primary outcome | |||||
| PANSS negative subscale score | −2.67 ± 2.14 | −0.72 ± 1.84 | 0.96 | 0.27 to 1.65 | 0.006 |
| Secondary outcomes | |||||
| PANSS total score | −5.28 ± 3.98 | −0.89 ± 4.39 | 1.02 | 0.33 to 1.72 | 0.003 |
| PANSS five-factor score | |||||
| PANSS-FSPS | −0.22 ± 0.73 | 0.06 ± 0.64 | 0.4 | −0.26 to 1.06 | 0.23 |
| PANSS-FSNS | −2.44 ± 2.59 | −0.56 ± 1.85 | 0.82 | 0.14 to 1.50 | 0.017 |
| Excited | −0.11± 0.32 | 0.11± 0.47 | 0.54 | −0.12 to 1.20 | 0.11 |
| Cognitive | −1.83 ± 1.15 | −0.56 ± 1.29 | 1.02 | 0.32 to 1.71 | <0.001 |
| Emotional/depressed | −0.11 ± 0.58 | 0.28 ± 1.13 | 0.42 | −0.24 to 1.09 | 0.2 |
| PANSS two-subdomain score of negative symptoms | |||||
| Exp Neg | −0.78 ± 1.00 | −0.56 ± 1.15 | 0.2 | −0.46 to 1.50 | 0.54 |
| Soc Amot | −2.17 ± 2.62 | −0.39 ± 1.42 | 0.82 | 0.15 to 1.51 | 0.016 |
| SANS score | −7.11 ± 5.65 | −2.17 ± 4.95 | 0.91 | 0.22 to 1.60 | 0.008 |
| Dual 2-back task accuracy, % | 30.39 ± 14.70 | 10.50 ± 10.00 | 1.55 | 0.80 to 2.29 | <0.001 |
| Variables | Active tACS | Sham | Cohen’s d | 95% CI | p a |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| Other secondary outcomes | |||||
| PSP | |||||
| Social useful activities | −0.44 ± 0.51 | −0.11 ± 0.32 | 0.76 | 0.08 to 1.43 | 0.03 |
| Personal and social relationships | −0.17 ± 0.38 | 0.06 ± 0.42 | 0.56 | −0.10 to 1.23 | 0.09 |
| Self-care | −0.28 ± 0.46 | 0.00 ± 0.69 | 0.47 | −0.20 to 1.13 | 0.16 |
| Disturbing and aggressive behavior | −0.28 ± 0.46 | −0.17 ± 0.51 | 0.22 | −0.43 to 0.88 | 0.5 |
| Global score | 3.72 ± 3.49 | 0.56 ± 2.83 | 0.97 | 0.28 to 1.66 | 0.01 |
| SUMD | |||||
| Awareness of disease | −0.62 ± 2.62 | 0.00 ± 0.00 | 0.33 | −0.33 to 0.98 | 0.32 |
| Awareness of positive symptoms | −0.00 ± 3.81 | 0.00 ± 0.00 | 0 | −0.65 to 0.65 | 1 |
| Awareness of negative symptoms | −3.09 ± 9.18 | −0.62 ± 2.62 | 0.36 | −0.30 to 1.02 | 0.28 |
| SRG-PSP | |||||
| Social useful activities | 0.78 ± 1.73 | −1.11 ± 2.78 | 0.8 | 0.12 to 1.48 | 0.02 |
| Personal and social relationships | −0.28 ± 2.14 | 0.61 ± 2.30 | 0.39 | −0.27 to 1.05 | 0.24 |
| Self-care | −0.11 ± 1.64 | −0.06 ± 2.07 | 0.03 | −0.63 to 0.68 | 0.94 |
| Disturbing and aggressive behavior | −0.22 ± 0.94 | −0.17 ± 2.43 | 0.03 | −0.63 to 0.68 | 0.94 |
| Global score | 0.61 ± 4.13 | −0.39 ± 6.40 | 0.18 | −0.47 to 0.84 | 0.58 |
| SAIQ | |||||
| Total score | 0.17 ± 4.62 | −3.83 ± 9.13 | 0.54 | −0.12 to 1.21 | 0.11 |
| Worry | −0.11 ± 3.92 | −2.28 ± 5.75 | 0.43 | −0.23 to 1.09 | 0.19 |
| Need treatment | 0.33 ± 1.71 | −1.00 ± 2.17 | 0.67 | −0.01 to 1.34 | 0.05 |
| Presence/outcome | −0.06 ± 2.51 | −0.67 ± 3.68 | 0.19 | −0.47 to 0.84 | 0.57 |
| BCIS | |||||
| BCIS-R | −1.17 ± 3.40 | 1.72 ± 5.53 | 0.62 | −0.05 to 1.28 | 0.07 |
| BCIS-C | −1.00 ± 2.57 | 1.78 ± 2.78 | 1.02 | 0.32 to 1.71 | <0.001 |
| R-C index | −0.17 ± 3.73 | −0.06 ± 6.30 | 0.02 | −0.63 to 0.67 | 0.95 |
| SQLS-R4 | |||||
| Total | −6.83 ± 13.60 | −2.61 ± 18.09 | 0.26 | −0.40 to 0.91 | 0.43 |
| Psychosocial | −4.39 ± 9.62 | −0.78 ± 11.07 | 0.34 | −0.32 to 1.00 | 0.3 |
| Vitality | −2.44 ± 6.31 | −1.83 ± 7.84 | 0.08 | −0.57 to 0.74 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Huang, C.C.-Y.; Chung, Y.-A.; Im, J.J.; Lin, Y.-Y.; Ma, C.-C.; Tzeng, N.-S.; Chang, H.-A. Online Left-Hemispheric In-Phase Frontoparietal Theta tACS for the Treatment of Negative Symptoms of Schizophrenia. J. Pers. Med. 2021, 11, 1114. https://doi.org/10.3390/jpm11111114
Chang C-C, Huang CC-Y, Chung Y-A, Im JJ, Lin Y-Y, Ma C-C, Tzeng N-S, Chang H-A. Online Left-Hemispheric In-Phase Frontoparietal Theta tACS for the Treatment of Negative Symptoms of Schizophrenia. Journal of Personalized Medicine. 2021; 11(11):1114. https://doi.org/10.3390/jpm11111114
Chicago/Turabian StyleChang, Chuan-Chia, Cathy Chia-Yu Huang, Yong-An Chung, Jooyeon Jamie Im, Yen-Yue Lin, Chin-Chao Ma, Nian-Sheng Tzeng, and Hsin-An Chang. 2021. "Online Left-Hemispheric In-Phase Frontoparietal Theta tACS for the Treatment of Negative Symptoms of Schizophrenia" Journal of Personalized Medicine 11, no. 11: 1114. https://doi.org/10.3390/jpm11111114
APA StyleChang, C.-C., Huang, C. C.-Y., Chung, Y.-A., Im, J. J., Lin, Y.-Y., Ma, C.-C., Tzeng, N.-S., & Chang, H.-A. (2021). Online Left-Hemispheric In-Phase Frontoparietal Theta tACS for the Treatment of Negative Symptoms of Schizophrenia. Journal of Personalized Medicine, 11(11), 1114. https://doi.org/10.3390/jpm11111114









